New Oral Anticoagulants and Their Reversal Agents
Highlights
- Long-term oral anticoagulant therapy includes vitamin K antagonists and new oral anticoagulants (NOACs).
- The introduction of NOACs in the treatment guidelines for atrial fibrillation has improved the quality of life of patients, as they do not require close monitoring or dose adjustments.
- NOACs are divided into two broad categories, depending on the mechanism of action: substances that directly inhibit the activity of thrombin (e.g., dabigatran) and substances that directly inhibit activated factor X (e.g., rivaroxaban, apixaban, edoxaban).
Abstract
:Highlights
- ✓
- Long-term oral anticoagulant therapy includes vitamin K antagonists and new oral anticoagulants (NOACs).
- ✓
- The introduction of NOACs in the treatment guidelines for atrial fibrillation has improved the quality of life of patients, as they do not require close monitoring or dose adjustments.
- ✓
- NOACs are divided into two broad categories, depending on the mechanism of action: substances that directly inhibit the activity of thrombin (e.g. dabigatran) and substances that directly inhibit activated factor X (e.g. rivaroxaban, apixaban, edoxaban).
Introduction
Atrial fibrillation – epidemiological data
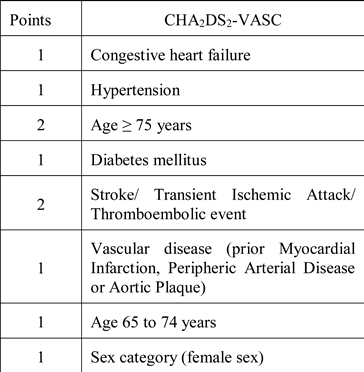
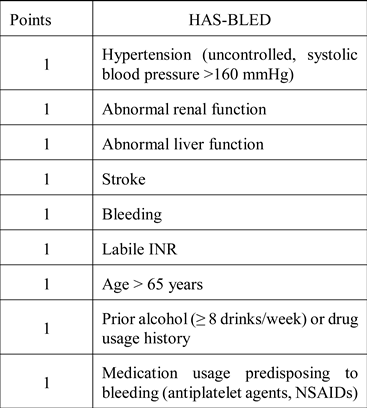
Anticoagulant medication in patients with atrial fibrillation
Discussions
New oral anticoagulants
New perspectives in the acute treatment of hemorrhage in patients taking NOACs: reversal agents Idarucizumab and Andexanet Alpha
Conclusions
Conflicts of Interest
References
- Ball, J.; Carrington, M.J.; McMurray, J.J.; Stewart, S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. 2013, 167, 1807–24. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, C.; Bălăceanu, A. Atrial fibrillation and comorbidities in very elderly patients. Arch Balk Med Union. 2015, 50, 190–3. [Google Scholar]
- Iancu, M.A.; Diaconu, C.; Dediu, G.; et al. An analysis of hypertensive male patients addressed to a primary practice. J Hypertens. 2016, 34, e323. [Google Scholar] [CrossRef]
- Available online: https://www.statista.com/statistics/236775/increase- in-life-expectancy-worldwide-by-country/ (accessed on 10 May 2018).
- Available online: https://www.statista.com/statistics/274514/life-expectancy-in-europe/ (accessed on 10 May 2018).
