Highlights
- A single value of 12 lymph nodes should not be viewed as a benchmark for quality of colon surgery.
- The current standards on colon resections need to be reevaluated; a more flexible algorithm should be developed for assessment of surgical performance.
Highlights
- A single value of 12 lymph nodes should not be viewed as a benchmark for quality of colon surgery.
- The current standards on colon resections need to be reevaluated; a more flexible algorithm should be developed for assessment of surgical performance.
Abstract
Introduction. Improving the quality of surgical resections by evaluating surgical specimens is probably the most important feedback a surgeon can receive. Moreover, prognosis of patients with colon cancer is based on achieving appropriate resection margins and assessment of lymph node status. For these reasons we aim to provide a retrospective analysis on colon cancer specimens operated by a single surgical team. Materials and Methods. 88 patients operated between 2013 and 2016 were included in the study. Data were gathered prospectively and assessed by multivariate analysis for the main variables (age, gender, tumor staging, specimen length, distance to closest resection margin, number of lymph nodes, and number of positive lymph nodes). Results. The mean number of lymph nodes excised was 31.9, with more after right colectomies (39.6) than after left colonic resections (29.1). The average specimen length was 29.2 cm after right colectomies, 35.6 cm after left hemicolectomies and 18 cm after segmental colectomies. There was a significant correlation between the number of lymph nodes, specimen length, and age of patients. Conclusion. Lymph node status is correlated with specimen length and age. The standard of 12 lymph nodes was achieved and surpassed, being comparable to the benchmark literature. Standards on colon resections need to be reevaluated as many surgeons are pressured by quality measurements which do not always reflect sound oncologic principles.
Introduction
The number of lymph nodes excised in colon cancer surgery has been found to be critical to the overall survival rate of patients [1,2]. Besides their staging role, the number of lymph nodes correlates with Kaplan- Meyer survival curves. Surgeons with proper lymph node yields have better prognostic outcomes than surgeons with statistics below standards values [3,4]. The importance of extensive lymph node resection is not based on the therapeutic role but rather on the diagnostic and staging significance, as more lymph nodes in the specimen will lead to better staging and subsequently improved treatment for patients [5,6,7,8,9]. A total of 12 retrieved lymph nodes are considered the standard of care. Variation in the numbers of retrieved nodes has been analyzed, and many factors have been proposed to influence the number of excised lymph nodes, including age, sex, tumor location, tumor stage, and the technique used for pathological assessment [10,11]. Perhaps the most important factor influencing appropriate lymph node excision is the surgeon him/herself. Technique of dissection, maintaining the correct anatomic plane, and central vascular ligation are all influencing factors that depend on the specific surgeon.
Following anatomical plane in the course of dissection is imperative for proper specimen retrieval, an idea supported by Total Mesorectal Excision (TME). Heald [12] has thoroughly described the correct excision plane in rectal surgery, with such specimens proving far superior to previous ones [13]. The logic of TME lies in respecting the embryological, avascular plane between the mesorectal and pelvic fascia, and with the advent of TME, rectal surgery has now become standardized. However, these planes may not be limited to the rectum, but rather can be found all along the colon such that Complete Mesocolic Excision (CME) has now been promoted as the standard surgical technique in colon surgery. Proponents of CME present superior lymph node counts and better oncological outcomes when CME is followed [14,15,16,17]. Others argue that CME is simply a D3 lymphadenectomy, which Japanese and Taiwanese surgeons had already been doing before CME was proposed. Even so, the necessity of a standard procedure in colon surgery is obvious and underscored by the variability of lymph node retrieval and specimen length among surgeons worldwide. Comparative studies of specimens before and after adopting CME by the same surgeons show superior outcomes under the latter conditions. Regardless of the procedure, CME or D3 lymphadenectomy, correct dissection, central ligation, respecting oncological margins, and retrieving a proper specimen are critical for all the lymph nodes identified for excision. Evaluating the specimen is based on the number of lymph nodes excised, as the number of lymph nodes is an independent prognostic and staging factor in colon cancer. For these reasons, a thorough review of one`s specimens regarding lymph node count is or should be a must for improving surgical standards.
