Introduction
Vulvar cancer is a malignant disease with low frequency, responsible for 5% of the total number of carcinomas in the scope of gynecology [
1], yet its incidence is growing. The debut of vulvar cancer varies in age; however, the majority of cases occur in women over 50, and the maximum incidence is between 65 and 70 years of age [
2]. Histologically, more than 85% of vulvar malignancies are squamous cell carcinomas [
2,
3]. Squamous cell cancer of the vulva is indolent, slowly growing with metastasis occurring late in the course of its evolution.
The natural evolution of the disease is characterized by local extension of the tumour, followed by lymphatic invasion. In general, the lymphatic invasion is organized, progressing towards the superficial inguinal lymph nodes, later towards the deep nodal chains (inguinal-femoral and pelvic).
Surgery is the main pillar of vulvar cancer therapy, but is also reserved for tumours that have not extended beyond the vulva [
4]. Surgery may involve a large local excision: radical partial vulvectomy or radical complete vulvectomy with excision of the vulva and inguinal and femoral lymph nodes [
2]. In the case of early vulvar cancer, surgery may be less extensive, consisting of a large excision or a simple vulvectomy. When the vulvar neoplasm extends into its neighbouring organs such as the urethra, vagina, or rectum, extensive surgical treatment is very important.
Complications of vulvar cancer surgery include: wound infection, sexual dysfunction, thrombosis and swelling, and secondary lymphedema after local-regional lymph node excision [
2]. In rare cases general surgeons will consider collaborating with a plastic surgeon when a wide excision is needed, resulting in a large defect of the tegument [
1].
Case Report
A 64-year-old patient with no significant past medical history presented with an imprecisely delineated ulcero-vegetative tumour of approximately 4 cm in diameter of the right labia majora. A total vulvectomy was performed with a favourable local evolution. Histopathological exam: microscopic structure of squamous cell carcinoma, moderately differentiated, ulcerated, with margins of resection without invading tumour cells. At 30 days post-op, the patient was scheduled for bilateral inguinal lymphadenectomy, but did not show up for her follow up appointment and post-operative chemotherapy treatment.
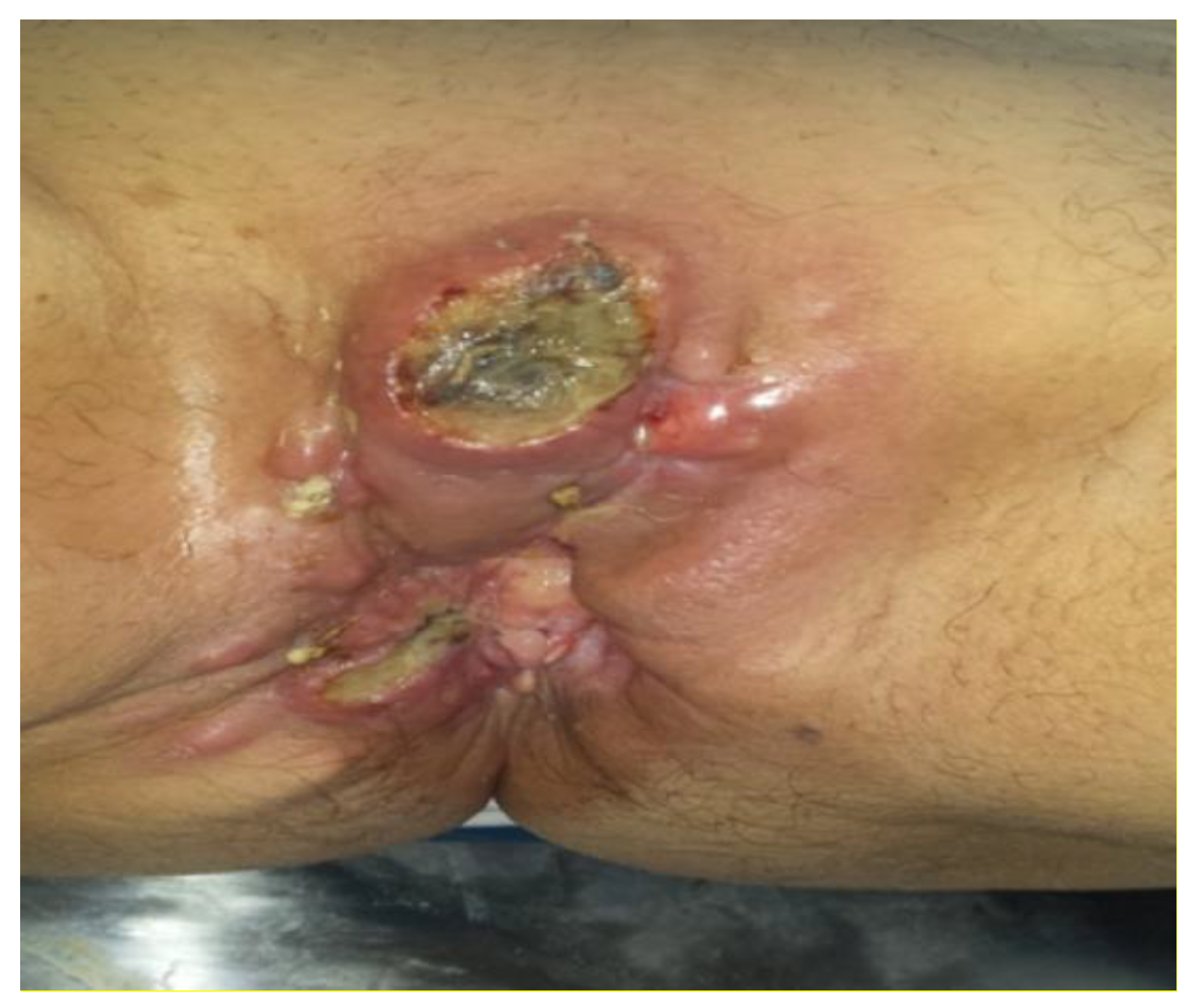
At 4 months post-operative, the patient presented with perineal pain and bowel transit disorder. A local examination found: perineal tumour formation which extended from the level of the pubic symphysis to the posterior perineum where it invaded the anal sphincter; at this level the tumour stretched the tissue of the right ischiorectal fossa forming several lumps in the skin (