- Diaconu, C.C.; Dediu, G.N.; Iancu, M.A. Drug-induced arterial hypertension, a frequently ignored cause of secondary hypertension: a review. Acta Cardiol. 2018. [CrossRef] [PubMed]
- Diaconu, C.; Bălăceanu, A.; Bartoş, D. Venous thromboembolism in pregnant woman – a challenge for the clinician. Central European Journal of Medicine. 2013, 8, 548–552. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hindricks, J.H.G.; Manolis, A.S.; Oldgren, J.; Popescu, B.A.; Schotten, U.; Van Putte, B.; Vardas, P. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016, 37, 2893–962. [Google Scholar] [CrossRef] [PubMed]
- Gallego, P.; Roldán, V.; Torregrosa, J.M.; Gálvez, J.; Valdés, M.; Vicente, V.; Marín, F.; Lip, G.Y. Relation of the HAS-BLED bleeding risk score to major bleeding, cardiovascular events and mortality in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2012, 5, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Wittkowsky, A.K. Novel oral anticoagulants and their role in clinical practice. Pharmacotherapy. 2011, 31, 1175–91 PMID: 22122180. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.; Jurk, K.; Schinzel, H. Direct oral anticoagulants in patients with chronic kidney disease: patient selection and special considerations. Int J Nephrol Renovasc Dis 2017, 10, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, C.; Bălăceanu, A.; Ghinescu, M. A neck mass that dissapears at compression: is it a reason for concern? Acta Medica Mediterranea 2015, 31, 339–341. [Google Scholar]
- Tincu, R.C.; Cobilinschi, C.; Tomescu, D.; Coman, L.; Tincu, I.; Diaconu, C.; Macovei, R.A. Favourable results for L-carnitine use in valproic acid acute poisoning. Farmacia. 2017, 65, 396–400. [Google Scholar]
- Diaconu, C.; Nastasă, A.; Zaki, A.R.; Arsalan, M. Type 2 diabetes: a driver for chronic heart failure. In In Proceedings of the 2nd International Conference on Interdisciplinary Management of Diabetes Mellitus and its Complications - INTERDIAB 2016 Proceedings; Serafinceanu, C., Negoiţă, O., Elian, V., Eds.; Editura Niculescu; pp. 201–210.
- Diaconu, C.; Bălăceanu, A.; Moroşan, E. Sepsis biomarkers: past, present and future. Farmacia. 2015, 63, 811–15. [Google Scholar]
- Dediu, G.; Diaconu, C.; Dumitrache Rujinski, S.; Iancu, A.; Bălăceanu, A.; Dina, I.; Bogdan, M. May inflammatory markers be used for monitoring the continuous positive airway pressure effect in patients with obstructive sleep apnea and arrhythmias? Med Hypotheses. 2018, 115, 81–86 PMID: 29685205. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Copeland, A.; Gangidine, M.; Schreiber- Gregory, D.; Ritter, E.M.; Durning, S.J. Factors associated with surgery clerkship performance and subsequent USMLE Step Scores. J Surg Educ. 2018, pii, S1931-7204(17)30830-9. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; Wang, S.; Alings, M.; Xavier, D.; Zhu, J.; Diaz, R.; Lewis, B.S.; Darius, H.; Diener, H.C.; Joyner, C.D.; Wallentin, L. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009, 361, 1139–51 PMID: 19717844. [Google Scholar] [CrossRef] [PubMed]
- Bratu, O.; Mischianu, D.; Spinu, D.; et al. Paraneoplastic syndrome in primitive retroperitoneal tumors. Chirurgia. 2013, 108, 26–31. [Google Scholar]
