Insights Into the Pathogenesis of Nicotine Addiction. Could a Salivary Biosensor Be Useful in Nicotine Replacement Therapy (NRT)?
Highlights
- Smoking addiction has strong pharmacological, psycho-social, and media support.
- The development of a double-effect salivary biosensor could represent a useful approach in nicotine addiction therapy.
Abstract
Highlights
- ✓ Smoking addiction has strong pharmacological, psycho-social, and media support.
- ✓ The development of a double-effect salivary biosensor could represent a useful approach in nicotine addiction therapy.
Introduction
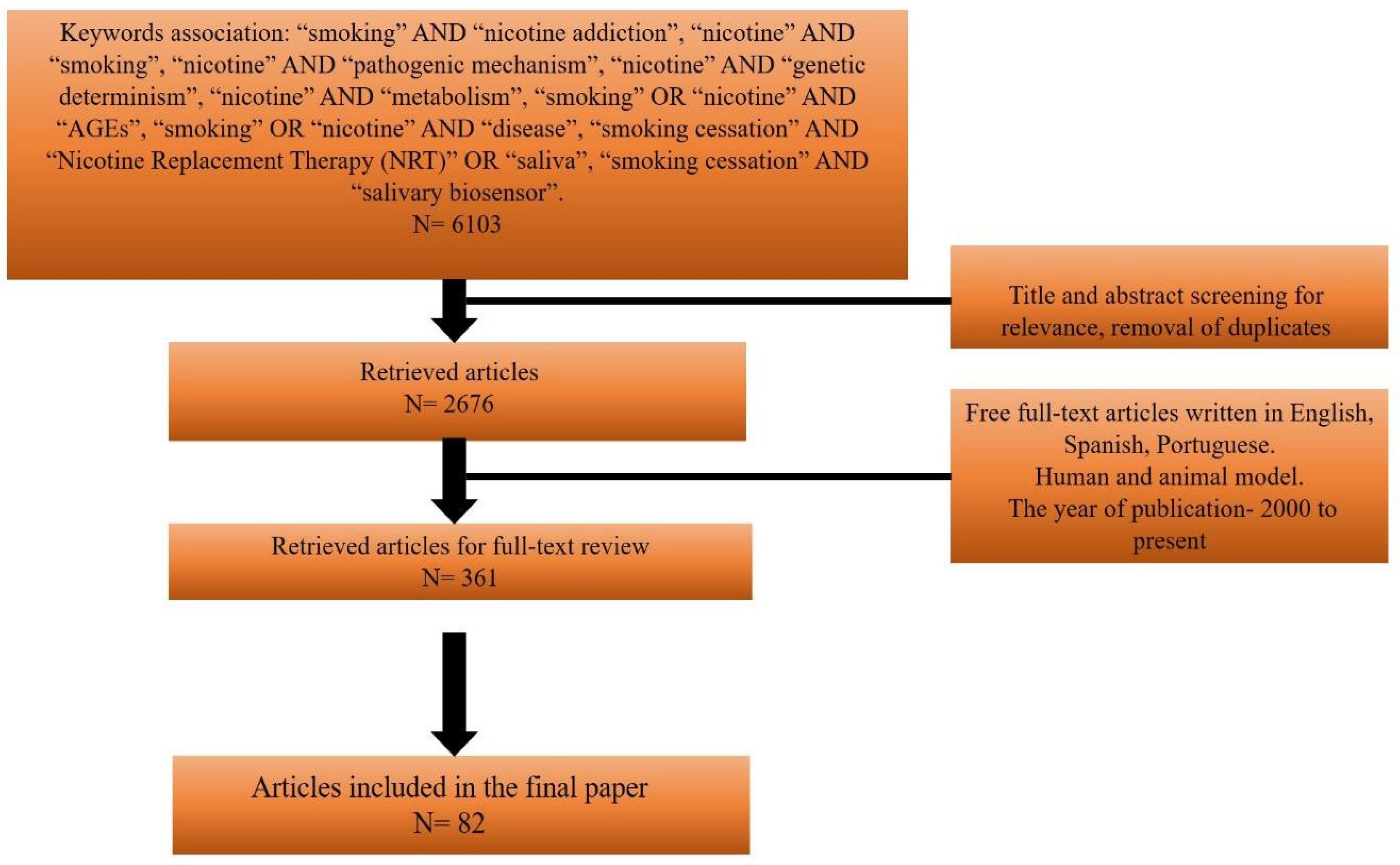
Materials and Methods
Results
1. Nicotine addiction
1.1. Nicotine and smoking
1.2. Pathogenic molecular mechanisms of nicotine addiction
1.3. Nicotine metabolism
1.4. Genetic determinism of nicotine addiction
1.5. Genetic determinism in smoking cessation
2. Social context and medical implications
3. The correlation between nicotine and Advanced Glycation End Products (AGEs)