Materials and Methods
We conducted a prospective study of patients diagnosed with colon cancer between 2013 and 2016 who were operated by a single surgical team at the 2nd Surgical Unit, Regional Oncology Institute, Iasi, Romania. Data were prospectively collected in an excel database and included age, sex, operation date, intraoperative diagnostic and findings, preoperative staging, preoperative radio-chemotherapy, preoperative CEA and CA19-9 values, and histopathology report (histologic diagnosis, pTNM, specimen length, distance to resection margins, number of lymph nodes and number of positive lymph nodes). Only patients that had all the aforementioned details were included in the study. Postoperatively, patients were followed up at 3, 6, 12 months and annually thereafter by tumor marker levels, abdominal ultrasound, chest x-ray, and CT scans. Patients were called in for control to assess possible local recurrence and overall survival according to the Institute’s follow-up protocols.
Data were grouped according to the type of procedure the patients underwent: right hemicolectomy, left hemicolectomy, segmental sigmoid resection, and transverse segmental colectomy. Rectal cancer cases were not included in the study. Specimens were measured after formalin fixation. No special techniques were used to harvest lymph nodes (e.g., fat clearance, blue dye injection). Multivariate data analysis was performed in Microsoft Excel using a logistic regression model; p<0.05 was considered statistically significant. Lymph Node Ratio (LNR) was calculated, defined by the ratio between positive lymph nodes and the total number of lymph nodes excised. Interquartile Range (IQR), mean, and min and max values were generated.
Results
Surgical technique
An open “lateral to medial” approach was used in all cases. In summary, for right colectomies the dissection commences with the mobilization of the gastrocolic ligament and entrance into the omental bursa where the middle colic artery is easily identified. Laterally, Toldt’s fascia is identified and dissected carefully to ensure an intact mesocolon (Figure 1). The superior mesenteric vein (SMV) is found and skeletonized on its lateral aspect. The ileocolic artery (ICA) and right colic artery (if present) are ligated on the left lateral border of the superior mesenteric vein. The superior mesenteric artery (SMA) is not dissected, thus preventing the risk of postoperative diarrhea related to iatrogenic injuries of the splanchnic nerves [18]. For tumors of the cecum and ascending colon, the middle colic artery is dissected and its right branch is ligated at its origin (Figure 1).
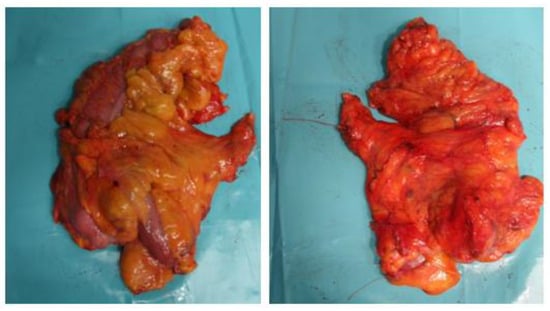
Figure 1.
Unfixed specimen of right colectomy. Thread left at the central vascular ligation (CVL) site.
For tumors of the hepatic flexure or proximal transverse colon, the middle colic artery is ligated at its origin and the right greater omentum is transected just below the gastroepiploic arcade. In left sided tumors, regardless of location, the inferior mesenteric artery (IMA) is ligated to about 1 cm from its origin in the abdominal aorta to prevent injuries to the preaortic plexus, while the inferior mesenteric vein (IMV) is ligated 1cm above the origin of the IMA for sigmoid carcinoma while ligation of the IMV at the inferior pancreatic border is done in left hemicolectomies and when further mobilization of the splenic flexure is needed for a tension-free anastomosis (Figure 2) [19,20,21].
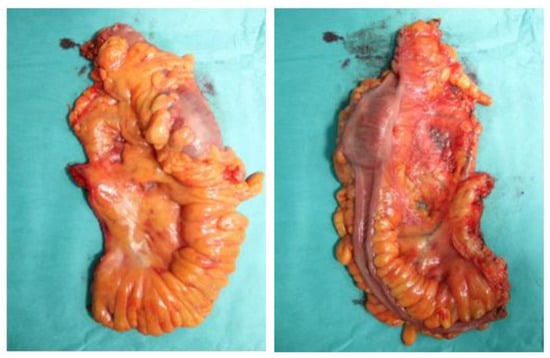
Figure 2.
