Pigmented Melanoma Cell Migration Study on Murine Syngeneic B16F10 Melanoma Cells or Tissue Transplantation Models
Highlights
- The syngeneic B16F10 melanoma tissue transplantation in C57BL/6 mice was a viable technique for the study of melanoma cell behavior.
- The melanic pigment loading level and the ability to remove the pigment seem to be defining elements of the migratory behavior and aggressiveness of the B16F10 melanoma cells.
Abstract
:Highlights
- The syngeneic B16F10 melanoma tissue transplantation in C57BL/6 mice was a viable technique for the study of melanoma cell behavior.
- The melanic pigment loading level and the ability to remove the pigment seem to be defining elements of the migratory behavior and aggressiveness of the B16F10 melanoma cells.
Abstract
Introduction
Materials and Methods
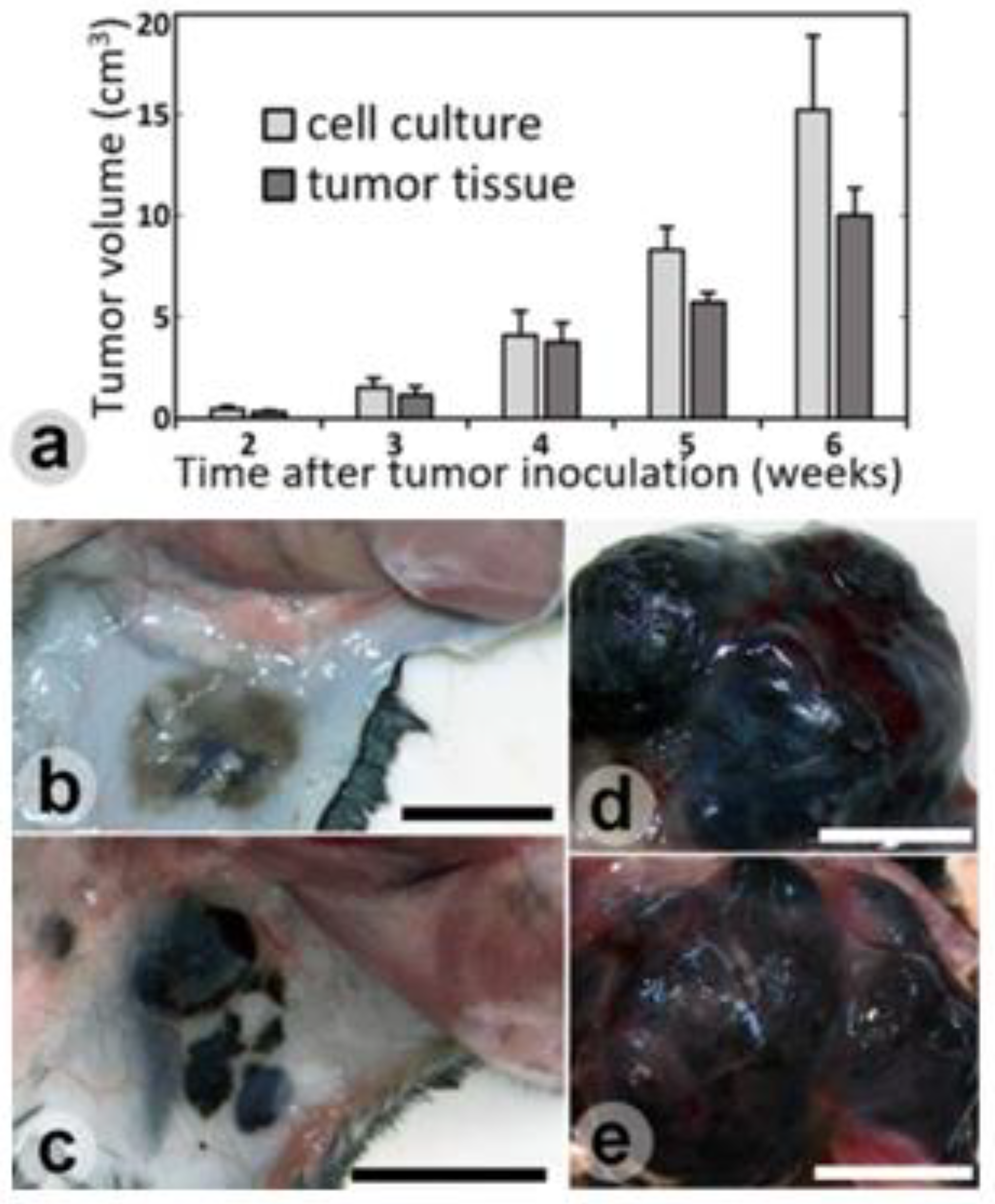
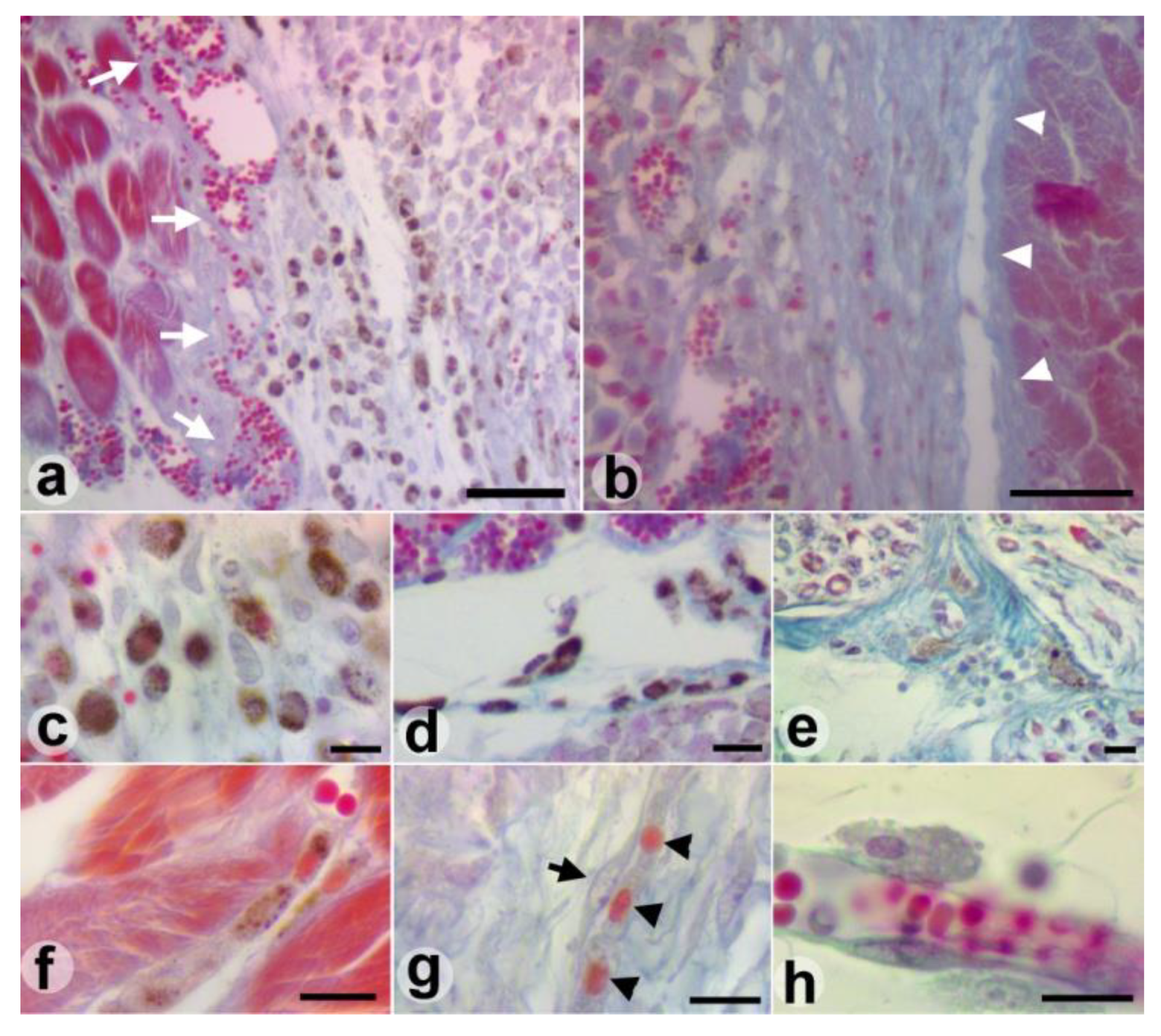
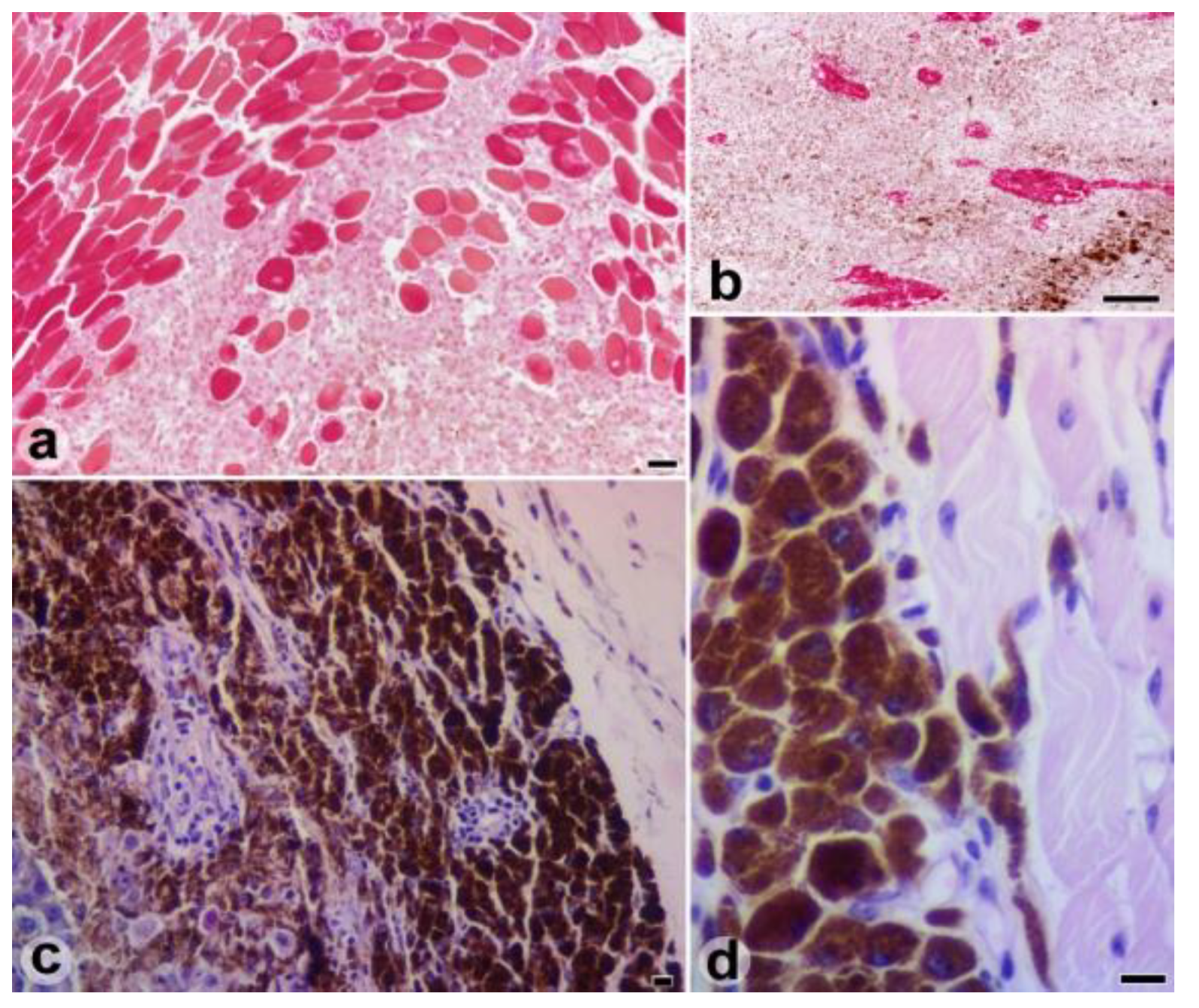
Results
Discussions
Conclusions
References
- Balch, C.M.; Soong, S.J.; Gershenwald, J.E.; et al. Prognostic factors analysis of 17,600 melanoma patients: Validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001, 19, 3622–3634. [Google Scholar] [CrossRef]
- Anbari, K.K.; Schuchter, L.M.; Bucky, L.P.; et al. Melanoma of unknown primary site: presentation, treatment, and prognosis-a single institution study. University of Pennsylvania Pigmented Lesion Study Group. Cancer. 1997, 79, 1816–1821. [Google Scholar] [CrossRef]
- Fidler, I.J. Selection of successive tumour lines for metastasis. Nat New Biol. 1973, 242, 148–149. [Google Scholar] [CrossRef]
- Caisová, V.; Vieru, A.; Kumžáková, Z.; et al. Innate immunity based cancer immunotherapy: B16-F10 murine melanoma model. BMC Cancer. 2016, 16, 940. [Google Scholar] [CrossRef] [PubMed]