4. Treatment strategy in nicotine addiction
4.1. Electronic cigarettes (ECs)—a healthier alternative?
4.2. Old habits die hard—perspectives?
Discussions
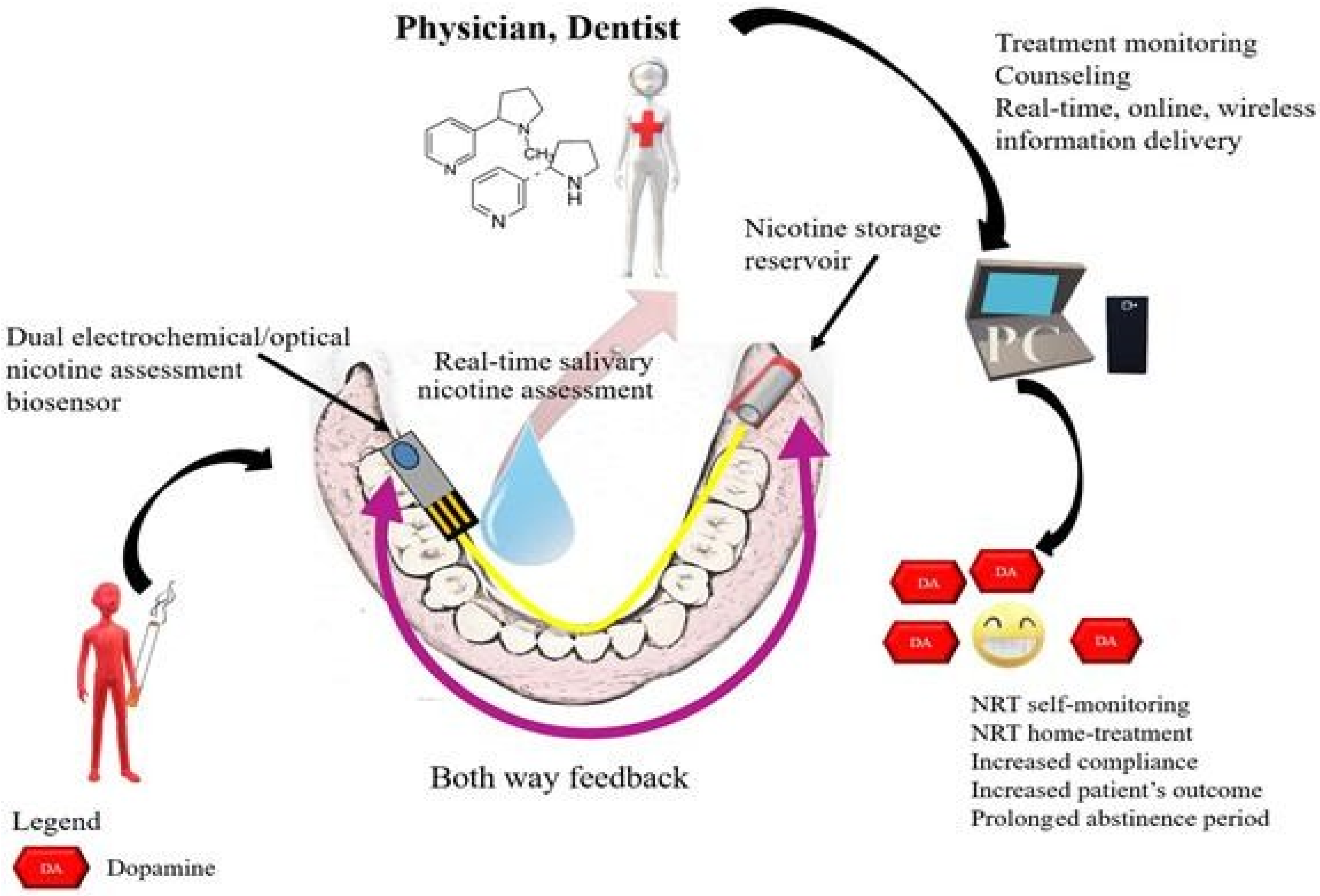
Biosensors, nicotine assessment and NRT
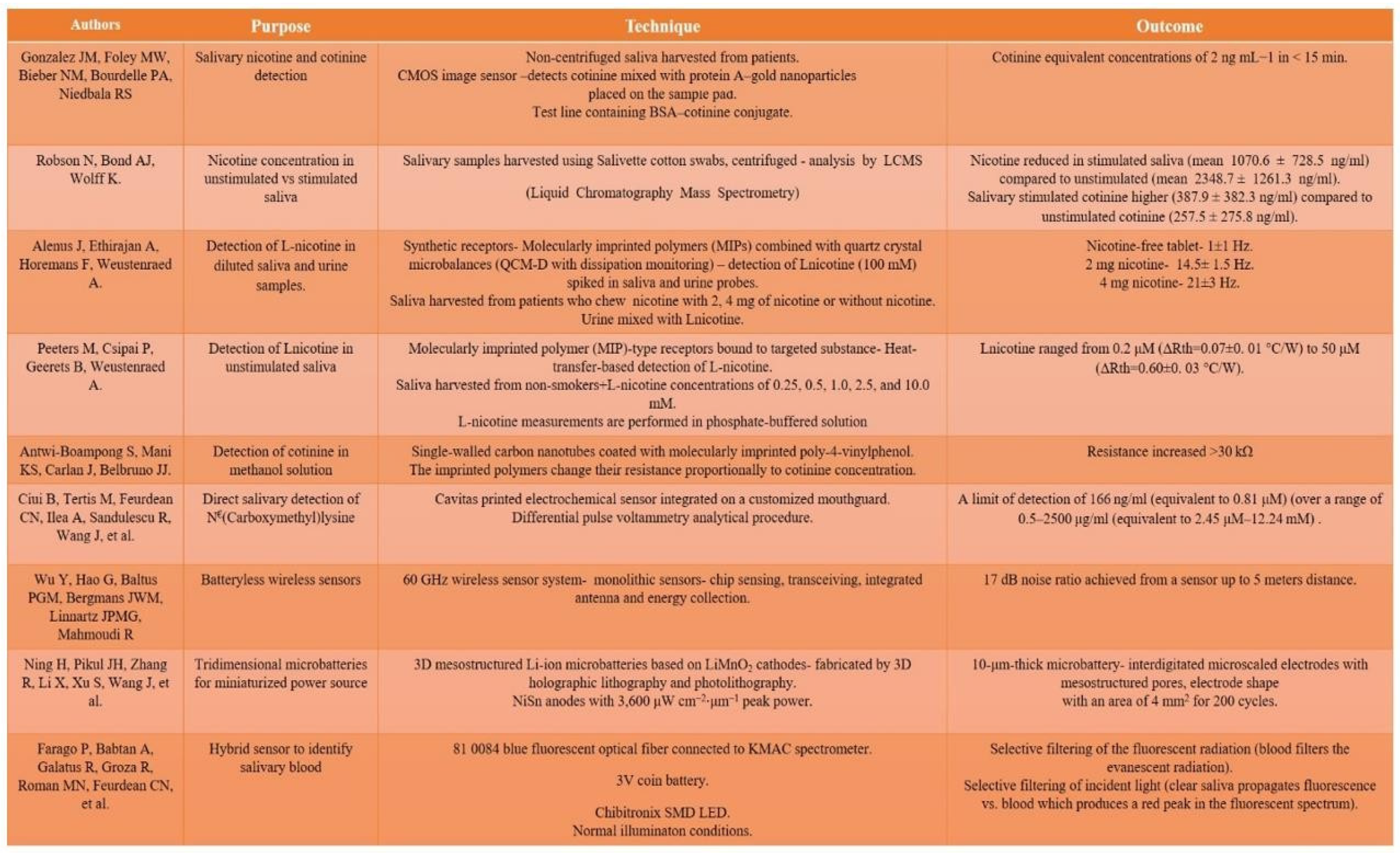 |
Conclusions
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Nicotine. [Internet]. [cited 2018 Nov 22]. Available online: https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&ns=NCI_Thesaurus&code=C691.