Unfixed specimen of left hemicolectomy.
Clinicopathological outcomes
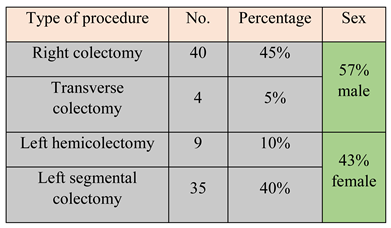
A total of 88 consecutive patients operated between 2013 and 2016 were included in the study. Only two patients received preoperative chemotherapy. According to the type of procedure, patients were split as follows: right colectomy (45%), transverse colectomy (5%), left hemicolectomy (10%), and left segmental colectomy (40%). Demographic data showed a mean age of 67.5 years and a slight male predominance, 57% compared to 43% (Table 1).

Table 1.
Split data according to procedure type and sex.
Pathologic staging showed the majority of cases were stage II (45.4%), followed by stage III (29.5%). The overwhelming majority of tumors were moderately differentiated (G2), accounting for 66% of all cases (Figure 3).
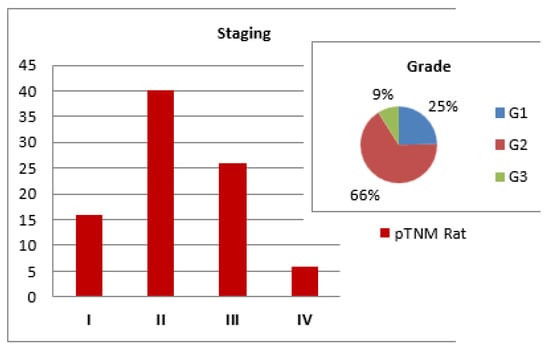
Figure 3.
Pathological staging of tumors.
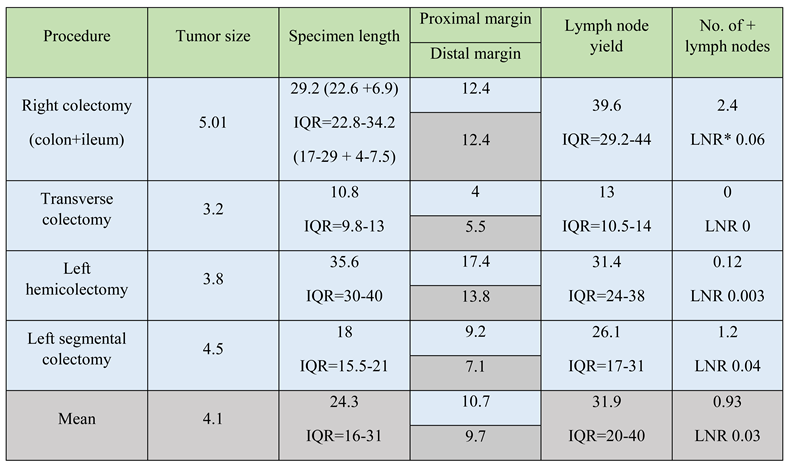
After fixation, specimens were measured. The mean tumor size was 4.1 cm. The mean specimen length was 24.3 cm with an Interquartile Range (IQR) of 16-31, with left hemicolectomies being the longest (35.6 cm, IQR 30-40) followed by right colectomy (29.2 cm, IQR 22.8-34.2) and left segmental colectomies (18 cm, IQR 15.5-21).
For right colectomies the resulting length is the sum between colon and ileal length with 22.6cm, IQR 17-29 being the average colon length and 6.9cm, IQR 4-7.5 the ileal length after right colectomies. Proximal and distal margins were approximately 10cm in most cases, except the distal margin after segmental colectomies, as ensuring more than 10 cm below the tumor would lead to unnecessary mesorectal excision and a low extraperitoneal anastomosis, thereby increasing complications rate.
