Unmodifiable Variables Related to Thyroid Cancer Incidence
Highlights
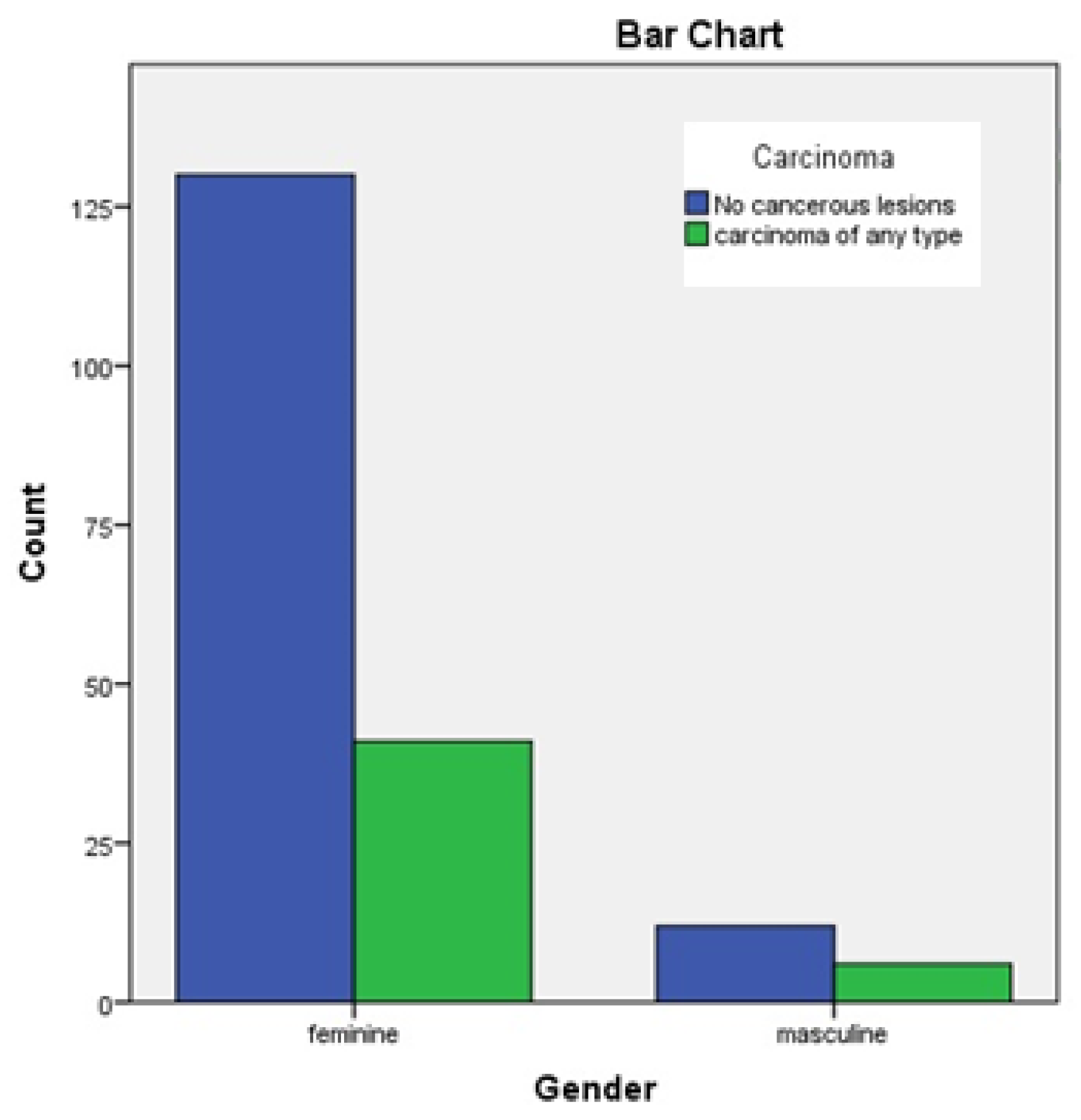
- The incidence of thyroid carcinoma seems to be higher for women than for men.
- Women and for younger patients present better survival, even when they present with advanced disease, as compared with older, male patients.
- Such prognostic indicators should be considered important factors in the therapeutic decision.
Abstract
:Highlights
- The incidence of thyroid carcinoma seems to be higher for women than for men.
- Women and for younger patients present better survival, even when they present with advanced disease, as compared with older, male patients.
- Such prognostic indicators should be considered important factors in the therapeutic decision.
