Introduction
The first authors that coined the term GIST were Mazur and Clarke in 1983, referring to distinct mesenchymal tumors that lack features characteristic of smooth muscles differentiation [
1]. In 1998, Kindblom et al. demonstrated that the actual origin’s cell of these tumors is a pluripotent mesenchymal stem cell programmed to differentiate into interstitial cells of Cajal, the gastrointestinal tract “pacemaker cells”—the cells responsible for initiating and coordinating gastrointestinal motility [
2]. The most important development that made GIST a unique clinical entity was the discovery of c-kit proto-oncogene in these tumors by Hirota and colleagues in 1998 [
3]. C-KIT has been demonstrated as a very specific and sensitive marker, with about 95% of GISTs express c-KIT.
Usually, GISTs appear in middle age, affecting males and females equally. They rarely occur in children or young adults, but when they do, an association with neurofibromatosis and Carney’s triad (gastric stromal tumor, extra adrenal paraganglioma and pulmonary chordoma) has been noted [
4].
A GIST can be located anywhere in the gastrointestinal tract. Small intestinal tumors occur in 20–30% of digestive tract tumors; duodenal tumors occur less than 5%. Tumors occurring in the angle of Treitz are even rarer, with most published studies being case reports [
5]. Most such cases occur sporadically, but some may occur in the context of a familial syndrome (Peutz-Jeghers syndrome or Crohn’s disease) [
6]. Patients with duodenal GIST have the symptoms such as abdominal pain, iron-deficiency anemia, gastrointestinal bleeding, or vomiting caused by an obstruction. A tumor of the angle of Treitz may also be an incidental finding during clinical examination.
Limited surgical resection or pancreatico-duodenectomy is the treatment of choice [
7]. Most tumors located at the angle of Treitz are gastrointestinal stromal tumors (GISTs) and only a few are adenocarcinomas.
From our broad experience with tumors located at the level of Treitz angle (10 cases reported so far), we selected this one for detailed analysis as it manifested as a GIST during immunochemistry exams. Although the surgical approach was optimal and the general outcome positive, this type of tumor poses challenges for the surgeon and raises several interesting points for discussion.
Material and Methods
This paper presents the case of a female patient, G.S., 54 years old, who was admitted to our clinic, Witting Clinical Hospital, General Surgery Department, for persistent abdominal pain in the epigastrium and left hypochondrium associated with asthenia, pallor of skin, weight loss of about 5 kg; symptoms occurred within the past 6 months. These symptoms were accompanied in the last 16 h by nausea and vomiting. The patient’s history was negative for alcohol, tobacco, or illicit drug use and her family history was unremarkable. The patient had no surgical history and was under treatment for hypertension (maximum value 195/110 mmHg) with Bisoprololum 10 mg (1 tablet daily), Quinalaprilum 10 mg (2 tablets daily), and Acetylsalicylic Acidum 75 mg (1 tablet daily).
On physical examination the patient was found under weight, pale and with tachycardia (pulse rate—110/min, blood pressure—130/85 mmHg; the belly was mobile with respiratory movements, painful spontaneous and on palpation in epigastrium, without palpable mass; auscultation revealed normal bowel sounds and the bowel was preserved. Rectal exam was normal.
Blood tests revealed a hemorrhagic anemia (Hgb = 8,2 gm/dl, Hct = 29,9% and MCV, MCH, MCHC in low levels), iron deficiency (serum iron = 36 mcg/dl), positive fecal occult blood test, slightly altered kidney function (BUN = 60 mg/dl, Creatinine = 1,34 mg/dl); other blood tests were within normal limits, including the levels of tumor markers (CEA, CA 19–9, α-fetoprotein).