- Rădulescu, D.; Balcangiu Stroescu, A.; Pricop, C.; Geavlete, B.; Negrei, C.; Bratu, O.; Ginghină, O.; Văcăroiu, I. Vitamin K influence on cardiovascular mortality in chronic hemodialysed patients. Revista de Chimie. 2017, 68, 52–54. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; Becker, R.C.; Nessel, C.C.; Paolini, J.F.; Berkowitz, S.D.; Fox, K.A.; Califf, R.M.; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011, 365, 883–91 PMID: 21830957. [Google Scholar] [CrossRef] [PubMed]
- Niculae, A.; Peride, I.; Vinereanu, V.; Rădulescu, D.; Bratu, O.; Geavlete, B.; Checheriță, I.A. Nephrotic syndrome secondary to amyloidosis in a patient with monoclonal gammopathy with renal significance (MGRS). Rom J Morphol Embryol. 2017, 58, 1065–8. [Google Scholar]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; Bahit, M.C.; Diaz, R.; Easton, J.D.; Ezekowitz, J.A.; Flaker, G.; Garcia, D.; Geraldes, M.; Gersh, B.J.; Golitsyn, S.; Goto, S.; Hermosillo, A.G.; Hohnloser, S.H.; Horowitz, J.; Mohan, P.; Jansky, P.; Lewis, B.S.; Lopez-Sendon, J.L.; Pais, P.; Parkhomenko, A.; Verheugt, F.W.; Zhu, J.; Wallentin, L. ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011, 365, 981–92. [Google Scholar] [CrossRef] [PubMed]
- Stanimir, M.; Chiutu, L.C.; Wese, S.; Milulescu, A.; Nemes, R.N.; Bratu, O. Mullerianosis of the urinary bladder: a rare case report and review of the literature. Rom J Morphol Embryol. 2016, 57, 849–852. [Google Scholar] [PubMed]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; Ruzyllo, W.; Ruda, M.; Koretsune, Y.; Betcher, J.; Shi, M.; Grip, L.T.; Patel, S.P.; Patel, I.; Hanyok, J.J.; Mercuri, M.; Antman, E.M.; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013, 369, 2093–104. [Google Scholar] [CrossRef] [PubMed]
- Radavoi, G.D.; Jinga, V.; Bratu, O.G.; Mischianu, D.L.D.; Pricop, C.; Mates, D.; Radoi VEUrsu, R.I.; Jinga, M.; Iordache, P. A comprehensive analysis of genome- association studies to identify prostate cancer susceptibility loci for the Romanian population. Rom J Morphol Embryol. 2016, 57, 467–75. [Google Scholar] [PubMed]
- Balaceanu, A.; Diaconu, C.; Aron, G. Budd-Chiari syndrome as an initial presentation of hepatocellular carcinoma – a case report. Med Ultrason. 2014, 16, 172–4. [Google Scholar] [CrossRef] [PubMed]
- Guteanu, R.; Bobocea, A.C.; Dumitrescu, M.; Miron, B.A. The role of mediastinoscopy for diagnosis of isolated mediastinal lymphadenopathies. J Clin Invest Surg. 2017, 2, 120–125. [Google Scholar] [CrossRef]
- Scarneciu, I.; Lupu, S.; Pricop, C.; Scarneciu, C. Morbidity and impact on quality of life in patients with indwelling ureteral stents: a 10-year clinical experience. Pak J Med Sci. 2015, 31, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Pricop, C.; Dragimir, S.; Mardari, B.; et al. Factors influencing recurrent reflux acute pyelonephritis in patients with JJ ureteral stent after discharge. Archives of Biological Sciences. 2014, 66, 1581–4. [Google Scholar] [CrossRef]
- Scarneciu, I.; Muntean, I.; Scarneciu, C.; et al. Diagnosis and renal lithiasis treatment using ultrasounds. Metalurgia International. 2010, 15, 112–5. [Google Scholar]
- Motofei, I.G.; Rowland, D.L.; Georgescu, S.R.; Tampa, M.; Baconi, D.; Stefanescu, E.; Baleanu, B.C.; Balalau, C.; Constantin, V.; Paunica, S. Finasteride adverse effects in subjects with androgenic alopecia: A possible therapeutic approach according to the lateralization process of the brain. J Dermatolog Treat. 2016, 27, 495–497. [Google Scholar] [CrossRef]