- Heckewelder, J.; Rechel, W. History, Manners and Customs of the Indian Nations who Once Inhabited Pennsylvania; Historical Society of Pennsylvania: Philadelphia, USA, 1881; p. 149. [Google Scholar]
- Appleby, J. The Relentless Revolution: A History of Capitalism. W.W. Norton & Company 2010, 131. [Google Scholar]
- Lewis, J.B.; Hirschi, K.M.; Arroyo, J.A.; Bikman, B.T.; Kooyman, D.L.; Reynolds, P.R. Plausible roles for RAGE in conditions exacerbated by direct and indirect (secondhand) smoke exposure. Int J Mol Sci. 2017, 18, 1–20. [Google Scholar] [CrossRef]
- DiFranza, J.; Ursprung, W.; Carlson, A. New insights into the compulsion to use tobacco from a case series. J Adolesc. 2010, 33, 209–214. [Google Scholar]
- DiFranza, J.R. Can tobacco dependence provide insights into other drug addictions? BMC Psychiatry. 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Van Voorhees, E.; Mitchell, J.; McClernon, F.; Beckham, J.; Kollins, S. Sex, ADHD symptoms, and smoking outcomes: An integrative model. Med Hypotheses. 2012, 78, 585–593. [Google Scholar]
- Johnstone, E.; Benowitz, N.; Cargill, A.; Jacob, R.; Hinks, L.; Day, I.; et al. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharmacol Ther. 2006, 80, 319–330. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Lessov-Schlaggar, C.N.; Swan, G.E.; Jacob, P. , 3rd. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006, 79, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Ashare, R.L.; Kable, J.W. Sex differences in time perception during smoking abstinence. Nicotine Tob Res. 2014, 17, 449–454. [Google Scholar]
- Dani, J.; Bertrand, D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007, 47, 699–729. [Google Scholar] [CrossRef]
- Herman, A.; DeVito, E.; Jensen, K.; Sofuoglu, M. Pharmacogenetics of nicotine addiction: Role of dopamine. Pharmacogenomics. 2014, 15, 221–234. [Google Scholar] [CrossRef]
- Molas, S.; DeGroot, S.R.; Zhao-Shea, R.; Tapper, A.R. Anxiety and Nicotine Dependence: Emerging Role of the Habenulo-Interpeduncular Axis. Trends Pharmacol Sci. 2017, 38, 169–180. [Google Scholar] [PubMed]
- Tomar, S.L.; Henningfield, J.E. Review of the evidence that pH is a determinant of nicotine dosage from oral use of smokeless tobacco. Tob Control. 1997, 6, 219–225. [Google Scholar]
- Tega, Y.; Yamazaki, Y.; Akanuma, S.; Kubo, Y.; Hosoya, K. Impact of Nicotine Transport across the Blood—Brain Barrier: Carrier-Mediated Transport of Nicotine and Interaction with Central Nervous System Drugs. Biol Pharm Bull. 2018, 41, 1330–1336. [Google Scholar] [PubMed]
- Hukkanen, J.; Jacob 3rd, P.; Benowitz, N. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005, 57, 79–115. [Google Scholar]
- Valentine, G.; Sofuoglu, M. Cognitive Effects of Nicotine: Recent Progress. Curr Neuropharmacol. 2017, 15, 403–414. [Google Scholar]
- Murphy, S.E.; Raulinaitis, V.; Brown, K.M. Nicotine 5—Oxidation and methyl oxidation by P450 2A enzymes. Drug Metab Dispos. 2005, 33, 1166–1173. [Google Scholar]
- Chen, X.; Owoseni, E.; Cederbaum, A.I.; Lu, Y. Nicotine enhances alcoholic fatty liver in mice: Role of CYP2A5. Arch Biochem Biophys. 2018, 657, 65–73. [Google Scholar] [CrossRef]
- Lu, Y.; Zhuge, J.; Wu, D.; Cederbaum, A. Ethanol Induction of CYP2A5: Permissive Role for CYP2E1 Yongke. DRUG Metab Dispos. 2011, 39, 330–336. [Google Scholar] [PubMed]
- Gubner, N.; Kozar-Konieczna, A.; Szoltysek-Boldys, I.; Slodczyk- Mankowska, E.; Goniewicz, J.; Sobczak, A.; et al. Cessation of Alcohol Consumption Decreases Rate of Nicotine Metabolism In Male Alcohol-Dependent Smokers. Drug Alcohol Depend. 2016, 163, 157–164. [Google Scholar]
- Zhou, X.; Zhuo, X.; Xie, F.; Kluetzman, K.; Shu, Y.; Humphreys, W.G.; et al. Role of CYP2A5 in the Clearance of Nicotine and Cotinine: Insights from Studies on a Cyp2a5 -null Mouse Model. J Pharmacol Exp Ther. 2010, 332, 578–587. [Google Scholar]