Regarding lymph node yield, the mean number of lymph nodes excised was 31.9 (IQR 20-40), with more after right colectomies (39.6, IQR 29.2-44) than left colectomies (31.4, IQR 24-38) for hemicolectomies, and 26.1 (IQR 17-31) for segmental colectomies. Regardless of the procedure, the recommended number of 12 lymph nodes was exceeded. The number of positive lymph nodes was 0.92 on average, with most found after right colectomies (2.4) and a mean lymph node ratio (LNR) of 0.003 (Table 2).

Table 2.
Pathological outcomes after colon resections. All values for tumor size, specimen length, proximal and distal margin are measured in centimeters. *LNR = Lymph Node Ratio.
On follow-up, we found no local recurrences in the studied group after a mean follow-up period of two years and three months (min of 4 months and 17 days, max of three years and 10 months). With regard to the overall survival rate in the studied period, we observed 86.6% rate at 4 years for stage I, 85.7% for stage II and 70% for stage III. For stage IV patients, the overall survival rate was 66.6% after 2 years, thereafter all patients deceased accounting for 0% survival rate at 4 years.
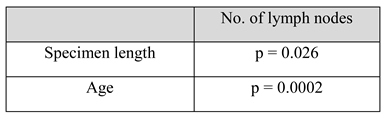
On assessing the lymph node retrieval during the studied timeframe, the results show a gradual increase in the number of nodes excised after right colectomies, reaching a peak of 45.6 in 2016. For left colic tumors, regardless of the operation, the number of nodes increased until 2015 (37.3). In 2016 there was a significant decrease to 25.5 nodes (Figure 4). Multivariate analysis of the studied variables (age, stage, tumor size, specimen length, lymph nodes, positive lymph nodes) showed a statistically significant correlation (p < 0.05) between the number of lymph nodes retrieved and age and specimen length. For patients over 65 years, the mean number of nodes was 35.9, while for patients under 65 the mean number of retrieved nodes was 29.5 (Table 3). Regarding tumor stage and its correlation with lymph node yield and specimen length, even though a statistically significant relationship was not found, more lymph nodes and longer specimens were found in stage II and III patients, where radicality is maximal (Figure 5).
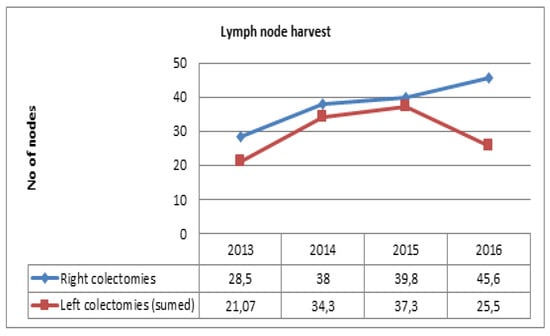
Figure 4.
Trend of lymph node yield over the studied four years.

Table 3.
Multivariate regression model shows p<0.05 when number of lymph nodes is compared to specimen length and patient age.
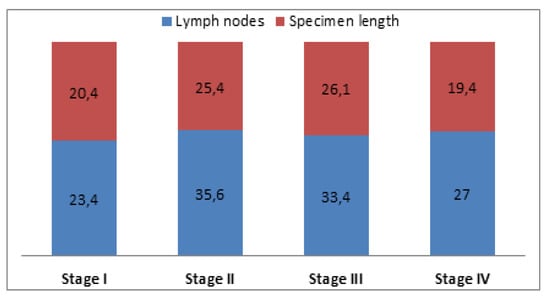
Figure 5.
Correlation between tumor stage with lymph nodes and specimen length. For specimen length values are in centimeters.
Discussion
In all cases we used an open approach with lateral to medial dissection. During the lateral dissection, care was taken to respect the embryologic planes between the mesocolon and Gerota’s fascia. In right colectomies, the ICA was ligated at the point where it crosses the SMV, opposed to the CME principles proposed by Hohenberger et al. [14] who indicated that the ICA and right colic artery should be ligated on the ventral aspect of the dissected SMA. In more than 60% of cases, the ICA crosses the IMV posteriorly making dissection more difficult [18]. Although Central Vascular Ligation (CVL) medial to the SMV would lead to extra length for the CVL distance, the oncological benefit is uncertain and dissection on the SMA’s versants can lead to injuries of splanchnic nerves and secondary severe diarrhea [18]; thus, we adhered to the presented protocol.