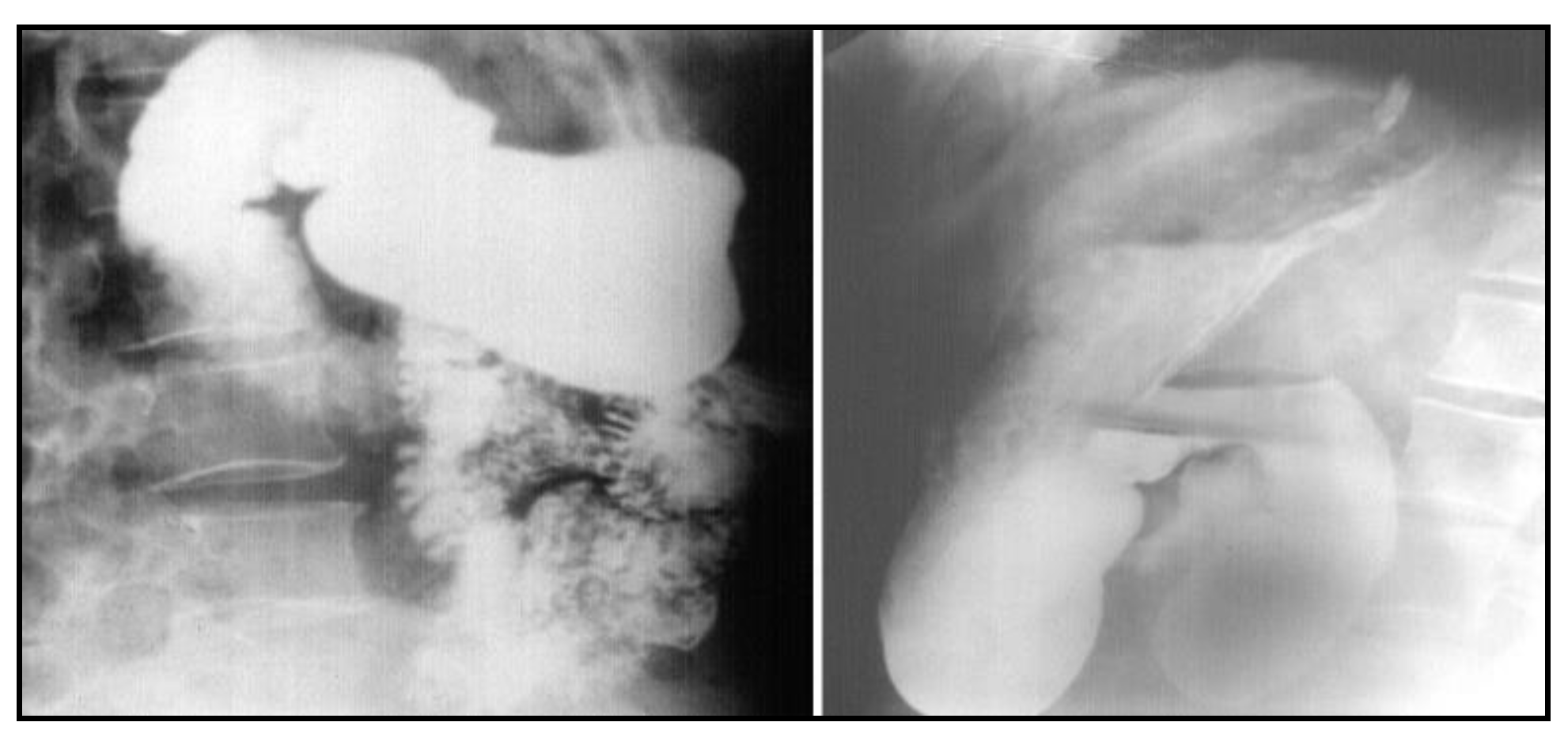
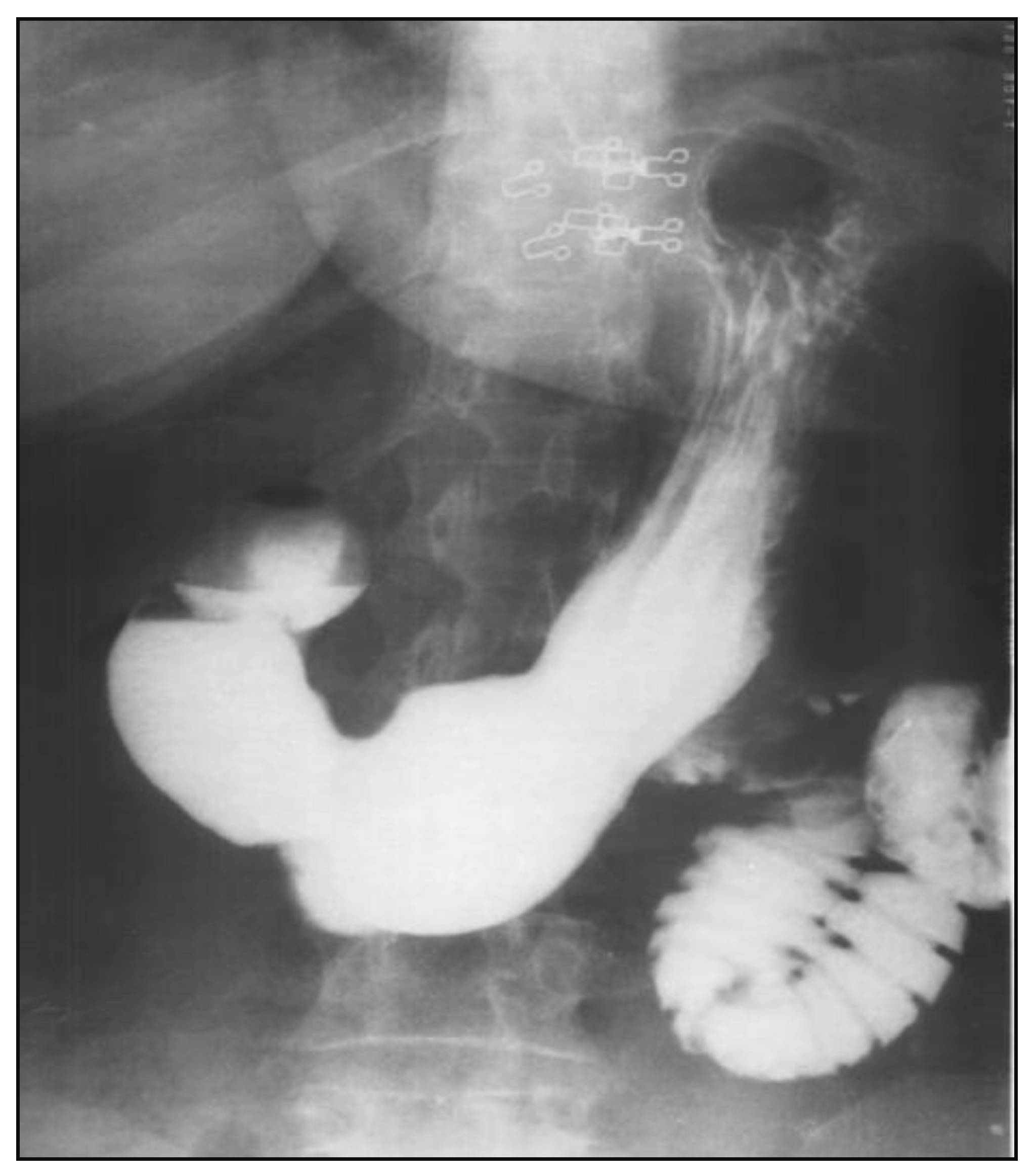
After nasogastric tube positioning, the upper endoscopy revealed dilated hypotonic stomach and duodenum with lesions of erosive gastritis and duodenitis. Using barium study of the upper gastrointestinal tract, we found a filling defect of the fourth portion of the duodenum, with dilated stomach and duodenum proximal to the site of obstruction; we also found gastroesophageal reflux without radiological signs of hiatal hernia (
Figure 1). Ultrasonography and CT scan showed no metastases.