Abstract
Introduction
Materials and methods
Results
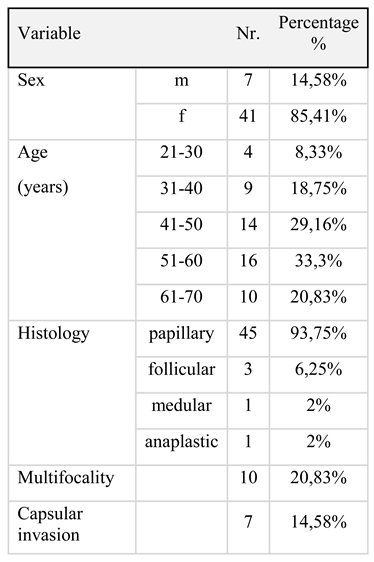
Discussion
Conclusions
Acknowledgments
References
- Davies, L.; Welch, H.G. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006, 295, 2164–2167. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.; Sala, C.; Flor, B.; Lledo, S. Efficacy and cost–effectiveness of the UltraCision harmonic scalpel in thyroid surgery: An analysis of 200 cases in a randomized trial. J Laparoendosc Adv Surg Tech A. 2004, 14, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Kilfoy, B.A.; Devesa, S.S.; Ward, M.H.; Zhang, Y.; Rosenberg, P.S.; Holford, T.R.; Anderson, W.F. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev. 2009, 18, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Weeks, K.S.; Kahl, A.R.; Lynch, C.F.; Charlton, M.E. Racial/ethnic differences in thyroid cancer incidence in the United States, 2007-2014. Cancer. 2018. [CrossRef] [PubMed]
- Negri, E.; Dal Maso, L.; Ron, E.; La Vecchia, C.; Mark, S.D.; Preston-Martin, S.; McTiernan, A.; Kolonel, L.; Yoshimoto, Y.; Jin, F.; Wingren, G.; Rosaria Galanti, M.; Hardell, L.; Glattre, E.; Lund, E.; Levi, F.; Linos, D.; Braga, C.; Franceschi, S. A pooled analysis of case– control studies of thyroid cancer. II. Menstrual and reproductive factors. Cancer Causes Control. 1999, 10, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.; Orsi, L.; Dubourdieu, D.; Rougier, Y.; Hemon, D.; Guenel, P. Role of goiter and of menstrual and reproductive factors in thyroid cancer: A population-based case–control study in New Caledonia (South Pacific), a very high incidence area. Am J Epidemiol. 2005, 161, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Cerfolio, R.J.; Bryant, A.S.; Scott, E.; Sharma, M.; Robert, F.; Spencer, S.A.; Garver, R.I. Women with pathologic stage I, II, and III non-small cell lung cancer have better survival than men. Chest. 2006, 130, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Naugler, W.E.; Sakurai, T.; Kim, S.; Maeda, S.; Kim, K.; Elsharkawy, A.M.; Karin, M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007, 317, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Cady, B.; Rossi, R. An expanded view of risk-group definition in differentiated thyroid carcinoma. 1988, 104, 947–953. [Google Scholar] [PubMed]
- Brierly, J.D.; Panzarella, T.; Tsang, R.W.; Gospodarowicz, M.K.; O’Sullivan, B. A comparison of different staging systems predictability of patient outcome. Cancer. 1997, 79, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Yager, J.D.; Liehr, J.G. Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol. 1996, 36, 203–232. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.L.; Chen, G.G.; Vlantis, A.C.; Tse, G.M.; Leung, B.C.; van Hasselt, C.A. Induction of thyroid papillary carcinoma cell proliferation by estrogen is associated with an altered expression of BclxL. Cancer J. 2005, 11, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, C.; Haselkorn, T.; Tekawa, I.S.; Friedman, G.D. Cohort study of thyroid cancer in a San Francisco Bay area population. Int J Cancer. 2001, 93, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Kruijff, S.; Petersen, J.F.; Chen, P.; Aniss, A.M.; Clifton-Bligh, R.J.; Sidhu, S.B.; Delbridge, L.W.; Gill, A.J.; Learoyd, D.; Sywak, M.S. Patterns of structural recurrence in papillary thyroid cancer. World J Surg. 2014, 38, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, T.; Zeng, W.; Wang, S.; Xiong, Y.; Liu, Z.; Huang, T. Reevaluating the prognostic significance of male gender for papillary thyroid carcinoma and microcarcinoma: A SEER database analysis. Sci Rep. 2017, 7, 11412. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferri, E.L.; Kloos, R.T. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001, 86, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Moagar-Poladian, S.; Folea, V.; Paunica, M. Competitiveness of EU member states in attracting EU funding for research and innovation. Romanian Journal of Economic Forecasting. 2017, 20, 150–167. [Google Scholar]
