Determination of Pyrrolizidine Alkaloids in Dietary Sources Using a Spectrophotometric Method
Highlights
- It is presented a simple and inexpensive method for the quantitative determination of PAs in honey and flour, with a quantification limit (0.174 μg/mL).
- This method is applicable for the determination of PAs from food sources at toxic levels for consumers.
Highlights
- It is presented a simple and inexpensive method for the quantitative determination of PAs in honey and flour, with a quantification limit (0.174 μg/mL).
- This method is applicable for the determination of PAs from food sources at toxic levels for consumers.
Abstract
Introduction
Materials and Methods
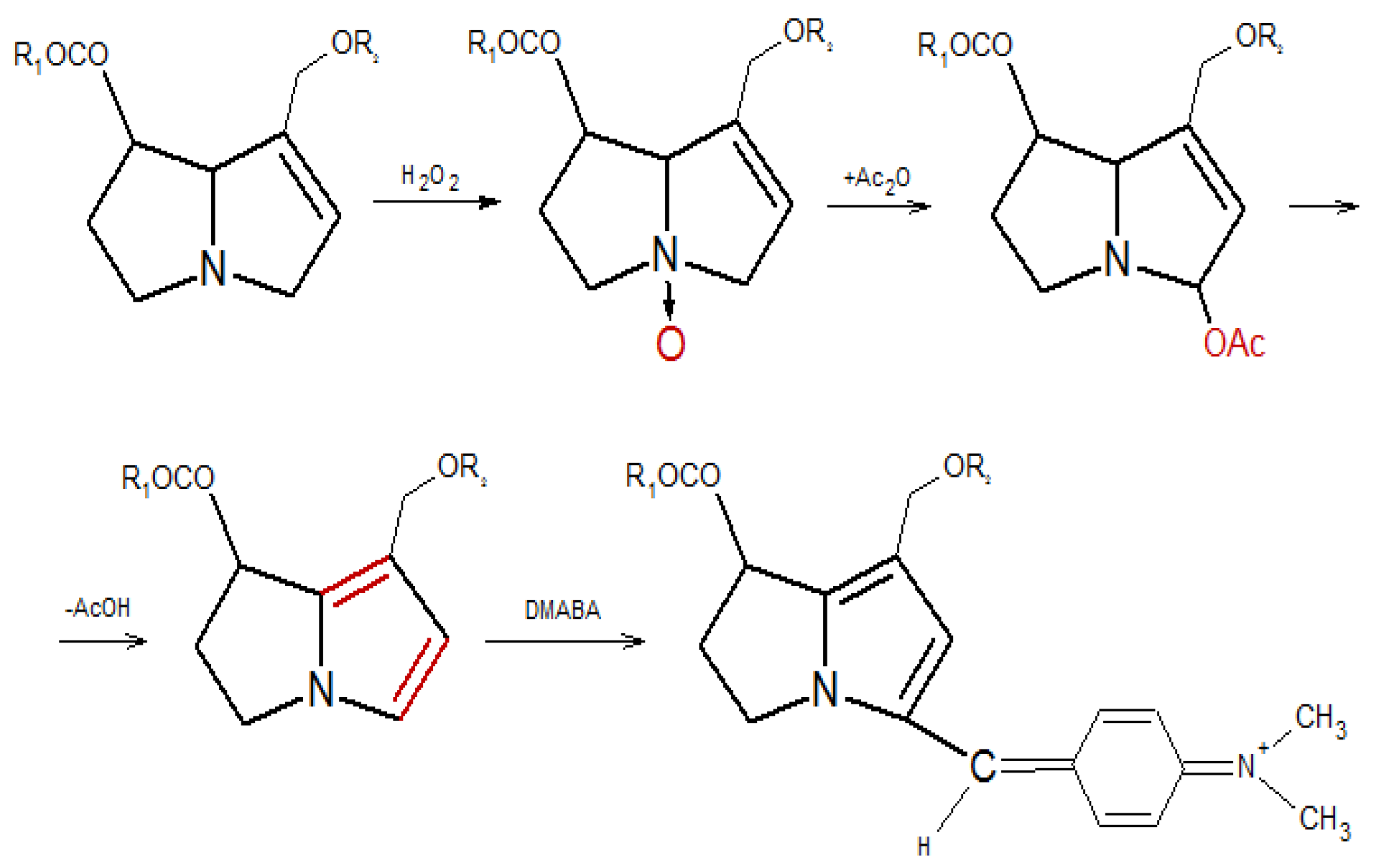
Principle
Materials
- −
- Standard solution
- −
- Samples
- −
- Reagents
Method
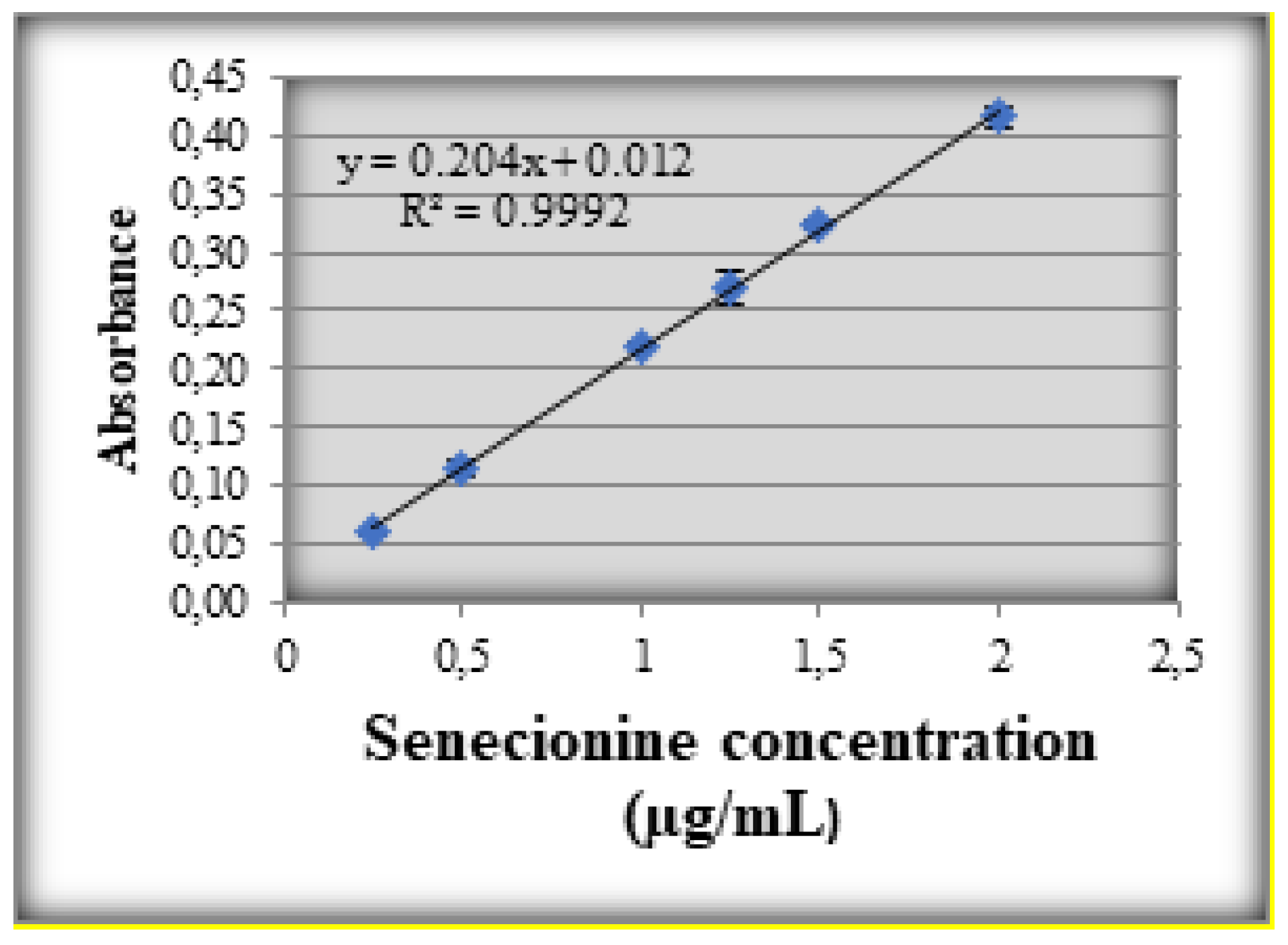
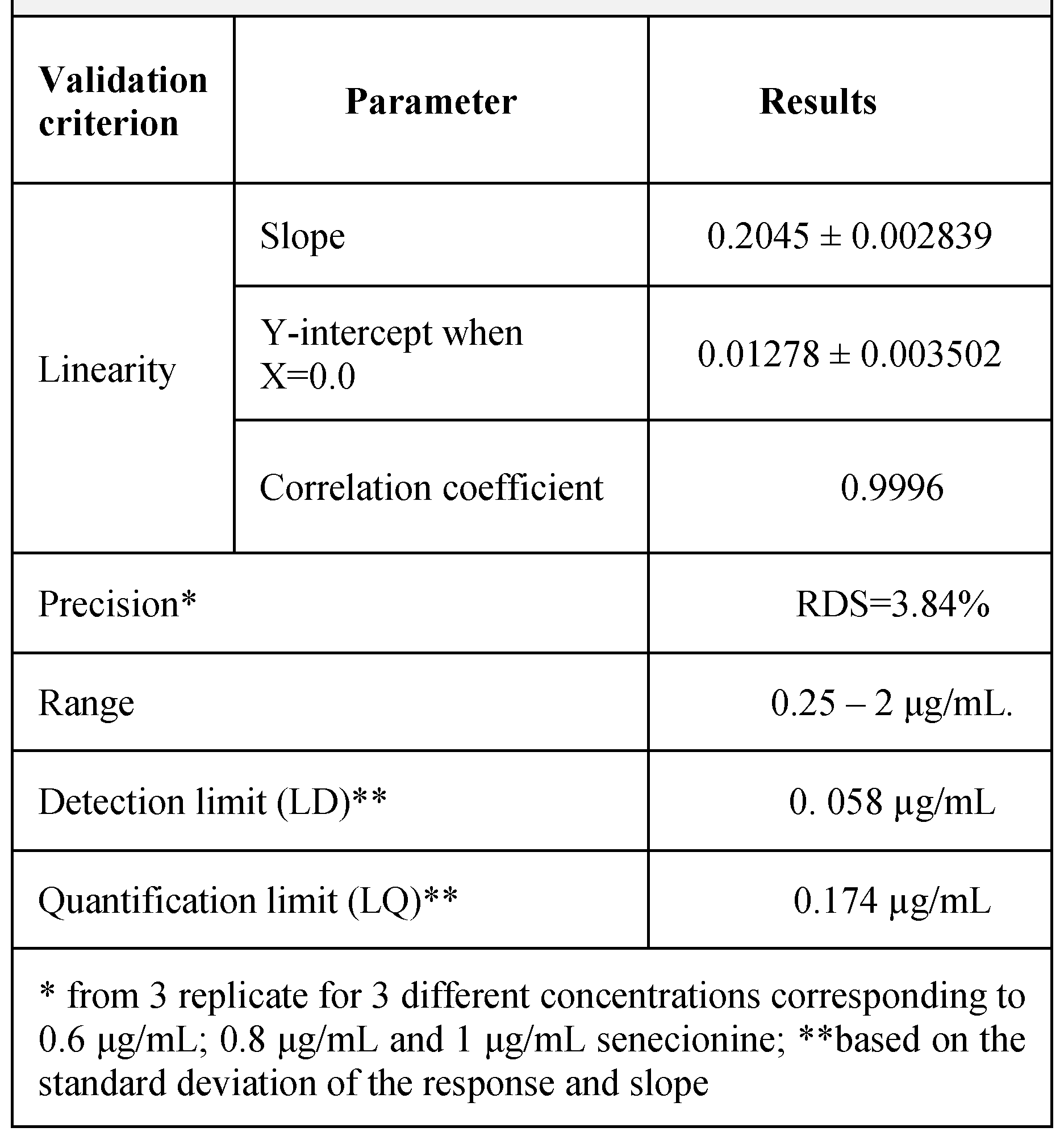
Results
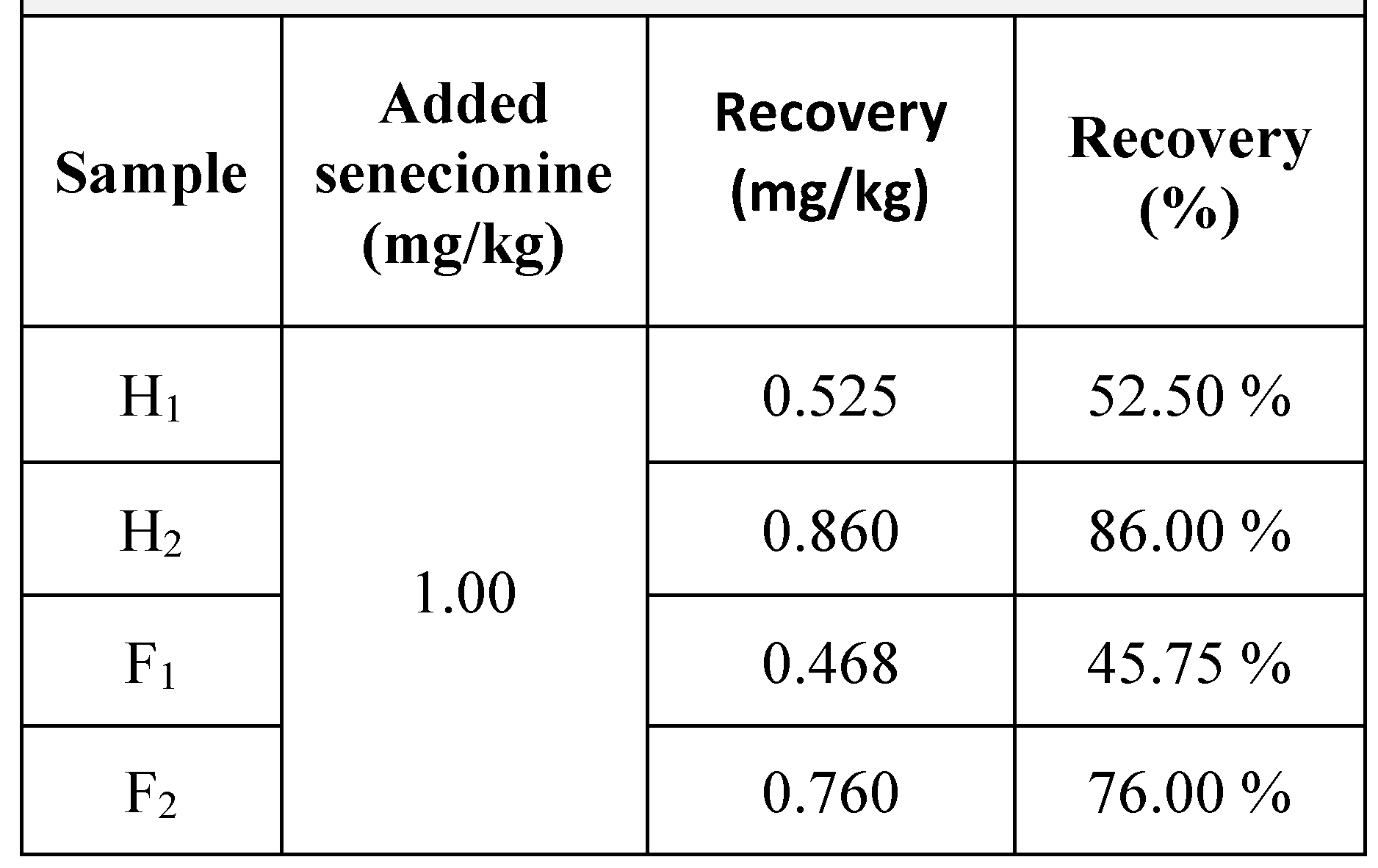 |
Discussions
Conclusions
Acronyms and abbreviations
- −
- GC-MS - gas chromatography coupled with mass spectrometry
- −