For the same reasons, the IMA was ligated at 1cm from its origin in left colon cancers. The quality of dissection and the integrity of the mesocolon is supported by the 0% local recurrence rate in the studied group. In our opinion a major risk factor for local recurrence is the violation of the mesocolic fascia through which tumor cells can spread and generate implants in the peritoneal cavity that may be responsible for local recurrence.
We believe that this is the most important technical factor, as CVL will only yield a plus of 2-3 lymph nodes at maximum with clinical relevance only in advanced stages. For these reasons we compared our data with Hohenberger, based on the view that a less aggressive operation ensures 0% local recurrences.
All the cases in this study were operated electively so maintaining correct dissection planes is straightforward compared with emergency procedures in patients with obstructive neoplasms where the risk of penetrating the mesocolic fascia is increased due to dilated bowels and disrupted anatomy [22].
Besides CVL, an important factor for high-quality colon surgery is the length of the specimen, longitudinal margins, and area of mesentery excised. A flaw of the study herein is the fact that specimens were shrunk by fixation when measured, thus comparisons with the literature are unreliable as most studies measured fresh specimens.
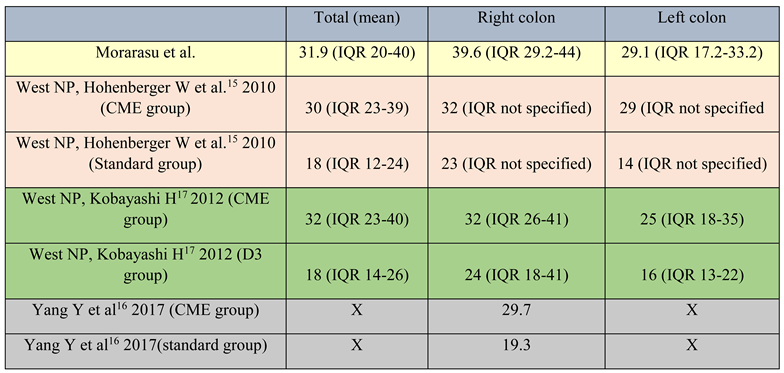
Even so, Yang Y, Wang J, Jin L et al. [16] reported a mean specimen length after right colectomies at CME standards of 28.6 cm (unfixed specimen), whereas in our study the mean length of the fixed specimen was 29.2 cm with an IQR of 22.8-34.2. The quality of specimens is not directly correlated to the specimen length. The length itself does not matter so much, but it is an indirect factor for the area of mesocolon excised. In this way, the length may reflect the quality of the final specimen (Table 4).

Table 4.
Lymph node yield literature comparison. Yang Y et al16 compared only right colon resections (CME vs standard specimens) hence the “X” marking for mean and left colon resections.
Lymphatic and vascular metastatic processes start early in the natural tumor evolution, and tumor cells disseminate via the lymphatic channels in close proximity to the supplying arteries. Besides the upstream spread following main arteries, tumor cells also disseminate longitudinally via the pericolic nodes which follow arc of Riolan. Studies showed that longitudinal spread of more than 10 cm is extremely rare and this limit should be sufficient for a curative procedure [18]. In our study the 10 cm limit is achieved in most cases besides sigmoid tumors for which the procedure was a segmental colectomy with shorter specimen length and proximal and distal margins of 9.2 cm and 7.1 cm respectively. Thus, the anastomosis remains intraperitoneally and without tension, although the IMA was ligated at its origin to ensure proper lymph node retrieval.
When compared with other studies, the mean lymph node count (31.9, IQR 20-40) is comparable to CME and D3 lymphadenectomy reports and higher than standard specimens, especially when split into right and left colon resections. The area of mesentery was not studied herein. However, given the number of nodes retrieved and specimen length with correct margins, an adequate segment of the mesentery was presumably excised in order to obtain these values (Table 4).