- Toniato, A.; Boschin, I.; Casara, D.; Mazzarotto, R.; Rubello, D.; Pelizzo, M. Papillary thyroid carcinoma: Factors influencing recurrence and survival. Ann Surg Oncol. 2008, 15, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Barbu, C.G.; Florin, A.; Neamţu, M.C.; Avramescu, E.T.; Terzea, D.; Miron, A.; Dănciulescu Miulescu, R.; Poiană, C.; Fica, S. Papillary thyroid carcinoma with anaplastic dedifferentiation in the lymph node metastasis—A rare form of presentation even for a tall cell variant. Rom J Morphol Embryol. 2015, 56, 527–531. [Google Scholar] [PubMed]
- Păun, D.L.; Poiană, C.; Petriş, R.; Radian, S.; Miulescu, R.D.; Constantinescu, G.; Orban, C. Multiple Endocrine Neoplasia Type 2A: Case Report. Chirurgia (Bucur). 2013, 108, 900–903. [Google Scholar] [PubMed]
- Gilliland, F.D.; Hunt, W.C.; Morris, D.M.; Key, C.R. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973-1991. Cancer. 1997, 79, 564–573. [Google Scholar] [PubMed]
- Brehar, A.C.; Terzea, D.C.; Ioachim, D.L.; Procopiuc, C.; Brehar, F.M.; Bulgăr, A.C.; Ghemigian, M.V.; Dumitrache, C. Cribriform-morular variant of papillary thyroid carcinoma at pediatric age—Case report and review of the literature. Rom J Morphol Embryol. 2016, 57, 531–537. [Google Scholar] [PubMed]
- Hundahl, S.A.; Fleming, I.D.; Fremgen, A.M.; Menck, H.R. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995. Cancer. 1998, 83, 2638–2648. [Google Scholar] [PubMed]
- Cho, J.S.; Yoon, J.H.; Park, M.H.; Shin, S.H.; Jegal, Y.J.; Lee, J.S.; Kim, H.K. Age and prognosis of papillary thyroid carcinoma: Retrospective stratification into three groups. J Korean Surg Soc. 2012, 83, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Radu, T.G.; Ciurea, M.E.; Mogoantă, S.Ş.; Busuioc, C.J.; Grosu, F.; Ţenovici, M.; Petrescu, I.O.; Vladu, I.M. Papillary thyroid cancer stroma—Histological and immunohistochemical study. Rom J Morphol Embryol. 2016, 57 (Suppl. 2), 801–809. [Google Scholar] [PubMed]

© 2018 by the author. 2018 Cornelia Nitipir, Lucian Alecu, Iulian Slavu, Raluca Tulin, Radu C. Jecan, Cristina Orlov, Silviu Pituru, Dana L. Stanculeanu, Razvan Hainarosie, Anca Pantea Stoian, Adrian Tulin.
Share and Cite
Nitipir, C.; Alecu, L.; Slavu, I.; Tulin, R.; Jecan, R.C.; Orlov, C.; Pituru, S.; Stanculeanu, D.L.; Hainarosie, R.; Stoian, A.P.; et al. Unmodifiable Variables Related to Thyroid Cancer Incidence. J. Mind Med. Sci. 2018, 5, 123-128. https://doi.org/10.22543/7674.51.P123128
Nitipir C, Alecu L, Slavu I, Tulin R, Jecan RC, Orlov C, Pituru S, Stanculeanu DL, Hainarosie R, Stoian AP, et al. Unmodifiable Variables Related to Thyroid Cancer Incidence. Journal of Mind and Medical Sciences. 2018; 5(1):123-128. https://doi.org/10.22543/7674.51.P123128
Chicago/Turabian StyleNitipir, Cornelia, Lucian Alecu, Iulian Slavu, Raluca Tulin, Radu C. Jecan, Cristina Orlov, Silviu Pituru, Dana L. Stanculeanu, Razvan Hainarosie, Anca Pantea Stoian, and et al. 2018. "Unmodifiable Variables Related to Thyroid Cancer Incidence" Journal of Mind and Medical Sciences 5, no. 1: 123-128. https://doi.org/10.22543/7674.51.P123128
APA StyleNitipir, C., Alecu, L., Slavu, I., Tulin, R., Jecan, R. C., Orlov, C., Pituru, S., Stanculeanu, D. L., Hainarosie, R., Stoian, A. P., & Tulin, A. (2018). Unmodifiable Variables Related to Thyroid Cancer Incidence. Journal of Mind and Medical Sciences, 5(1), 123-128. https://doi.org/10.22543/7674.51.P123128