- HPTLC - high performance thin layer chromatography
- −
- LC-MS - liquid chromatography coupled with mass spectrometry
- −
- PAs - pyrrolizidine alkaloids
- −
- RDS – relative standard deviation
- †
- In Memoriam: The authors would like to respectfully dedicate this article to Ms. Mihaela Ilie, who passed away on 1 January 2018.
Conflict of interest disclosure
References
- Fu, P.P.; Xia, Q.; Lin, G.; Chou, M.W. Pyrrolizidine alkaloids – genotoxicity, metabolism enzymes, metabolic activation and mechanisms. Drug Metab Rev. 2004, 36, 1–55. [Google Scholar] [CrossRef] [PubMed]
- WHO-IPCS (World Health Organisation- International Programme on Chemical Safety). Pyrrolizidine alkaloids. Environmental Health Criteria 1988, 80. Available online: http://www.inchem.org/documents/ehc/ehc/ehc080.htm.
- Şeremet, O.C.; Bărbuceanu, F.; Ionică, F.E.; Margină, D.M.; Guţu, C.M.; Olaru, O.T.; Ilie, M.; Gonciar, V.; Negreş, S.; Chiriţă, C. Oral toxicity study of certain plant extracts containing pyrrolizidine alkaloids. Rom J Morphol Embryol. 2016, 57, 1017–23. [Google Scholar] [PubMed]
- Mattocks, A.R. Chemistry and toxicology of pyrrolizidine alkaloids; Academic Press: London, New York, 1986. [Google Scholar]
- EFSA (European Food Safety Authority), Scientific Opinion on Pyrrolizidine alkaloids in food and feed. The EFSA Journal. 2011, 9, 2406–40. [CrossRef]
- Committee on Herbal Medicinal Products. Public statement on the use of herbal medicinal products containing toxic, unsaturated pyrrolizidine alkaloids (EMA/HMPC/893108/2011), 2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2014/12/WC500179559.pdf.