- Eberting, C.L.; Shrayer, D.P.; Butmarc, J.; Falanga, V. Histologic progression of B16 F10 metastatic melanoma in C57BL/6 mice over a six-week time period: distant metastases before local growth. J Dermatol. 2004, 31, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Sonoda, K.; Fujii, K.; Ishikawa, K.; Shiraishi, N.; Kitano, S. A convenient murine model for the study of intra-abdominal lymph node metastasis. Oncol Rep. 2004, 12, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, G.I.; Monahan, K.B.; Torrice, C.; Bear, J.E.; Sharpless, N.E. Metastasis in an orthotopic murine model of melanoma is independent of RAS/RAF mutation. Melanoma Res. 2010, 20, 361–371. [Google Scholar] [CrossRef]
- Lugassy, C.; Zadran, S.; Bentolila, L.A.; et al. Angiotropism, pericytic mimicry and extravascular migratory metastasis in melanoma: an alternative to intravascular cancer dissemination. Cancer Microenviron. 2014, 7, 139–152. [Google Scholar] [CrossRef]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef]
- Bugyik, E.; Renyi-Vamos, F.; Szabo, V.; et al. Mechanisms of vascularization in murine models of primary and metastatic tumor growth. Chin J Cancer 2016, 35, 19. [Google Scholar] [CrossRef]
- Bentolila, L.A.; Prakash, R.; Mihic-Probst, D.; et al. Imaging of Angiotropism/Vascular Co-Option in a Murine Model of Brain Melanoma: Implications for Melanoma Progression along Extravascular Pathways. Sci Rep 2016, 6, 23834. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.S. What is the optimal rodent model for anti- tumor drug testing? Cancer Metastasis Rev. 1998- 1999, 17, 301–304. [Google Scholar]
- Killion, J.J.; Radinsky, R.; Fidler, I.J. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1998- 1999, 17, 279–284. [Google Scholar] [CrossRef]
- Hoffman, R.M. Orthotropic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999, 17, 343–359. [Google Scholar] [PubMed]
- Fidler, I.J.; Nicolson, G.L. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J Natl Cancer Inst. 1976, 57, 1199–1202. [Google Scholar]