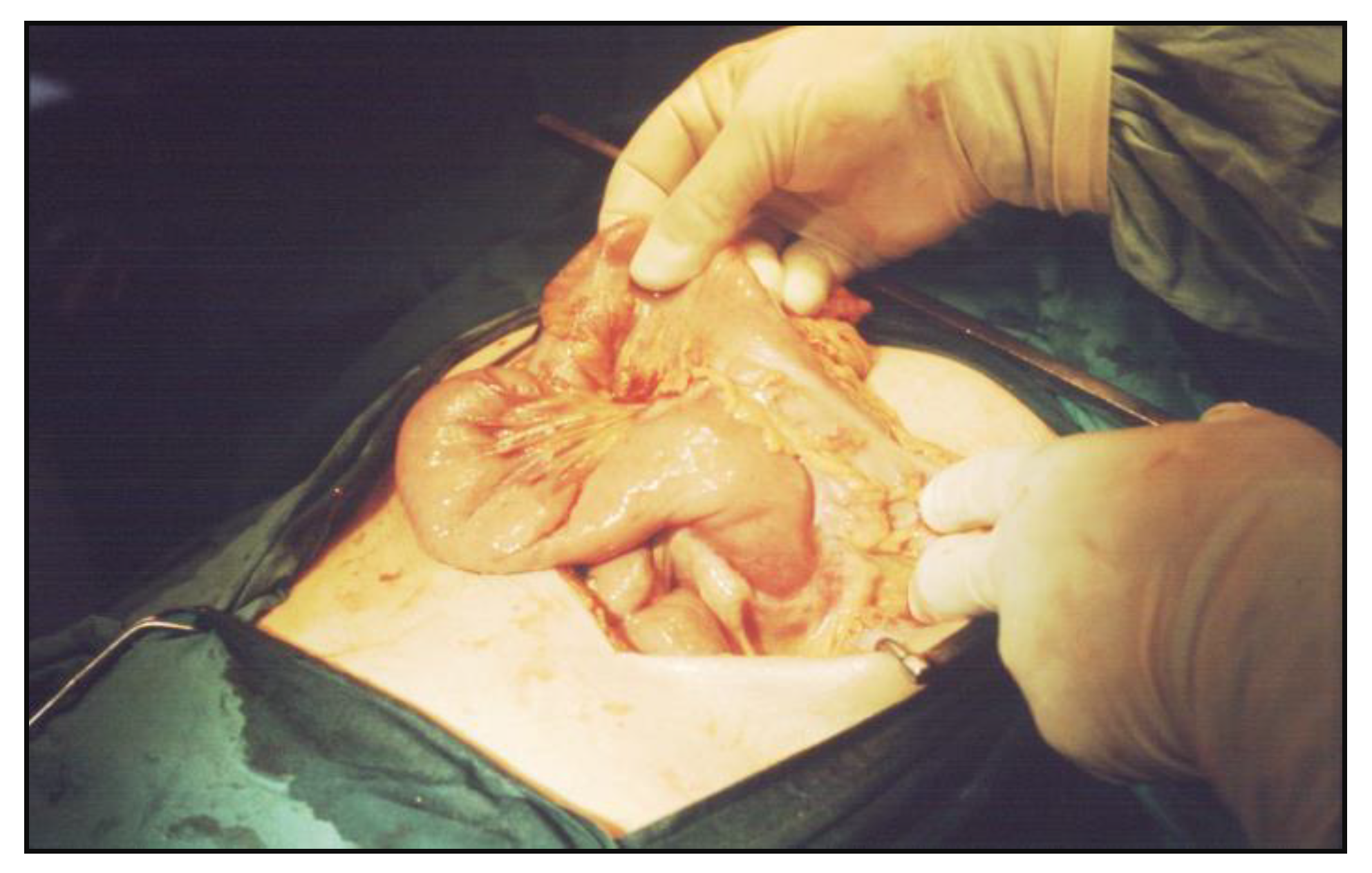
Surgery was recommended after correcting the anemia and electrolyte balance. A midline laparotomy was chosen, revealing a tumor of about 2 cm in diameter, with a circumscribed mass shape was found at the fourth part of the duodenum, developed mostly in the lumen, with extensive mesenteric fibrosis in the near of the Treitz’ angle and important distension of the stomach and duodenum. No macroscopic involvement of regional lymph nodes or liver and no direct invasion of the pancreas had occurred (
Figure 2).