- Molyneux, R.J.; Gardner, D.L.; Colegate, S.M.; Edgar, J.A. Pyrrolizidine alkaloid toxicity in livestock: a paradigm for human poisoning? Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011, 28, 293–307. [Google Scholar] [CrossRef]
- Seremet, O.C.; Olaru, O.T.; Ilie, M.; Negres, S.; Balalau, D. HPTLC Evaluation Of The Pyrollizidine Alkaloid Senecionine In Certain Phytochemical Products. Farmacia. 2013, 61, 756–63. [Google Scholar]
- Kempf, M.; Wittig, M.; Reinhard, A.; von der Ohe, K.; Blacquière, T.; Raezke, K.P.; Michel, R.; Schreier, P.; Beuerle, T. Pyrrolizidine alkaloids in honey: comparison of analytical methods. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011, 28, 332–47. [Google Scholar] [CrossRef]
- Seremet, O.C.; Olaru, O.T.; Gutu, C.M.; Nitulescu, G.M.; Ilie, M.; Negres, S.; Zbarcea, C.E.; Purdel, C.N.; Spandidos, D.A.; Tsatsakis, A.M.; Coleman, M.D.; Margina, D.M. Toxicity of plant extracts containing pyrrolizidine alkaloids using alternative invertebrate models. Mol Med Rep. 2018, 17, 7757–63. [Google Scholar] [CrossRef]
- Kakar, F.; Akbarian, Z.; Leslie, T.; Mustafa, M.L.; Watson, J.; van Egmond, H.P.; Omar, M.H.; Mofleh, J. An outbreak of hepatic veno-occlusive disease in Western Afghanistan associated with exposure to wheat flour contaminated with pyrrolizidine alkaloids. J Toxicol. 2010, 2010, 313280. [Google Scholar] [CrossRef]
- Shar, Z.; Chippa, H.; Hussain, N.; Arain, M.; Khan, A.; et al. Spectrophotometric Determination of Caffeine in Selected Pakistani Beverages. J Food Processing & Beverages. 2017, 5, 1–4. [Google Scholar]
- Begum, J.; Rao, K.S.; Rambabu, C. Assay of yohimbine chloride in bulk samples and pharmaceutical formulations by extractive spectrophotometry. Asian Journal of Chemistry. 2006, 18, 1417–22. [Google Scholar]
- Vachnadze, V.; Dzhakeli, E.Z.; Dadeshidze, I.A.; Kintsurashvili, L.G. Quantitative spectrophotometric determination of alkaloids in roots of Vinca herbacea. Pharmaceutical Chemistry Journal. 2010, 44, 199–201. [Google Scholar] [CrossRef]
- Birecka, H.; Catalfamo, J.L.; Eisen, N. A sensitive method for detection and quantitative determination of pyrrolizidine alkaloids. Phytochemistry. 1981, 20, 343–4. [Google Scholar] [CrossRef]
- Mattocks, A.R. Spectrophotometric determination of unsaturated pyrrolizidine alkaloids. Anal Chem. 1967, 39, 443–7. [Google Scholar] [CrossRef]
- Şeremet, O.C.; Olaru, O.T.; Ilie, M.; Negreş, S.; Bălălău, D. Phytotoxicity Assessment of Certain Phytochemical Products Containing Pyrrolizidine Alkaloids. Acta Medica Marisiensis. 2013, 59, 250–253. [Google Scholar] [CrossRef]
- Roeder, E.; Liu, K.; Mütterlein, R. Quantitative photometrische Bestimmung der Pyrrolizidinalkaloide in Symphyti Radix. Fresenius J Anal Chem. 1992, 343, 621–624. [Google Scholar] [CrossRef]
- Motofei, I.G.; Rowland, D.L.; Georgescu, S.R.; Tampa, M.; Baconi, D.; Stefanescu, E.; Baleanu, B.C.; Balalau, C.; Constantin, V.; Paunica, S. Finasteride adverse effects in subjects with androgenic alopecia: A possible therapeutic approach according to the lateralization process of the brain. J Dermatolog Treat. 2016, 27, 495–497. [Google Scholar] [CrossRef]
- Şeremet, O.C.; Olaru, O.T.; Bălălău, D.; Negreş, S. The effect of certain plant extracts containing pyrrolizidine alkaloids on Lactuca sativa radicle growth. Rom J Biopys. 2014, 24, 1–9. [Google Scholar]
- Mattocks, A.R. Spectrophotometric determination of pyrrolizidine alkaloids – some improvements. Anal Chem. 1968, 40, 1749–50. [Google Scholar] [CrossRef]
- Barko Bartkowski, J.P.; Wiedenfeld, H.; Roeder, E. Quantitative Photometric Determination of Senkirkine in Farfarae Folium. Phytochemical Analysis. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- ICH-Q2 (R1) Validation of Analytical Procedures: Text and Methodology, 1995 (CPMP/ICH/381/95). Available online: http://www.ema.europa.eu/ docs/en_GB/document_library/Scientific_guidelin e/2009/09/WC500002662.pdf.