The number of lymph nodes increased over the given years, especially in right colectomies where the value of CME is most necessary. In 2016 there was a significant decrease, probably due to the increase in segmental colectomies over left hemicolectomies, where even though a central lymphadenectomy is performed, the area of mesentery is reduced (and number of nodes) to preserve more colon for proper anastomosis. The increase could be explained by several factors including better surgical techniques or improved pathological assessment, considering that the group of pathologists who worked on these cases are structured as a group dedicated to oncological pathology and allow a major part of their effort for correct sampling of the specimen (Figure 4). In the multivariate analysis, we found that the number of lymph nodes is statistically correlated (p<0.05) with specimen length and age (Figure 5). As expected a longer specimen with its surrounding mesentery will contain more nodes. Regarding age, the correlation could be explained by the physiological involution of lymph nodes or by the fact that in older patients, comorbidities may limit the extent of surgery.
Our results show the mean number of retrieved lymph nodes exceeded the standard of 12. However, from our experience, judging the surgical quality based on one value is highly stressful and prone to bias. Excising 12 lymph nodes may be critical to a correct diagnosis but it is not necessarily a marker of high- quality surgery. Such operative stressors should be more flexible and assessed with consideration for human factors and pathological biases.
Conclusions
This retrospective analysis shows quality figures after colon resections, comparable to the standard literature. More self-evaluations of clinico-pathological outcomes will show a more accurate picture of surgical quality and will ease the path to establishing operative gold-standards in colon cancer. A single value of 12 lymph nodes should not be viewed as a benchmark for quality surgery. A more flexible algorithm should be developed for assessment of surgical performance, a fact that should have impact not only on the patient evolution but also on the surgeon stress and workplace stability [23,24].
References
- Ong, M.L.H.; Schofield, J.B. Assessment of lymph node involvement in colorectal cancer. World J Gastrointest Surg. 2016, 8, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L. Lymph Node Counts and Survival Rates After Resection for Colon and Rectal Cancer. Gastrointest Cancer Res. 2009, 3 (Suppl. 1), S33–S55. [Google Scholar] [PubMed]
- Dimofte, G.; Târcoveanu, E.; Taraşi, M.; Panait, C.; Lozneanu, G.; Nicolescu, S.; Porumb, V.; Grigoraş, O. Mean number of lymph nodes in colonic cancer specimen: possible quality control index for surgical performance. Chirurgia (Bucur). 2011, 106, 759–764. [Google Scholar] [PubMed]
- Kotake, K.; Honjo, S.; Sugihara, K.; Hashiguchi, Y.; Kato, T.; Kodaira, S.; Muto, T.; Koyama, Y. Number of lymph nodes retrieved is an important determinant of survival of patients with stage II and stage III colorectal cancer. Jpn J Clin Oncol. 2012, 42, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Stracci, F.; Bianconi, F.; Leite, S.; Liso, A.; La Rosa, F.; Lancellotta, V.; van de Velde, C.J.; Aristei, C. Linking surgical specimen and examined lymph nodes in colorectal patients. Eur J Surg Oncol. 2016, 42, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Lavy, R.; Hershkovitz, Y.; Muhamad, A.; Sandbank, J.; Halevy, A. Re-examining Distal Resections in Colon Cancer. Isr Med Assoc J. 2017, 19, 696–699. [Google Scholar] [PubMed]