Figure 1). The right vaginal introitus was invaded by the tumour.
Ulcerations of approximately 2 cm in diameter situated in the pubic symphysis were found to have pathological secretions. No local-regional adenopathy was palpated. The diagnosis of recurrent vulvar carcinoma was established. Complementary laboratory exams (CT thorax, abdomen and pelvis, abdominal ultrasound, pelvic and chest radiograph) showed no general extension of the disease.
Surgical intervention was decided and a median laparotomy, terminal left iliac anus, and excision of the tumour was performed: with an additional 3 cm from the macroscopic margins of the tumour.
The tumour was raised up to the level of the bony plane of the pubic symphysis; laterally, to the level of the ischial tuberosity, and posteriorly to the level of the levator ani muscle, followed by removal of tumoral formations in the lower vagina and anal canal with anal sphincter (
Figure 2). We then sutured the anterior wall of the rectum to the rear wall of the vagina and rear wall of the rectum to the posterior perineal skin. We were left with a large defect of skin anterior and posterior to the perineum.
The post-operative evolution was favourable with granulation tissue of the remaining wound (
Figure 3). However, the patient suffered severe episodes of depression with suicidal ideations, making it necessary to order a psychological consultation.
In addition, it was decided to cover the remaining wound 30 days post-operatively via plastic surgery. Wound culture results found Acinobacter spp sensible to Imipenem. Histopathological exam: poorly differentiated squamous cell carcinoma with margins of resection showing no tumour cells. We biopsied the granulation tissue from the wound and the histopathological exam showed no tumour cells.
Before the plastic surgery, in order to perform a musculocutaneous flap, we conducted a Doppler ultrasound to identify the exact position of the perforator artery of the deep femoral artery, a perforator which would be sacrificed and its anatomical variants. The exam was conducted with the patient’s legs in abduction, knee flexion at about 120°, and forced adduction of the thigh to highlight the gracilis and long Adductor (position “frog leg”). Also in this position we performed preoperative markings which were as follows: inguinal ligament, the insertion site of the vascular pedicle, the body of the gracilis muscle, the island of skin overlying (which should not exceed 8–9 × 18–20 cm) and possible anatomic variations in vasculature.