- Benowitz, N.L.; Hukkanen, J.; Peyton, J.I. Nicotine Chemistry, Metabolism, Kinetics and Biomarkers. Handb Exp Pharmacol. 2009, 192, 29–60. [Google Scholar]
- Arany, I.; Hall, S.; Dixit, M. Age-dependent sensitivity of the mouse kidney to chronic nicotine exposure. Nat Publ Gr. 2017, 82, 822–828. [Google Scholar] [CrossRef][Green Version]
- Lan, X.; Lederman, R.; Eng, J.M.; Shadafarin, S.; Shoshtari, M.; Saleem, M.A.; et al. Nicotine Induces Podocyte Apoptosis through Increasing Oxidative Stress. PLoS ONE. 2016, 11, e0167071. [Google Scholar] [CrossRef] [PubMed]
- Sharp, B.M.; Chen, H. Neurogenetic Determinants and Mechanisms of Addiction to Nicotine and Smoked Tobacco. Eur J Neurosci. 2018, 1–16. [Google Scholar] [CrossRef]
- Jensen, K.P.; Smith, A.H.; Herman, A.I.; Farrer, L.A.; Kranzler, H.R.; Sofuoglu, M.; et al. A protocadherin gene cluster regulatory variant is associated with nicotine withdrawal and the urge to smoke. Mol Psychiatry. 2017, 22, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Salloum, N.C.; Buchalter, E.L.F.; Chanani, S.; Espejo, G.; Mahjabeen, S.; Trillo, L.; et al. From genes to treatments: A systematic review of the pharmacogenetics in smoking cessation. Pharmacogenomics. 2018, 19, 861–871. [Google Scholar]
- Vremaroiu-Coman, D.A.; Alexescu, T.G.; Negrean, V.; Milaciu, M.V.; Buzoianu, A.D.; Ciumărnean, L.; et al. Ethical aspects of smoking cessation among the population from Transylvania. Balneo Res J. 2018, 9, 254–259. [Google Scholar] [CrossRef]
- Agaku, I.T.; Omaduvie, U.T.; Filippidis, F.T.; Vardavas, C.I. Cigarette design and marketing features are associated with increased smoking susceptibility and perception of reduced harm among smokers in 27 EU countries. Tob Control 2015, 24, e233–e240. [Google Scholar] [CrossRef]
- Meneses-gaya ICDe Zuardi, A.W.; Loureiro, S.R.; Alexandre, J.; Crippa, D.S. Psychometric properties of the fagerstrom test (Systematic review). J Bras Pneumol. 2009, 35, 73–82. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. INFORME OMS SOBRE LA EPIDEMIA MUNDIAL DE TABAQUISMO, 2011 Advertencia sobre los peligros del tabaco. 2011; 1–3. Available online: http://apps.who.int/iris/bitstream/10665/70681/1/WH O_NMH_TFI_11.3_spa.pdf.
- Budin, C.E.; Marginean, C.; Bordea, I.R.; Enache, L.S.; Enache, E.L.; Grigorescu, B.L.; et al. The Influence of Smoking on Nicotine Exposure Biomarkers and Inflammatory Profile Among Foster Care Teenagers, Romania. Rev CHIM (Bucharest). 2019, 12, 3659–3663. [Google Scholar] [CrossRef]
- Pérez-Rubio, G.; Sansores, R.; Ramírez-Venegas, A.; Camarena, Á.; Pérez-Rodríguez, M.; Falfán-Valencia, R. Nicotine Addiction Development: From Epidemiology to Genetic Factors. Rev Invest Clin. 2015, 67, 333–343. [Google Scholar] [PubMed]
- Todea, D.A.; Cosma, C.; Dicu, T.; Rosca, L.; Cucos, A.; Risteiu, M.; et al. Lung cancer risk induced by residential radon in CLUJ and Alba Counties, ROMANIA. Environ Eng Manag J. 2013, 12, 1281–1285. [Google Scholar]
- Martín Ruiz, A.; Rodríguez Gómez, I.; Rubio, C.; Revert, C.; Hardisson, A. Efectos tóxicos del tabaco. Rev Toxicol. 2004, 21, 64–71. [Google Scholar]
- Moga, M.; Boşca, A.B.; Soriţău, O.; Băciuţ, M.; Lucaciu, O.; Virag, P.; et al. Nicotine cytotoxicity on the mesenchymal stem cells derived from human periodontium. Rom Biotechnol Lett. 2016, 21, 11632–11641. [Google Scholar]
- Moga, M.; Bosca, A.B.; Bondor, C.I.; Ilea, A.; Lucaciu, O.P.; Ionel, A.; et al. Assessment of the correlations between nicotine dependence, exhaled carbon monoxide levels and oral hygiene status: An observational study. Clujul Med [Internet]. 2017, 90, 99. [Google Scholar] [CrossRef] [PubMed]
- Magalãsh, J.; Appel, H.; Duarte, J. Involvement of advanced glycation end products in the pathogenesis of diabetic complications: The protective role of regular physical activity. Eur Rev Aging Phys Act. 2008, 5, 17–29. [Google Scholar]