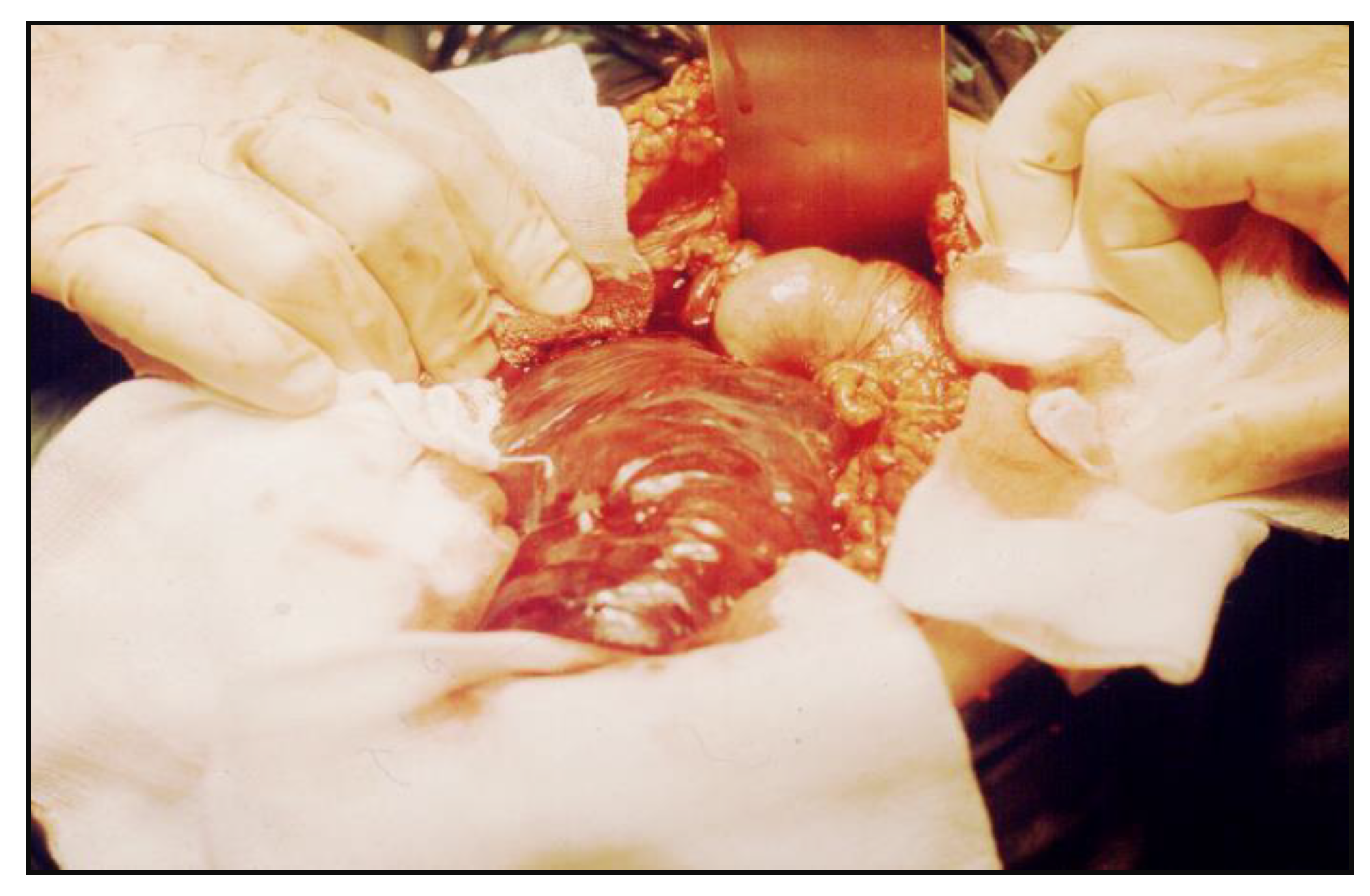
The tumor was resected with 5 cm of uninvolved duodenal margins, and an end-to-side
anastomosis was performed between the descending duodenum and the proximal jejunum (
Figure 3).
