Self-Reported Nutritional Status, Executive Functions, and Cognitive Flexibility in Adults
Highlights
- Healthy nutrition is significantly related to cognitive flexibility in adults
- Gender and nutrition status are influential factors on executive cognitive functions and mental flexibility
Abstract
Highlights
- ✓ Healthy nutrition is significantly related to cognitive flexibility in adults
- ✓ Gender and nutrition status are influential factors on executive cognitive functions and mental flexibility
Introduction
Nutrition, executive functions and cognitive flexibility
Theoretical frameworks
The present study
Materials and Methods
Participants
Instruments
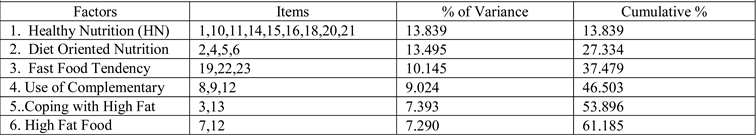
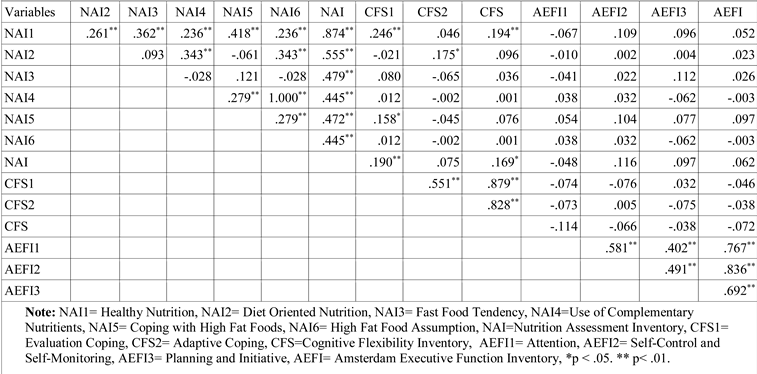
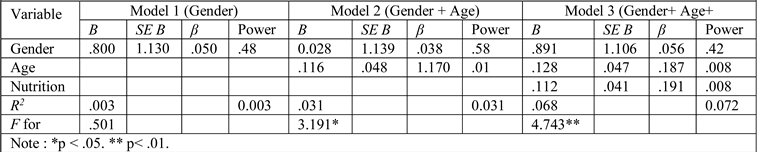
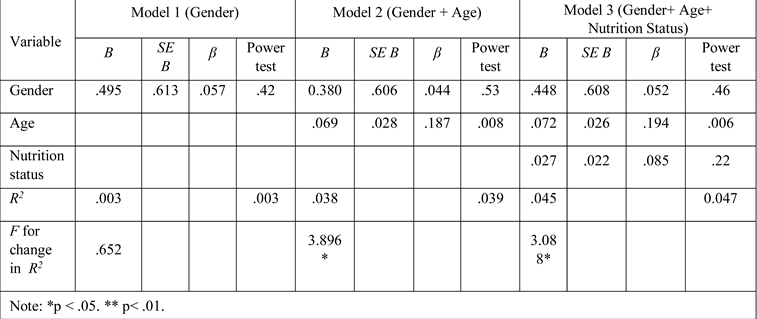
Results
Discussion
Conclusions
List of abbreviations
- Amsterdam Executive Function Inventory (AEFI)
- Cognitive Flexibility Scale (CFS)
- Coping with High Fat Foods (CHFF)
- Diet Oriented Nutrition (DON)
- Fast Food Tendency (FFT)
- High Fat Food Assumption (HFFA)
- Healthy Nutrition (HN)
- Nutrition Assessment Inventory (NAI)
- Saturated Fat (SF)
- Use of Complementary Nutritients (UCN)
Acknowledgments
References
- Ogden, J. The psychology of eating: From healthy to disordered behavior. A John Wiley & Sons, Ltd., Publication: United Kingdom. 2011. Available online: http://www.ikiu.ac.ir/public-files/profiles/items/090ad_1446739747.