- Azadbakht, M.; Talavaki, M. Qualitative and quantitative determination of pyrrolizidine alkaloids of wheat and flour contaminated with Senecio in Mazandaran province farms. Iranian Journal of Pharmaceutical Research. 2003, 2, 179–183. [Google Scholar]
- Diaconu, C.C.; Dragoi, C.M.; Bratu, O.G.; Neagu, T.P.; Pantea Stoian, A.; Cobelschi, P.C.; Nicolae, A.C.; Iancu, M.A.; Hainarosie, R.; Stanescu, A.M.A.; Socea, B. New approaches and perspectives for the pharmacological treatment of arterial hypertension. Farmacia 2018, 66, 408–415. [Google Scholar] [CrossRef]
- Deinzer, M.L.; Thomson, P.A.; Burgett, D.M.; Isaacson, D.L. Pyrrolizidine alkaloids: their occurrence in honey from tansy ragwort (Senecio jacobaea L.). Science. 1977, 195, 497–9. [Google Scholar] [CrossRef] [PubMed]
- Crews, C.; Startin, J.R.; Clarke, P.A. Determination of pyrrolizidine alkaloids in honey from selected sites by solid phase extraction and HPLC-MS. Food Additives and Contaminants. 1997, 14, 419–428. [Google Scholar] [CrossRef]
- Betteridge, K.; Cao, Y.; Colegate, S.M. Improved method for extraction and LCMS analysis of pyrrolizidine alkaloids and their N-oxides in honey: Application to Echium vulgare honeys. J Agric Food Chem. 2005, 53, 1894–902. [Google Scholar] [CrossRef] [PubMed]
 |
© 2018 by the author. 2018 Oana C. Șeremet, Octavian T. Olaru, Mihaela Ilie, Claudia M. Guțu, Mihai G. Nițulescu, Camelia Diaconu, Catalina Motofei, Denisa Margină, Simona Negreș, Cristina E. Zbârcea, Emil Ștefănescu
Share and Cite
Șeremet, O.C.; Olaru, O.T.; Ilie, M.; Guțu, C.M.; Nițulescu, M.G.; Diaconu, C.; Motofei, C.; Margină, D.; Negreș, S.; Zbârcea, C.E.; et al. Determination of Pyrrolizidine Alkaloids in Dietary Sources Using a Spectrophotometric Method. J. Mind Med. Sci. 2018, 5, 294-299. https://doi.org/10.22543/7674.52.P294299
Șeremet OC, Olaru OT, Ilie M, Guțu CM, Nițulescu MG, Diaconu C, Motofei C, Margină D, Negreș S, Zbârcea CE, et al. Determination of Pyrrolizidine Alkaloids in Dietary Sources Using a Spectrophotometric Method. Journal of Mind and Medical Sciences. 2018; 5(2):294-299. https://doi.org/10.22543/7674.52.P294299
Chicago/Turabian StyleȘeremet, Oana C., Octavian T. Olaru, Mihaela Ilie, Claudia M. Guțu, Mihai G. Nițulescu, Camelia Diaconu, Catalina Motofei, Denisa Margină, Simona Negreș, Cristina E. Zbârcea, and et al. 2018. "Determination of Pyrrolizidine Alkaloids in Dietary Sources Using a Spectrophotometric Method" Journal of Mind and Medical Sciences 5, no. 2: 294-299. https://doi.org/10.22543/7674.52.P294299
APA StyleȘeremet, O. C., Olaru, O. T., Ilie, M., Guțu, C. M., Nițulescu, M. G., Diaconu, C., Motofei, C., Margină, D., Negreș, S., Zbârcea, C. E., & Ștefănescu, E. (2018). Determination of Pyrrolizidine Alkaloids in Dietary Sources Using a Spectrophotometric Method. Journal of Mind and Medical Sciences, 5(2), 294-299. https://doi.org/10.22543/7674.52.P294299