- Herlyn, M.; Fukunaga-Kalabis, M. What is a good model for melanoma? J Invest Dermatol. 2010, 130, 911–912. [Google Scholar] [CrossRef]
- Zbytek, B.; Carlson, J.A.; Granese, J.; Ross, J.; Mihm, M.C.; Slominski, A. Current concepts of metastasis in melanoma. Expert Rev Dermatol. 2008, 3, 569–585. [Google Scholar]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011, 147, 275–292. [Google Scholar]
- Mishra, H.; Mishra, P.K.; Ekielski, A.; Jaggi, M.; Iqbal, Z.; Talegaonkar, S. Melanoma treatment: from conventional to nanotechnology. J Cancer Res Clin Oncol. 2018, 144, 2283–2302. [Google Scholar]
- Mîndrilă, I.; Buteică, S.A.; Mihaiescu, D.E.; Badea, G.; Fudulu, A.; Mărgăritescu, D.N. Fe3O4/salicylic acid nanoparticles versatility in magnetic mediated vascular nanoblockage. J Nanopart Res. 2016, 18, 10. [Google Scholar]
- Zhou, Z.; Zheng, C.; Li, S.; et al. AFM nanoindentation detection of the elastic modulus of tongue squamous carcinoma cells with different metastatic potentials. Nanomedicine 2013, 9, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Park, S. Nano-mechanical phenotype as a promising biomarker to evaluate cancer development, progression, and anti-cancer drug efficacy. J Cancer Prev. 2016, 21, 73–80. [Google Scholar] [CrossRef]
- Sarna, M.; Zadlo, A.; Czuba-Pelech, B.; Urbanska, K. Nanomechanical Phenotype of Melanoma Cells Depends Solely on the Amount of Endogenous Pigment in the Cells. Int J Mol Sci. 2018, 19, 607. [Google Scholar] [CrossRef]
- Pinner, S.; Jordan, P.; Sharrock, K.; et al. Intravital imaging reveals transient changes in pigment production and Brn2 expression during metastatic melanoma dissemination. Cancer Res. 2009, 69, 7969–7977. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kawamura, T.; Furuhashi, M.; Tsukamoto, K.; Shimada, S. Improving chemotherapeutic drug penetration in melanoma by imatinib mesylate. J Dermatol Sci. 2008, 51, 190–199. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, H.; Zhang, J.; et al. Safety and Efficacy of Transplantation with Allogeneic Skin Tumors to Treat Chemically-Induced Skin Tumors in Mice. Med Sci Monit 2016, 22, 3113–3123. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Radisky, D. Putting tumours in context. Nat Rev Cancer. 2001, 1, 46–54. [Google Scholar] [CrossRef]