Access to the third and fourth part of the duodenum was gained by complete mobilization of the Treitz’s angle from the left side of the proximal jejunum to the right side of the angle, by cutting the ligament of Treitz.
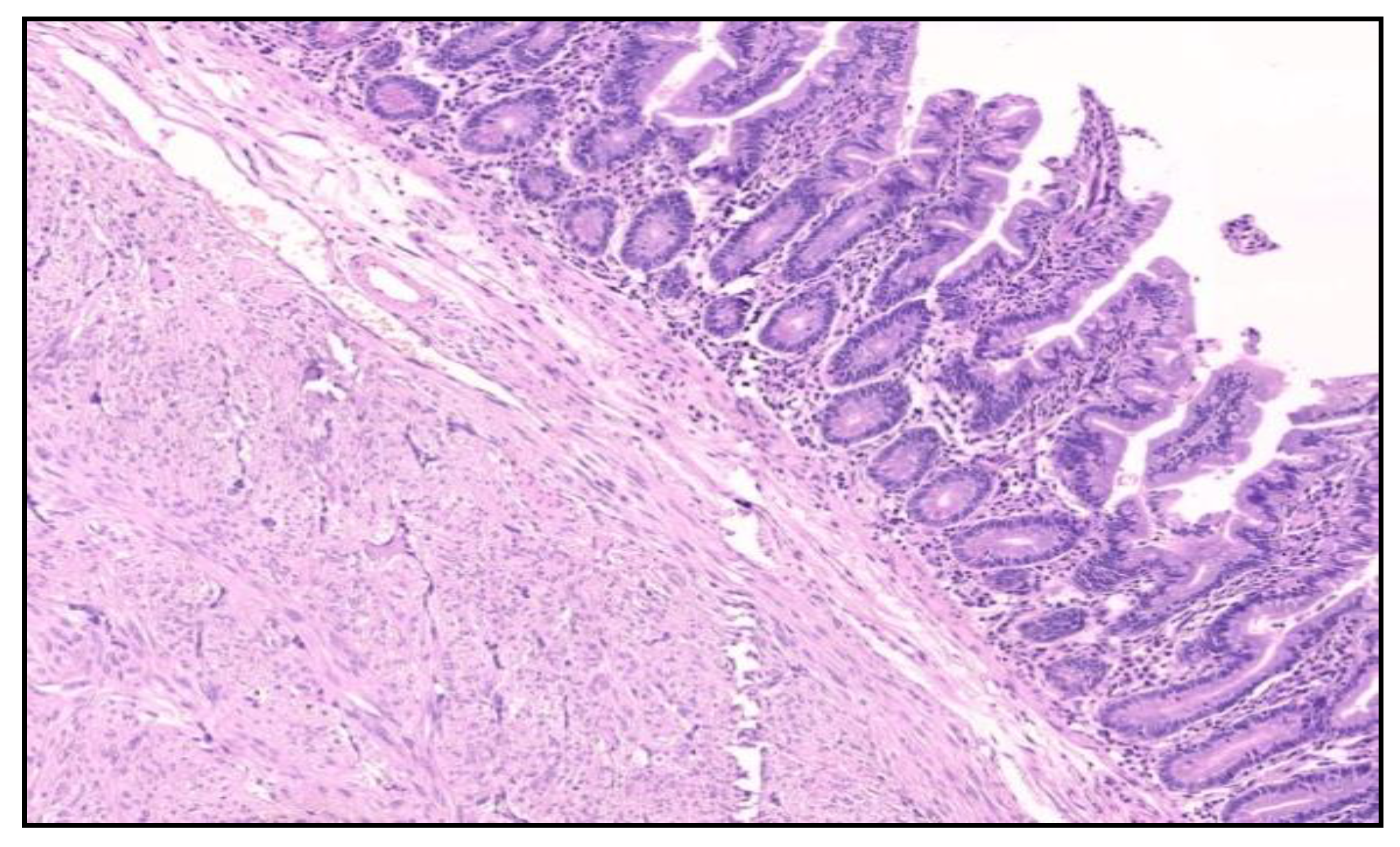
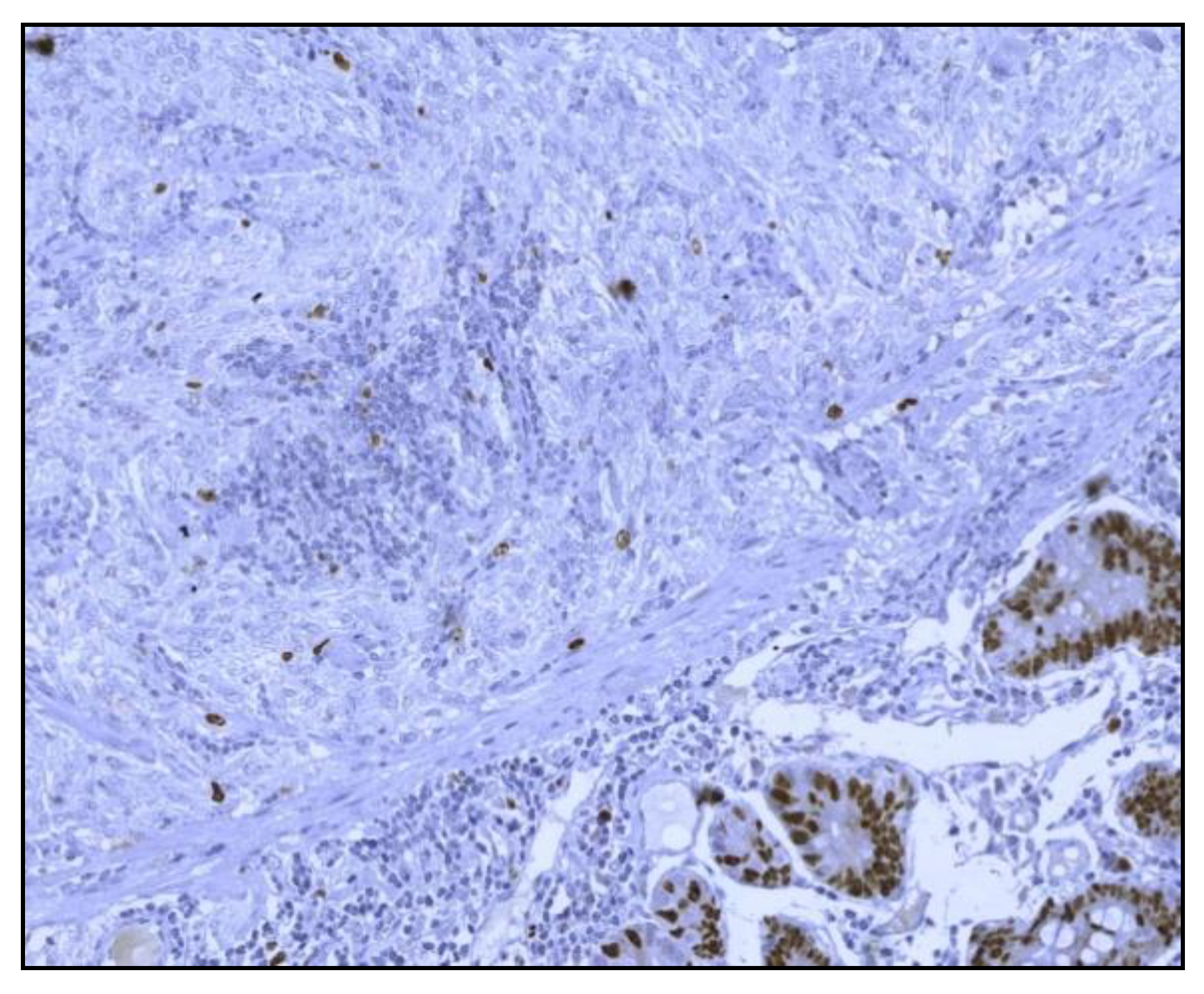
The histological examination of the specimen demonstrated an intramural small tumor −2 cm in diameter with intraluminal growth and obstruction. The final diagnosis was a high-grade ulcerated GIST with classic spindle cells, clear resection margins and no lymph node invasion (
Figure 4). Mitotic activity was 12/50 on HPF (High-Power Fields). Ki67 proliferation marker labels 10% of the nuclei (
Figure 5).