- Ilea, A.; Băbţan, A.M.; Boşca, B.A.; Crişan, M.; Petrescu, N.B.C.M.; Sainz, R.M.; Gerlach, J.Q.C.R. Advanced glycation end products (AGEs) in oral pathology. Arch Oral Biol. 2018, 18, 22–30. [Google Scholar]
- Sanders, N.T.; Dutson, D.J.; Durrant, J.W.; Lewis, J.B.; Wilcox, S.H.; Winden, D.R.; et al. Cigarette smoke extract (CSE) induces RAGE-mediated inflammation in the Ca9-22 gingival carcinoma epithelial cell line. Arch Oral Biol. 2017, 80, 95–100. [Google Scholar] [CrossRef]
- Chapman, S.; Mick, M.; Hall, P.; Mejia, C.; Sue, S.; Abdul Wase, B.; et al. Cigarette smoke extract induces oral squamous cell carcinoma cell invasion in a receptor for advanced glycation end-products-dependent manner. Eur J Oral Sci. 2018, 126, 33–40. [Google Scholar] [CrossRef]
- Reynolds, P.R.; Kasteler, S.D.; Schmitt, R.E.; Hoidal, J.R. Receptor for advanced glycation end-products signals through ras during tobacco smoke-induced pulmonary inflammation. Am J Respir Cell Mol Biol. 2011, 45, 411–418. [Google Scholar] [CrossRef]
- Marinucci, L.; Balloni, S.; Fettucciari, K.; Bodo, M.; Talesa, V.N.; Antognelli, C. Nicotine induces apoptosis in human osteoblasts via a novel mechanism driven by H2O2and entailing Glyoxalase 1-dependent MG-H1 accumulation leading to TG2-mediated NF-kB desensitization: Implication for smokers-related osteoporosis. Free Radic Biol Med. 2018, 117, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Van Waateringe, R.P.; Mook-Kanamori, M.J.; Slagter, S.N.; Van Der Klauw, M.M.; Van Vliet-Ostaptchouk, J.V.; Graaff, R.; et al. The association between various smoking behaviors, cotinine biomarkers and skin autofluorescence, a marker for advanced glycation end product accumulation. PLoS ONE. 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Proctor, R. Why did the Nazis have the world’s most aggressive anti-cancer campaign? Endeavour. 1999, 2, 76–79. [Google Scholar]
- Budin, C.E.; Alexescu, T.G.; Bordea, I.R.; Gherginescu, M.C.; Aluas, M.; Grigorescu, B.L.; et al. Nicotine Addiction Objective in Educational Programs for Smoking Prevention in Young People. REV CHIM (Bucharest). 2019, 6, 2168–2172. [Google Scholar]
- Rollema, H.; Chambers, L.; Coe, J.; Glowa, J.; Hurst, R.; Lebel, L.; et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007, 52, 985–994. [Google Scholar] [PubMed]
- Falcone, M.; Cao, W.; Bernardo, L.; Tyndale, R.F.; Loughead, J.; Lerman, C. Brain Responses to Smoking Cues Differ Based on Nicotine Metabolism Rate. Biol Psychiatry. 2016, 80, 190–197. [Google Scholar]
- Lu, W.; Chappell, K.; Walters, J.A.E.; Jacobson, G.A.; Patel, R.; Schüz, N.; et al. The effect of varenicline and nicotine patch on smoking rate and satisfaction with smoking: An examination of the mechanism of action of two pre- quit pharmacotherapies. Psychopharmacology (Berl). 2017, 234, 1969–1976. [Google Scholar]
- Przulj, D.; Wehbe, L.; McRobbie, H.; Hajek, P. Progressive nicotine patch dosing prior to quitting smoking: Feasibility, safety, and effects during the pre-quit and post-quit periods. Addiction. 2019, 114, 515–522. [Google Scholar]
- Burkett, J.S. The use of the nicotine inhaler in smoking cessation. J Am Acad Nurse Pr. 2006, 18, 83–91. [Google Scholar] [CrossRef]
- Schneider, N.G.; Olmstead, R.E.; Franzon, M.A.; Lunell, E. The nicotine inhaler. Clinical Pharmacokinetics and Comparison with Other Nicotine Treatments. Clin Pharmacokinet. 2001, 40, 661–684. [Google Scholar]
- Caldwell, B.O.; Adamson, S.J.; Crane, J. Combination Rapid-Acting Nicotine Mouth Spray and Nicotine Patch Therapy in Smoking Cessation. Nicotine &Tobacco Res. 2014, 16, 1356–1364. [Google Scholar]
- Tonnesen, P.; Lauri, H.; Perfekt, R.; Mann, K.; Batra, A. Efficacy of a nicotine mouth spray in smoking cessation: A randomised, double blind trial. Eur Respir J. 2012, 40, 548–554. [Google Scholar] [CrossRef]
- Wallstrom, M.; Nilsson, F.; Hirsch, J. A randomized, double-blind, placebo-controlled clinical evaluation of a nicotine sublingual tablet in smoking cessation. Addiction. 2000, 95, 1161–1171. [Google Scholar] [PubMed]