- Hardt, J.; Buhr, H.J.; Klinger, C.; Benz, S.; Ludwig, K.; Kalff, J.; Post, S. Quality indicators for colon cancer surgery: Evidence-based development of a set of indicators for the outcome quality. Chirurg. 2018, 89, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Betge, J.; Harbaum, L.; Pollheimer, M.J.; Lindtner, R.A.; Kornprat, P.; Ebert, M.P.; Langner, C. Lymph node retrieval in colorectal cancer: determining factors and prognostic significance. Int J Colorectal Dis. 2017, 32, 991–998. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.R.; Renehan, A.G.; O’Dwyer, S.T.; Haboubi, N.Y. Lymph node harvest in colon and rectal cancer: Current Considerations. World J Gastrointest Surg. 2012, 4, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Cisz, K.C.; Moreira, A.Z.; Fialho, L.O.; Aguero, H.J.V.; Paiva, D.D.; Oliveira, A.V.; Paulo, F.L. Lymph nodes identification after colorectal cancer resections. Arq Bras Cir Dig. 2011, 24, 103–106. [Google Scholar] [CrossRef]
- Dedavid e Silva, T.L.; Damin, D.C. Lymph node ratio predicts tumor recurrence in stage III colon cancer. Rev Col Bras Cir. 2013, 40, 463–470. [Google Scholar] [PubMed]
- Heald, R.J. The ‘Holy Plane’ of rectal surgery. J R Soc Med. 1988, 81, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Heald, R.J.; Moran, B.J.; Ryall, R.D.; Sexton, R.; MacFarlane, J.K. Rectal Cancer. The basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998, 133, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation – tehnical notes and outcome. Colorectal Dis. 2009, 11, 354–364. [Google Scholar] [CrossRef] [PubMed]
- West, N.P.; Hohenberger, W.; Weber, K.; Perrakis, A.; Finan, P.J.; Quirke, P. Complete Mesocolic Excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 2010, 28, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, J.; Lin, L.; Li, J.; Chen, G.; Wang, K.; Zhao, X.; Guocong, W.; Zhang, Z. Surgical and pathological outcomes of complete mesocolic excision compared with conventional surgery in right colon cancers. Int J Clin Exp Med. 2017, 10, 11616–11625. [Google Scholar]
- West, N.P.; Kobayashi, H.; Takahashi, K.; Perrakis, A.; Weber, K.; Hohenberger, W.; Sugihara, K.; Quirke, P. Understanding optimal colon cancer surgery: comparison of japanese D3 resection and european complete mesocolic excision with central vascular ligation. J Clin Oncol. 2012, 30, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Sondenaa, K.; Quirke, P.; Hohenberger, W.; Sugihara, K.; Kobayashi, H.; Kessler, H.; Brown, G.; Tudyka, V.; D’Hoore, A.; Kennedy, R.H.; West, N.P.; Kim, S.H.; Heald, R.; Storli, K.E.; Nesbakken, A.; Moran, B. The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery. Int J Colorectal Dis. 2014, 29, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Culligan, K.; Remzi, F.H.; Soop, M.; Coffey, J.C. Review of nomenclature in colonic surgery – proposal of a standardized nomenclature based on mesocolic anatomy. Surgeon. 2013, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.K.; Kim, Y.W.; Han, Y.D.; Cho, M.S.; Hur, H.; Min, B.S.; Lee, K.Y. Complete mesocolic excision and central vascular ligation for colon cancer: principle, anatomy surgical technique and outcomes. Surg Oncol. 2016, 25, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, N.; Griniatsos, J. Complete mesocolic excision: techniques and outcomes. World J Gastrointest Oncol. 2015, 7, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Iancu, C.; Osian, G.; Mocan, L.; Mocan, T.; Zaharie, F.; Todea-Iancu, D.; Bala, O.; Bodea, R.; Al-Hajjar, N.; Pop, F.; Puia, I.C.; Graur, F.; Muteanu, D.; Vlad, L. Management of colorectal resections for treatment of neoplastic intestinal occlusions. Experience of surgery clinic No III, Cluj-Napoca. Chirurgia (Bucur). 2008, 103, 45–51. [Google Scholar] [PubMed]
- Păunică, M.; Pitulice, I.C.; Ștefănescu, A. International migration from public health systems. Case of Romania. Amfiteatru Economic. 2017, 19, 742–756. [Google Scholar]
- Aslan, D.; Bordea, A.; Burcoș, T. Anastomotic leakage after sphincter-sparing surgery in a young woman diagnosed with low rectal cancer – case report. J Clin Invest Surg. 2017, 2, 45–53. [Google Scholar] [CrossRef]
© 2018 by the author. 2018 Stefan Morarasu, Tudor Frunza, Karina Bilavschi, Ana Maria Patrascu, Sorinel Lunca, Gabriel Dimofte