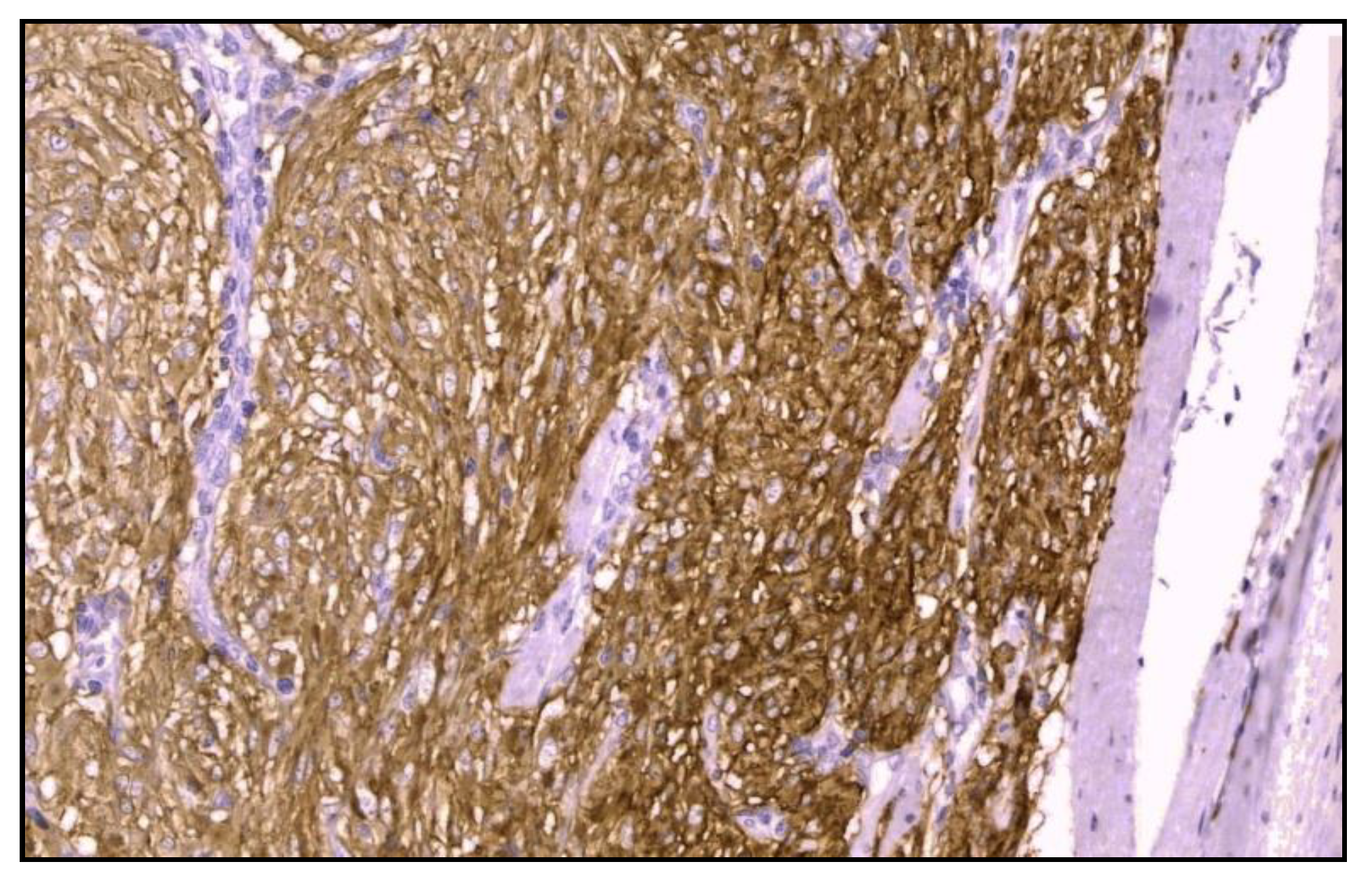
Immunohistochemistry was positive for CD117/c-kit and CD34 immunomarkers (
Figure 6) and negative for desmin, vimentin, muscle-specificactin, smooth muscle actin, S-100protein and neuron-specific enolase.
The patient’s postoperative course passed without any problems. An oncologist colleague started adjuvant treatment with inhibitors of tyrosine kinase receptors—IMATINIBMESYLATE 400mg once daily for 2 years.
The patient is disease-free after a 1.5 year follow-up. A recent barium gastrointestinal follow up study demonstrated a widely patent anastomosis with a normal mucosal pattern between the proximal duodenum and jejunum (
Figure 7) and ultrasoudsonography and CT scan do not highlight pathological lesions.
Discussion
The maximum incidence places GIST at gastric level (50–70%) and small intestine (20–30%), being followed by colon and rectal locations (10%), epi-ploon and mesenteric areas, esophagus (5%) and retroperitoneal space (5%). As already pointed out, duodenal tumors occur less than 5%, and tumors occurring in the angle of Treitz are even rarer [
5].
The epidemiology of GISTs cannot be completely estimated. The average age of patients with GISTs is 53 years and only 5% are under the age of 30. Duodenal GISTs commonly involve the second part of the duodenum followed by the third, fourth, and first part [
8].
Duodenal GISTs are relatively small in size, with the median reported to be 4 cm in contrast to the median of gastric and small intestinal GISTs of 6 to 7 cm, respectively. Duodenal GISTs that are detected early when they are smaller are amenable to relatively minor resection or excisional surgery. They may also have a favorable prognosis in comparison to GISTs from other sites of the gastrointestinal tract [
8].
Patients with duodenal GIST have the symptoms that may include abdominal pain, iron-deficiency anemia, gastrointestinal bleeding, obstructive jaundice, palpable mass or vomiting caused by an obstruction. In our case, a high bowel obstruction was caused by the tumor with location in the Treitz’s angle, but we note a previous six month evolution of gastrointestinal bleeding and iron-deficiency anemia. A tumor of the angle of Treitz may also be an incidental finding.
A large percentage of duodenal GISTs are localized in the third and fourth part of the duodenum and may not be found through standard upper endoscopy. Intraluminal growth is visualized on barium radiography as a distinct, smooth filling defect; ulceration or depression at the peak of the protrusion can be observed. Endoscopic ultrasound is helpful when the tumors are submucosal or arise from an intramural or extramural structure [
9,
10]. CT scan and MRI are the optimal imaging tests for description of the primary lesion, the local extension, and metastasis.