- Pye, R.J.; Pemberton, D.; Tovar, C.; et al. A second transmissible cancer in Tasmanian devils. Proc Natl Acad Sci U S A. 2016, 113, 374–379. [Google Scholar] [CrossRef]
- Aung, P.P.; Nagarajan, P.; Prieto, V.G. Regression in primary cutaneous melanoma: etiopathogenesis and clinical significance. Lab Invest 2017, 97, 657–668. [Google Scholar] [CrossRef]
- Lazova, R.; Pawelek, J.M. Why do melanomas get so dark? Exp Dermatol. 2009, 18, 934–938. [Google Scholar] [CrossRef]
- D’Ischia, M.; Wakamatsu, K.; Napolitano, A.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [PubMed]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Flach, E.H.; Rebecca, V.W.; Herlyn, M.; Smalley, K.S.; Anderson, A.R. Fibroblasts contribute to melanoma tumor growth and drug resistance. Mol Pharmacol. 2011, 8, 2039–2049. [Google Scholar]
- Zhou, L.; Yang, K.; Andl, T.; Wickett, R.R.; Zhang, Y. Perspective of Targeting Cancer-Associated Fibroblasts in Melanoma. J Cancer. 2015, 6, 717–726. [Google Scholar] [CrossRef]
- Löffek, S.; Zigrino, P.; Angel, P.; Anwald, B.; Krieg, T.; Mauch, C. High invasive melanoma cells induce matrix metalloproteinase-1 synthesis in fibroblasts by interleukin-1alpha and basic fibroblast growth factor- mediated mechanisms. J Invest Dermatol. 2005, 124, 638–643. [Google Scholar]
- Marques, P.C.; Diniz, L.M.; Spelta, K.; Nogueira, P.S.E. Desmoplastic melanoma: a rare variant with challenging diagnosis. A Bras Dermatol. 2019, 94, 82–85. [Google Scholar]
- Sarna, M.; Zadlo, A.; Hermanowicz, P.; Madeja, Z.; Burda, K.; Sarna, T. Cell elasticity is an important indicator of the metastatic phenotype of melanoma cells. Exp Dermatol. 2014, 23, 813–818. [Google Scholar]