- Thomas, S.; Makris, M. The reversal of anticoagulation in clinical practice. Clin Med (Lond). 2018, 18, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Davlouros, P.; Giamouzis, G.; Giannakoulas, G.; Pipilis, A.; Tsivgoulis, G.; Parissis, J. Direct Oral Anticoagulants in Nonvalvular Atrial Fibrillation: Practical Considerations on the Choice of Agent and Dosing. Cardiology. 2018, 140, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Milling TJJr Eiklboom, J.W.; Gibson, C.M.; Curnutte, J.T.; Gold, A.; Bronson, M.D.; Lu, G.; Conley, P.B.; Verhamme, P.; Schmidt, J.; Middeldorp, S.; Cohen, A.T.; Beyer-Westendorf, J.; Albaladejo, P.; Lopez-Sendon, J.; Goodman, S.; Leeds, J.; Wiens, B.L.; Siegal, D.M.; Zotova, E.; Meeks, B.; Nakamya, J.; Lim, W.T.; Crowther, M.; ANNEXA-4 Investigators. The ANNEXA-4 Investigators. Andexanet alfa for acute major bleeding associated with factor Xa Inhibitors. N Engl J Med. 2016, 375, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, C.C.; Manea, M.; Iancu, M.A.; Stanescu, A.M.A.; Socea, B.; Spinu, D.A.; Marcu, D.; Bratu, O.G. Hyponatremia in patients with heart failure: a prognostic marker. Revista de Chimie. 2018, 69, 1071–74. [Google Scholar] [CrossRef]
- Ansell, J.E.; Bakhru, S.H.; Laulicht, B.E.; Steiner, S.S.; Grosso, M.A.; Brown, K.; Dishy, V.; Lanz, H.J.; Mercuri, M.F.; Noveck, R.J.; Costin, J.C. Single-dose ciraparantag safely and completely reverses anticoagulant effects of edoxaban. Thromb Haemos. 2017, 117, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, C.C.; Dragoi, C.M.; Bratu, O.G.; Neagu, T.P.; Pantea Stoian, A.; Cobelschi, P.C.; Nicolae, A.C.; Iancu, M.A.; Hainarosie, R.; Stanescu, A.M.A.; Socea, B. New approaches and perspectives for the pharmacological treatment of arterial hypertension. Farmacia. 2018, 66, 408–15. [Google Scholar] [CrossRef]
© 2018 by the author. 2018 Crista L. Laslo1, Anca Pantea Stoian, Bogdan Socea, Dan N. Păduraru, Oana Bodean, Laura I. Socea, Tiberiu Paul Neagu, Ana Maria Alexandra Stănescu, Dragoș Marcu, Camelia C. Diaconu.
Share and Cite
Laslo, C.L.; Stoian, A.P.; Socea, B.; Păduraru, D.N.; Bodean, O.; Socea, L.I.; Neagu, T.P.; Stanescu, A.M.A.; Marcu, D.; Diaconu, C.C. New Oral Anticoagulants and Their Reversal Agents. J. Mind Med. Sci. 2018, 5, 195-201. https://doi.org/10.22543/7674.52.P195201
Laslo CL, Stoian AP, Socea B, Păduraru DN, Bodean O, Socea LI, Neagu TP, Stanescu AMA, Marcu D, Diaconu CC. New Oral Anticoagulants and Their Reversal Agents. Journal of Mind and Medical Sciences. 2018; 5(2):195-201. https://doi.org/10.22543/7674.52.P195201
Chicago/Turabian StyleLaslo, Crista L., Anca Pantea Stoian, Bogdan Socea, Dan N. Păduraru, Oana Bodean, Laura I. Socea, Tiberiu Paul Neagu, Ana Maria Alexandra Stanescu, Dragoș Marcu, and Camelia C. Diaconu. 2018. "New Oral Anticoagulants and Their Reversal Agents" Journal of Mind and Medical Sciences 5, no. 2: 195-201. https://doi.org/10.22543/7674.52.P195201
APA StyleLaslo, C. L., Stoian, A. P., Socea, B., Păduraru, D. N., Bodean, O., Socea, L. I., Neagu, T. P., Stanescu, A. M. A., Marcu, D., & Diaconu, C. C. (2018). New Oral Anticoagulants and Their Reversal Agents. Journal of Mind and Medical Sciences, 5(2), 195-201. https://doi.org/10.22543/7674.52.P195201