Surgical treatment represents one of the most efficient approaches to cure a GIST. The possibility of a real, life-saving resection gives these types of tumors a positive overall prognosis. This trend for a positive outcome remains even when there is local spread to adjacent organs if a true radical and complex excision is performed during the initial surgery. However, this is not the case for a GIST that has already become metastatic; a different clinical outcome should be expected, with a very low survival rate.
The tumor relapse rate or the possibility of a metastatic behavior occurs in about 50% of cases, even if radical excision of the primary tumor is performed; the responsible factors include: tumor fracture during intraoperative manipulation or performing a palliative resection or even a sub-standard so-called extended resection, with remnant margins positive for tumoral traces.
Primary tumor resection allows for a survival rate of about one year from the main surgery, and the main sites for tumoral relapse are the liver, the great omentum and the peritoneal surface. The basic rules of classic oncologic surgery should be applied with the utmost attention, such as tumoral-free remnant margins, a monolith-like dissection that takes into the same block the primary tumor and its local spread to the nearby organs, avoidance of the peritoneal pollution with tumor debris, and minimizing the lymphatic resection, since these tumors do not take the lymphatic route for a wide metastatic spread.
All GISTs are considered as malignant, even the intramural with a diameter of less than 2 cm, and therefore should be resected; in this regard, a laparoscopic approach is allowed.
The evolution of GIST tumors has been estimated, based on studies considering a combination of clinical and anatomo-pathological factors. Strong evidence demonstrates these tumors are influenced by their location within the digestive tract. Thus, the tumors located at the level of the small bowel tend to develop a malignant action more frequently than gastric tumors, and tumors developing at the level of the colon or rectum tend to display a more metastatic behavior. Some studies, based on a large scale clinical research, point out some markers for a severe prognosis: aneuploidy (proven by the means of flux- cytometry): size of the tumor, coagulation based necrosis, a low score for Ki67 marker and peritoneal spreading during the initial surgery [
11,
12].
At the time of their first diagnosis, almost 25–30% of all GISTs are malignant as the feature secondary metastasis (most often at the level of liver) or are spread locally. As malignant evolution is unpredictable and benign development is less likely, the rest of the tumors have been approached with different levels of medical aggression.
In surgical practice, when we discuss the risk of aggressivity, we take into consideration both the size of the tumor and its mitotic index. Therefore we now recognize GISTs that are rated as very low, low, intermediate, or high risk of aggression, based on their potential of developing metastases and relapse [
13].
Benign characteristics of these tumors are: a size less than 5 cm of the tumor, a low mitotic index of less than 5 mitoses per 20 High Power Field, Nikon technology, a 40x optical magnification, a 10x objective. The mitotic index is interpreted in close relationship with the cellular type, nuclear modifications, and cellularity.
Targeted therapy with inhibitors of tyrosine kinase receptors (IMATINIB) has been introduced for the management of advanced and metastatic tumors. Primary resistance of these tumors to the Imatinib™ is noted at about 5%, and secondary resistance develops due to some second-stage mutations of the tirozin-kinazic receptor [
14,
15,
16]; a possible way to work around this problem is to increase the 24 h dosage or the surgical intervention to remove the remnant tumor. Some authors cite partial clinical resistance with a remission of a tumor clone while other tumors yet develop.
Sometimes simple resection with a free margin of about 2 cm provides the edge necessary to defeat the malignant evolution of a case and provide full recovery with no relapse at the 5-year mark.
Conclusions
Based on our experience, the reasons behind the rarity of gastrointestinal stromal tumors could reside in one or all the following: the high speed of the digestive transit, the alkaline pH of the small bowel, the reduced bacterial population diversity and concentration in the small bowel, less aggressive liquid content of the small bowel, high concentration of IgA at this level, with a proven local protective role and, finally, a high content of benzipren-hidroxylaze enzyme with high anticarcinogenetic potential.
Surgical resection is the primary treatment for GIST tumors and the only therapy offering a chance of cure, although there is evidence that IMATINIB-based therapy has significant potential.
GISTs located at the level of duodenum appear slow-growing and exhibit a limited propensity for both metastatic and local spread; such favorable biologic characteristics mandate an aggressive surgical approach to this disease, aimed at complete tumor excision with negative histologic margins.
The location and histology of the tumors permitted a pancreas-preserving segmental duodenectomy, a real benefit for a tumor located at the level of Treitz’s angle, the operative approach chosen allowing the patient to develop very well in the postoperative stage with a general favorable outcome in the long term.
One of the goals of this paper was to showcase an extremely rare tumor located at an extremely rare level of the small bowel that could well end up in a diagnosis trap. Also, the rarity of these cases, their evolutive particularities, survival rate, the histopathological or immunohistochemistry aspects, and the therapeutic plan are points of equal interest for this study as well.