The surgery was performed with spinal anaesthesia and started with an incision close to the origin of the body of the adductor longus muscle, and medial and posterior to the gracilis muscle. Dissection continued painstakingly, repairing the vascular-nervous packet found between the initial portion of the adductor longus and adductor magnus muscles at approximately 9 cm from the inguinal ligament in the appropriate place according to preoperative ultrasound Doppler marking.
An incision was made at the level of the insertion of the gracilis muscle identified at the level of the pes anserinus tendon (“goose foot” conjoined tendons of sartorius, gracilis, semitendinosus muscles), allowing for the identification of the whole projection of the body of the muscles simply by applying traction to the tendon with a retractor. The dissection was meticulously continued, identifying posteriorly the semi-membranosus muscle, antero-laterally the adductor longus muscle, and distally the sartorius muscle and the oblique fibers of the adductor magnus muscle (
Figure 4).
The vasculo-nervous pedicle was dissected with care, easily identifying the afferent motor branches of the obturator nerve. What followed was sectioning of the insertion of the gracilis tendon being that it was uncovered by the island of skin, thus trying to avoid the distal 1/3 with its vascularization from a branch of the saphenous artery which was subsequently sacrificed; skin island measuring 8 × 22 cm.
The dissection continued from proximal towards distal and from anterior towards posterior, finding in this case an inconsistent arterial branch which vascularizes the middle 1/3 of the gracilis (not the skin island) being sacrificed.
We gradually raised the island flap, paying close attention to the many ligations of the perforators of the adductor magnus and semimembranosus muscles. The flap was constantly kept wet by saline compresses heated to about 32 °C, the maximum temperature that allows vasodilation. Once raised, the composite flap was rotated anti-clockwise about 160° to the level of the excision defect which was debrided in advance.
After obtaining hemostasis at the level of the donor site and surgical lavage with antiseptic solution, a draining tube was placed, and the donator site was sutured in two planes with separate everted sutures (PGA 3.0) and separate Blair Donati sutures (polypropylene 4.0), using care to avoid tension at the level of the vasculo-nervous pedicle. Also, at the receiving area, a drainage tube was placed using sutures in two planes: everted separate sutures (PGA 3.0) and separate Blair Donati sutures (
Figure 5).
Post-operative evolution was favourable with no local and general complications. Drainage tubes were removed 5 days postoperatively, and the patient was discharged, with the flap undergoing integration.
Discussion
Most vulvar squamous cell carcinomas are determined by the origin of the vulvar epidermis; most injuries develop in the labia, especially the labia majora [
2]. Over 80% of recurrences are in the first 2 years after therapy and may be local or remote [
3]. Although treatment of the primary lesion is well established, surgical treatment of recurrent local-regional lesions poses difficulties in excision, and more so in reconstruction.
Local reconstruction is problematic due to the remaining large defect of skin; therefore, using the gracilis muscle in a flap is an important option. The gracilis muscle is an adductor and a rotator of the thigh and a flexor leg. It originates from a thin aponeurosis from the anterior margins of the lower half of the symphysis pubis and the upper half of the pubic arch; the muscle’s fibers run vertically downward, ending in a rounded tendon to join the pes anserinus [
4,
5]. On the anterior surface of the gracilis muscle you can spot the sartorius muscle, and the posterior surface is bordered by the semitendinosus and semimembranosus muscles. Deep within the gracilis muscle is the adductor magnus, and along the entire anterior-lateral length is the adductor longus muscle, which is the most important landmark during surgery. The adductor longus tendon along with the body of the gracilis muscle forms a real prominence when the thigh is forcefully adducted [
6,
7].
Gracilis vasculature is provided by a vascular pedicle which originates from the deep femoral artery and enters the predominantly muscular body 9–10 cm from the inguinal ligament. An artery with a smaller calibre and inconsistent can be identified at the middle 1/3 of the muscular body [
8]. In the lower part of the muscle body and the overlying tissue, vasculature is provided by a branch of the saphenous artery. In the case of this composite flap, arterial anastomoses and collateral vessels are minimal, yet the principle perforating artery from the deep femoral artery can assure the survival of the entire musculature as well as the skin island [
9]. Motor innervation is provided by a branch of the obturator nerve that can be identified near the main perforating artery of the deep femoral artery, and sensory innervation is provided by medial cutaneous nerve of the thigh [
10]. The main anatomical variant on which the functionality of the flap is dependent is represented by a perforating artery from the deep femoral artery that runs too deep, more than 15 cm, making it impossible to optimally rotate the flap [
11,
12].