- Saravia, R.; Ten-Blanco, M.; Grande, M.T.; Maldonado, R.; Berrendero, F. Anti-inflammatory agents for smoking cessation? Focus on cognitive deficits associated with nicotine withdrawal in male mice. Brain Behav Immun 2018, 1–12. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30391635%0Ah. [CrossRef] [PubMed]
- Johnson, M.W.; Garcia-Romeu, A.; Johnson, P.S.; Griffiths, R.R. An online survey of tobacco smoking cessation associated with naturalistic psychedelic use. J Psychopharmacol. 2017, 31, 841–850. [Google Scholar] [CrossRef]
- Klinsophon, T.; Thaveeratitham, P.; Sitthipornvorakul, E.; Janwantanakul, P. Effect of exercise type on smoking cessation: A meta-analysis of randomized controlled trials. BMC Res Notes. 2017, 10, 1–21. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Tampa, M.; Paunica, S.; Balalau, C.; Constantin, V.; Paunica, G.; Motofei, I. Distribution of post-finasteride syndrome in men with androgenic alopecia; ESDR-Congress. Journal of Investigative Dermatology 2015, 135, S40. [Google Scholar]
- Fiore, M.; Jaén, C.; Baker, T. Treating tobacco use and dependence: 2008 Update. In Quick reference guide for Clinicians; Department of Health and Human Services, Public Health Service: Rockville, MD, USA, 2009. [Google Scholar]
- Andersson, P.; Johannsen, A. Dental patients’ perceptions and motivation in smoking cessation activities. Acta Odontol Scand. 2016, 74, 285–290. [Google Scholar] [CrossRef]
- Chu, K.; Escobar-viera, C.G.; Matheny, S.J.; Davis, E.M.; Primack, B.A. Tobacco cessation mobile app intervention (Just Kwit ! study): Protocol for a pilot randomized controlled pragmatic trial. Trials. 2019, 20, 127. [Google Scholar] [CrossRef]
- Baskerville, N.B.; Struik, L.L.; Dash, D. Crush the Crave: Development and Formative Evaluation of a Smartphone App for Smoking Cessation Corresponding Author. JMIR MHealth UHealth. 2018, 6, 1–14. [Google Scholar] [CrossRef]
- Masaki, K.; Tateno, H.; Kameyama, N.; Morino, E. Impact of a Novel Smartphone App (CureApp Smoking Cessation) on Nicotine Dependence: Prospective Single-Arm Interventional Pilot Study. JMIR MHealth UHealth. 2019, 7, e12694. [Google Scholar] [PubMed]
- Dutra, T.; Formagini, B.; Ervilha, R.R.; Gomide, H.P.; Ronzani, T.M. A review of smartphone apps for smoking cessation available in Portuguese. Reports Public Heal. 2017, 33, 1–10. [Google Scholar]
- Bengalli, R.; Ferri, E.; Labra, M.; Mantecca, P. Lung toxicity of condensed aerosol from E-CIG liquids: Influence of the flavor and the in vitro model used. Int J Environ Res Public Health. 2017, 14, 1–14. [Google Scholar]
- DeVito, E.E.; Krishnan-Sarin, S. E-cigarettes: Impact of E-Liquid Components and Device Characteristics on Nicotine Exposure. Curr Neuropharmacol. 2017, 15, 438–459. [Google Scholar] [CrossRef]
- Health, P. Public Health England (2015) E-cigarettes: An evidence update. 2015. [Google Scholar]
- Löhler, J.; Wollenberg, B. Are electronic cigarettes a healthier alternative to conventional tobacco smoking? Eur Arch Oto-Rhino-Laryngology. 2019, 276, 17–25. [Google Scholar]
- Ogden, M.W.; Marano, K.M.; Jones, B.A.; Morgan, W.T.; Stiles, M.F. Switching from usual brand cigarettes to a tobacco-heating cigarette or snus: Part 2. Biomarkers of exposure. Biomarkers. 2015, 20, 391–403. [Google Scholar]
- Gonzalez, J.M.; Foley, M.W.; Bieber, N.M.; Bourdelle, P.A.; Niedbala, R.S. Development of an ultrasensitive immunochromatography test to detect nicotine metabolites in oral fluids. Anal Bioanal Chem. 2011, 400, 3655–3664. [Google Scholar]
- Moore, M.R.; Mason, M.J.; Brown, A.R.; Garcia, C.M.; Seibers, A.D.; Stephens, C.J. Remote biochemical veri fi cation of tobacco use: Reducing costs and improving methodological rigor with mailed oral cotinine swabs. Addict Behav. 2018, 87, 151–154. [Google Scholar] [CrossRef]