- Roman, I. The Psychology of Nutritional Behaviour and Children's Nutrition Education. Procedia-Social and Behavioral Sciences. 2014, 149, 819–824. [Google Scholar] [CrossRef]
- Lieberman, H.R.; Spring, B.J.; Garfield, G.S. The behavioral effects of food constituents: strategies used in studies of amino acids, protein, carbohydrate and caffeine. Nutr Rev 1986, 44 (Suppl. S61-70). [Google Scholar] [CrossRef]
- Kaplan, R.J.; Greenwood, C.E.; Winocur, G.; Wolever, T.M. Dietary protein, carbohydrate, and fat enhance memory performance in the healthy elderly. Am J Clin Nutr. 2001, 74, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Kalmijn, S.; van Boxtel, M.P.; Ocké, M.; Verschuren, W.M.; Kromhout, D.; Launer, L.J. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004, 62, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.N.; Joyner, M.J. Sugar highs and lows: the impact of diet on cognitive function. J Physiol. 2012, 590, 2831. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Wisner, K.L. Nutrition and depression: implications for improving mental health among childbearing-aged women. Biol Psychiatry. 2005, 58, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pinilla, F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Spaccavento, S.; Del Prete, M.; Craca, A.; Fiore, P. Influence of nutritional status on cognitive, functional and neuropsychiatric deficits in Alzheimer's disease. Arch Gerontol Geriatr. 2009, 48, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.M.; Murray, A.J.; Holloway, C.J.; Carter, E.E.; Kemp, G.J.; Codreanu, I.; Brooker, H.; Tyler, D.J.; Robbins, P.A.; Clarke, K. Short-term consumption of a high-fat diet impairs whole-body efficiency and cognitive function in sedentary men. FASEB J. 2011, 25, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C.; Scarmeas, N. The good, bad, and ugly? How blood nutrient concentrations may reflect cognitive performance. Neurology 2012, 78, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.L.; Silbert, L.C.; Howieson, D.; Dodge, H.H.; Traber, M.G.; Frei, B.; Kaye, J.A.; Shannon, J.; Quinn, J.F. Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology 2012, 78, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Francis, H.; Stevenson, R. The longer-term impacts of Western diet on human cognition and the brain. Appetite. 2013, 63, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Scholey, A.B. Nutritional influences on human neurocognitive functioning. Front Hum Neurosci. 2014, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Dauncey, M.J. Nutrition, the brain and cognitive decline: insights from epigenetics. Eur J Clin Nutr. 2014, 68, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R. Exercise, nutrition and the brain. Sports Med. 2014, 44 (Suppl. S1), S47–56. [Google Scholar] [CrossRef] [PubMed]
- Best, T.; Dye, L. Nutrition for brain health and cognitive performance; CRC Press. Taylor & Francis Group, 2015; ISBN 9781466570023. [Google Scholar]
- Khan, N.A.; Raine, L.B.; Drollette, E.S.; Scudder, M.R.; Hillman, C.H. The relation of saturated fats and dietary cholesterol to childhood cognitive flexibility. Appetite. 2015, 93, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.S.; Tovar, A.; Jayasuriya, A.T.; Welker, E.; Schober, D.J.; Copeland, K.; Dev, D.A.; Murriel, A.L.; Amso, D.; Ward, D.S. The relationship between physical activity and diet and young children's cognitive development: A systematic review. Prev Med Rep. 2016, 3, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Maslow, A.H. A theory of human motivation. Psychological review. 1943, 50, 370–396. [Google Scholar] [CrossRef]
- Nobuyuki, S. The psychology of eating from the point of view of experimental, social, and applied psychology. Psychology in Russia: State of the art. 2014, 7, 14–22. [Google Scholar]