The gracilis flap is primarily used to cover defects at the perineum, including vaginal, penile, and scrotal reconstruction [
5]. It is the flap of choice for total vaginal reconstruction and of the abdominal-perineal area [
8]. It can also be used to cover the defects of the medial 2/3 of the inguinal and suprapubic regions. Rarely is it used to cover decubitus pressure ulcers at the ischial level, especially in spastic paralysis, when some of the gracilis muscle mass is preserved. The gracilis flap also lends itself very well to a free transfer, which is particularly useful for osteomyelitis of the distal tibia due to its narrow and long configuration [
6]. It can also be used as an interposition flap for vesico-vaginal or recto-vaginal fistulas [
6,
8].
We paid particular attention to the skin island of the distal 1/3 of the flap to allow a period of “delay” to accommodate integration of circulation in the flap. The same cannot be said about the muscular body, whose only source of vascularization located in the proximal third is sufficient. If constructed and operated with caution, this flap is extremely versatile and safe compared to other local variants that are suited for the same defects (inferior gluteal fasciocutaneous flap, inferior rectus abdominis fasciocutaneous flap, fasciocutaneous advancement flap type “V-Y” from the biceps femoris).
Depending on the surgeon’s preference and the local needs, the flap can be used as an island flap without a cutaneous pedicle, as an island flap with a cutaneous pedicle, or as a free transfer [
13].
In deciding to use this type of flap, we considered several drawbacks that could endanger the viability of the flap. The main disadvantage is the tendency of vascular spasm of the pedicle due to torsion or inadequate maneuvering, resulting in a marked and irreversible suffering of the cutaneous island. This risk can be avoided by thorough dissection of the vascular pedicle, often requiring splitting of the intermuscular septum and skeletonization the vascular pedicle. However, the muscular body is not prone to this risk. Another drawback is constituted by poor vascular network of the cutaneous island at the inferior 1/3, often necessitating a period of adaptation [
5].
Low insertion of the vascular pedicle at the level of the muscle body (below 15 cm) is another serious disadvantage, making the flap unusable. Yet, in such a case, the use of this flap should not be excluded, because contra-laterally, the vascular source may be at the desired distance from the inguinal ligament (9–10 cm).
The main advantages of using this type of flap are its increased versatility, mobility, convenience of dissection, and low donor site morbidity.
It should be noted that the surface of the cutaneous island cannot exceed 8–9 cm x 18–20 cm and the position of this island must cover the body of the muscle along its entire length. Special attention should be given to the distal third of the cutaneous island flap which has its own source of vasculature as mentioned before: the branch of the saphenous artery. Even if the donor site has low morbidity, attention should be paid to cutaneous tension at the level of the vascular pedicle; if increased it can lead to the failure of this intervention.
Conclusions
Local recurrence is a major problem in vulvar cancer surgery, most relapses occurring within the first two years from the operation, despite neoadjuvant and adjuvant cancer therapy and negative resection margins. Re-excision with wide tumour free margins is a potentially curative and palliative solution (infected tumours, bleeding). Surgical resection of the relapse can pose a delicate situation for surgeons, being that local reconstruction is sometimes a necessity. If a large excision is made, the patient may suffer a significant psychological impact, sometimes requiring counselling; local reconstruction significantly improves the patient’s comfort. The immediate postoperative complication is ischemia of the flap (vascular micro thrombi, vascular compression due to hematoma etc.), thereby requiring its removal. Long-term evolution is given by the appearance of local relapse, even if local excision is made within oncological limits and curettage and biopsy of the granulation tissue is negative for tumour cells. If curettage biopsy of the granulated wound after excision of tumour relapse is positive for tumour cells, local reconstruction is contraindicated.
Recurrent vulvar carcinoma treatment is realised with the help of a complex multidisciplinary team: oncologist, general surgeon, plastic surgeon, radiologist oncologist, pathologist and psychiatrist. Excision and re-excision of vulvar carcinoma may result in the total abolition of local perineal anatomy.