- Robson, N.; Bond, A.J.; Wolff, K. Salivary nicotine and cotinine concentrations in unstimulated and stimulated saliva. African J Pharm Pharmacol. 2010, 4, 61–65. [Google Scholar]
- Alenus, J.; Ethirajan, A.; Horemans, F.; Weustenraed, A. Molecularly imprinted polymers as synthetic receptors for the QCM-D-based detection of L -nicotine in diluted saliva and urine samples. Anal Bioanal Chem. 2013, 405, 642079–6487. [Google Scholar]
- Peeters, M.; Csipai, P.; Geerets, B.; Weustenraed, A. Heat- transfer-based detection of L -nicotine, histamine, and serotonin using molecularly imprinted polymers as biomimetic receptors. Anal Bioanal Chem. 2013, 405, 6453–6460. [Google Scholar] [PubMed]
- Antwi-Boampong, S.; Mani, K.S.; Carlan, J.; Belbruno, J.J. A selective molecularly imprinted polymer- carbon nanotube sensor for cotinine sensing. J Mol Recognit. 2014, 27, 57–63. [Google Scholar] [PubMed]
- Bordea, I.R.; Sîrbu, A.; Lucaciu, O.; Ilea, A.; Câmpian, R.S.; Todea, D.A.; Alexescu, T.G.; Aluaș, M.; Budin, C.; Pop, A.S. Microleakage—The Main Culprit in Bracket Bond Failure? J Mind Med Sci. 2019, 6, 86–94. [Google Scholar] [CrossRef]
- Ciui, B.; Tertis, M.; Feurdean, C.N.; Ilea, A.; Sandulescu, R.; Wang, J.; et al. Cavitas electrochemical sensor toward detection of N -epsilon (carboxymethyl) lysine in oral cavity. Sensors Actuators B Chem [Internet]. 2019, 281, 399–407. [Google Scholar] [CrossRef]
- Wu, Y.; Hao, G.; Baltus, P.G.M.; Bergmans, J.W.M.; Linnartz, J.P.M.G.; Mahmoudi, R. System concept for a wireless sensor system with monolithic sensors. In Proceedings of the 21st ProRISC Workshop of the STWICT Conference, Veldhoven, The Netherlands, 18–19 November 2010; pp. 1–2. [Google Scholar]
- Ning, H.; Pikul, J.H.; Zhang, R.; Li, X.; Xu, S.; Wang, J.; et al. Holographic patterning of high-performance on-chip 3D lithium-ion microbatteries. Proc Natl Acad Sci USA 2015, 112, 6573–6578. [Google Scholar]
- Farago, P.; Babtan, A.; Galatus, R.; Groza, R.; Roman, M.N.; Feurdean, C.N.; et al. A Side-polished Fluorescent Fiber Sensor for the Detection of Blood in the Saliva. Meditech. 2018, 17–20. [Google Scholar]


© 2019 by the author. 2019 Anida-Maria Bäbtan, Nausica B. Petrescu, Anca Ionel, Bianca A. Bosca, Willi A. Uriciuc, Claudia N. Feurdean, Codruta I. Miricä, Roxana Bordea, Viorel Micläus, Flavia Ruxanda, Adina D. Todea, Te-odora G. Alexescu, Radu S. Câmpian, Aranka Ilea1
Share and Cite
Băbțan, A.-M.; Petrescu, N.B.; Ionel, A.; Boșca, B.A.; Uriciuc, W.A.; Feurdean, C.N.; Mirică, C.I.; Bordea, R.; Miclăuș, V.; Ruxanda, F.; et al. Insights Into the Pathogenesis of Nicotine Addiction. Could a Salivary Biosensor Be Useful in Nicotine Replacement Therapy (NRT)? J. Mind Med. Sci. 2019, 6, 196-209. https://doi.org/10.22543/7674.62.P196209
Băbțan A-M, Petrescu NB, Ionel A, Boșca BA, Uriciuc WA, Feurdean CN, Mirică CI, Bordea R, Miclăuș V, Ruxanda F, et al. Insights Into the Pathogenesis of Nicotine Addiction. Could a Salivary Biosensor Be Useful in Nicotine Replacement Therapy (NRT)? Journal of Mind and Medical Sciences. 2019; 6(2):196-209. https://doi.org/10.22543/7674.62.P196209
Chicago/Turabian StyleBăbțan, Anida-Maria, Nausica B. Petrescu, Anca Ionel, Bianca A. Boșca, Willi A. Uriciuc, Claudia N. Feurdean, Codruța I. Mirică, Roxana Bordea, Viorel Miclăuș, Flavia Ruxanda, and et al. 2019. "Insights Into the Pathogenesis of Nicotine Addiction. Could a Salivary Biosensor Be Useful in Nicotine Replacement Therapy (NRT)?" Journal of Mind and Medical Sciences 6, no. 2: 196-209. https://doi.org/10.22543/7674.62.P196209
APA StyleBăbțan, A.-M., Petrescu, N. B., Ionel, A., Boșca, B. A., Uriciuc, W. A., Feurdean, C. N., Mirică, C. I., Bordea, R., Miclăuș, V., Ruxanda, F., Todea, A. D., Alexescu, T. G., Câmpian, R. S., & Ilea, A. (2019). Insights Into the Pathogenesis of Nicotine Addiction. Could a Salivary Biosensor Be Useful in Nicotine Replacement Therapy (NRT)? Journal of Mind and Medical Sciences, 6(2), 196-209. https://doi.org/10.22543/7674.62.P196209