- Davis, C.M. Results of the self-selection of diets by young children. Can Med Assoc J. 1939, 41, 257–261. [Google Scholar] [PubMed]
- Shepherd, R. Handbook of the psychophysiology of human eating; John Wiley, 1989; ISBN 13: 978-0471914952. [Google Scholar]
- Birch, L.L. Development of food preferences. Annu Rev Nutr. 1999, 19, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Snoek, H.M.; Engels, R.C.; Janssens, J.M.; van Strien, T. Parental behaviour and adolescents’ emotional eating. Appetite. 2007, 49, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.E.; Paul, C.; Pizzo, B.; Riegel, K. Practice does make perfect: A longitudinal look at repeated taste exposure. Appetite. 2008, 51, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.; Moore, L.; Smith, A.; Murphy, S.; Smith, C. Health locus of control and value for health as predictors of dietary behaviour. Psychology and Health. 1994, 10, 54–41. [Google Scholar] [CrossRef]
- Thompson, M.M.; Zanna, M.P.; Griffin, D.W. Let's not be indifferent about (attitudinal) ambivalence. In Ohio State University series on attitudes and persuasion, Attitude strength: Antecedents and consequences; Petty, R.E., Krosnick, J.A., Eds.; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 1995; Volume 4, pp. 361–386. [Google Scholar]
- Arvola, A.; Vassallo, M.; Dean, M.; Lampila, P.; Saba, A.; Lähteenmäki, L.; Shepherd, R. Predicting intentions to purchase organic food: The role of affective and moral attitudes in the Theory of Planned Behaviour. Appetite. 2008, 50, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.H. The health belief model and personal health behavior. Health Education Monographs. 1974, 2, 324–508. [Google Scholar] [CrossRef]
- Champion, V.L.; Skinner, C.S. Glanz, K., Rimer, B., Viswanath, K., Eds.; The health belief model. In Health behavior and health education, 4th ed.; Jossey-Bass: San Francisco, CA, USA; pp. 45–65.
- Jones, C.L.; Jensen, J.D.; Scherr, C.L.; Brown, N.R.; Christy, K.; Weaver, J. The health belief model as an explanatory framework in communication research: exploring parallel, serial, and moderated mediation. Health Commun. 2015, 30, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Hill, A.J.; Lawton, C.L. Neurochemical factors involved in normal and abnormal eating in humans. In Handbook of the psychophysiology of human eating; 1989; pp. 85–112. [Google Scholar]
- Van der Elst, W.; Ouwehand, C.; van der Werf, G.; Kuyper, H.; Lee, N.; Jolles, J. The Amsterdam Executive Function Inventory (AEFI): psychometric properties and demographically corrected normative data for adolescents aged between 15 and 18 years. J Clin Exp Neuropsychol. 2012, 34, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.M.; Anderson, C.M. The cognitive flexibility scale: Three validity studies. Communication Reports. 1998, 11, 1–9. [Google Scholar] [CrossRef]
- Martin, M.M.; Rubin, R.B. A new measure of cognitive flexibility. Psychological reports. 1995, 76, 623–626. [Google Scholar] [CrossRef]
© 2008 by the author. 2008 Siamak Khodarahimi
Share and Cite
Khodarahimi, S. Self-Reported Nutritional Status, Executive Functions, and Cognitive Flexibility in Adults. J. Mind Med. Sci. 2018, 5, 210-217. https://doi.org/10.22543/7674.52.P210217
Khodarahimi S. Self-Reported Nutritional Status, Executive Functions, and Cognitive Flexibility in Adults. Journal of Mind and Medical Sciences. 2018; 5(2):210-217. https://doi.org/10.22543/7674.52.P210217
Chicago/Turabian StyleKhodarahimi, Siamak. 2018. "Self-Reported Nutritional Status, Executive Functions, and Cognitive Flexibility in Adults" Journal of Mind and Medical Sciences 5, no. 2: 210-217. https://doi.org/10.22543/7674.52.P210217
APA StyleKhodarahimi, S. (2018). Self-Reported Nutritional Status, Executive Functions, and Cognitive Flexibility in Adults. Journal of Mind and Medical Sciences, 5(2), 210-217. https://doi.org/10.22543/7674.52.P210217