- Thomas, N.E.; Kricker, A.; Waxweiler, W.T.; et al. Comparison of clinicopathologic features and survival of histopathologically amelanotic and pigmented melanomas: A population-based study. JAMA Dermatol. 2014, 150, 1306–1314. [Google Scholar] [CrossRef]
- Vernali, S.; Waxweiler, W.T.; Dillon, P.M.; et al. Association of Incident Amelanotic Melanoma with Phenotypic Characteristics, MC1R Status, and Prior Amelanotic Melanoma. JAMA Dermatol. 2017, 153, 1026–1031. [Google Scholar]

© 2019 by the authors. 2019 Maria-Cristina Predoi, Ion Mîndrilă, Sandra Alice Buteică, Ovidiu Marcel Mărginean, Bogdan Mîndrilă, Mihaela Niculescu
Share and Cite
Predoi, M.-C.; Mîndrilă, I.; Buteică, S.A.; Mărginean, O.M.; Mîndrilă, B.; Niculescu, M. Pigmented Melanoma Cell Migration Study on Murine Syngeneic B16F10 Melanoma Cells or Tissue Transplantation Models. J. Mind Med. Sci. 2019, 6, 327-333. https://doi.org/10.22543/7674.62.P327333
Predoi M-C, Mîndrilă I, Buteică SA, Mărginean OM, Mîndrilă B, Niculescu M. Pigmented Melanoma Cell Migration Study on Murine Syngeneic B16F10 Melanoma Cells or Tissue Transplantation Models. Journal of Mind and Medical Sciences. 2019; 6(2):327-333. https://doi.org/10.22543/7674.62.P327333
Chicago/Turabian StylePredoi, Maria-Cristina, Ion Mîndrilă, Sandra Alice Buteică, Ovidiu Marcel Mărginean, Bogdan Mîndrilă, and Mihaela Niculescu. 2019. "Pigmented Melanoma Cell Migration Study on Murine Syngeneic B16F10 Melanoma Cells or Tissue Transplantation Models" Journal of Mind and Medical Sciences 6, no. 2: 327-333. https://doi.org/10.22543/7674.62.P327333
APA StylePredoi, M.-C., Mîndrilă, I., Buteică, S. A., Mărginean, O. M., Mîndrilă, B., & Niculescu, M. (2019). Pigmented Melanoma Cell Migration Study on Murine Syngeneic B16F10 Melanoma Cells or Tissue Transplantation Models. Journal of Mind and Medical Sciences, 6(2), 327-333. https://doi.org/10.22543/7674.62.P327333


