Materials and Methods
This study was conducted on a lot of 103 consecutive esophageal cancers, admitted in the Second General Surgery Clinic, Emergency County Hospital No. 1 of Craiova, between January 2007 to December 2019. Six patients presented with advanced cancer of the cervical esophagus with a poor general condition and the preoperative assessment was very limited, therefore they were excluded from the study, which finally was conducted on a lot of 97 patients. Our clinic is not specialized in esophageal surgery, but two surgical teams present special interest in esophageal surgery and they were responsible for most of the surgical resections.
There were two distinct periods: between 2007 and 2014, when some of the imaging modalities lack from various reasons (with special reference to thoracic CT and esophageal endoscopic ultrasound). The second period started with 2014 and majority of the cases benefitted from the preoperative imaging modalities. The therapeutic team was complex and included (but not limited to) gastroenterologists, surgeons, a medical imaging team, pathologists and oncologist. The main sign that oriented the diagnostic examination was dysphagia, presented in all cases; only one patient presented a slight discomfort on deglutition, the rest of the patients presented with clear signs of esophageal obstruction. Significant weight loss (over 5 kg in less than a month) was found in 47 cases (48.45%), while anemia was present in 33 of the cases (34.02%).
All patients have given their written consent for the surgical therapy and disease related data to be published. Also, the approval of the Ethics Committee of the hospital was obtained in order to use the patient’s data and publish the study.
All patients with suggestive signs of esophageal obstruction were submitted to endoscopic examination. The endoscopy evaluated the distance from the teeth and the upper pole of the tumor, the morphologic type of the tumor, the size and the circumferential extent of the tumor; the endoscopy was completed with biopsy in all cases.
The preoperative staging protocol included (but not limited to) thoracic and abdominal CT and esophageal endoscopic ultrasound. However, the protocol was complete in only 39 cases (40.2%) (both CT and endoscopic ultrasound were performed), for the rest of the cases the preoperative staging being incomplete (only CT or endoscopic ultrasound or none of them available). In cases without CT the preoperative staging was based on plain thoracic X-ray and abdominal ultrasound.
As sequence, after the endoscopic confirmation of the esophageal cancer, 78 patients (80.4%) were submitted to abdominal and thoracic CT. If metastases were not detected, 42 patients (43.29%) were submitted to endoscopic esophageal ultrasound. Four patients were subsequently submitted to PET-CT, and in 6 cases laparoscopy was performed for pre-thoracotomy staging; thoracoscopy for preoperative evaluation wasn’t available in this study. PET-CT did not change the therapeutic strategy in our statistic, and due to the small number of cases this staging modality wasn’t included in the study. All the cases were reviewed and staged according to the 7th edition of the TNM classification of malignant tumors (there were no cTx and cMx).
After the preoperative workup, 74 (76.28%) patients were submitted to surgery with potential curative intent (thoracotomy and/or laparotomy). In the rest of the cases (23 cases – 23.71%), preoperatively considered unresectable (T4b tumors, distant metastases or poor general condition which counterindicated the thoracotomy), a laparotomy was performed with the only purpose of establishing a nutritional pathway (gastrostomy or jejunostomy), this not being considered as a failure of the preoperative staging.
During this study there were identified two different periods related to the preoperative imaging modalities availability: between 2007 and 2014, when the preoperative staging was incomplete in most of the cases, and the period started with 2014, when the imaging modalities were more largely available.
In cases admitted between 2007-2014 (51 cases) the CT was unavailable in 19 cases, while the endoscopic ultrasound was available in only 11 cases. Between 2015-2019 (46 cases) the thoracic and abdominal CT was available for all the patients while endoscopic ultrasound was performed in 31 cases.
Abdominal and thoracic CT was performed using a Siemens Somatom Emotion 16 slice tomographic equipment. Endoscopic ultrasound was performed using a linear Olympus UCT 180 (Exera II line and Aloka ultrasound) device and a linear EG-3870UTK Pentax echoendoscope (Pentax line and Hitachi ultrasound with elastography software), at the Research Gastroenterology and Hepatology Center from Craiova. The pathologic examination was performed in all cases, on the resected specimens (when it was possible) or endoscopic biopsy specimens (usually examination consisted in haematoxilin-eosin technique).
The esophageal cancer patient’s data were prospectively collected and registered in a Microsoft Excel table; however, many data related to patients have been completed subsequently, when we have started this study. The studied cases included only esophageal cancers, and Siewert type I and II esogastric junction cancers; the Siewert type III esogastric junction cancers were excluded after initial diagnostic protocol, surgery and pathologic examination.
Statistics were performed using the MedCalc Software, ver. 18.5, the data being transferred from Excel after proper adjustments. For comparison of the data, the chi square test for contingency tables was used.
The main parameters discussed were the accuracy, the specificity and sensitivity of the preoperative imaging modalities in avoiding useless thoracotomies and laparotomies, using well known formulas.
Results
During the studied period, there were 97 cases of thoracic and abdominal esophageal carcinomas addressed to our Clinic, aged between 51-89 years, average 65.44±7.23 years: 86 males (88.65%), average age 64.93±9.27 years old, and 11 females (11.34%) average age 69.45±5.86 years old.
Tumor’s topography was as follows: upper thoracic esophagus 19 cases, middle thoracic esophagus 36 cases and lower esophagus 42 cases, and the pathologic diagnosis was squamous esophageal carcinoma in 68 cases and adenocarcinoma in 29 cases. There were two distinct periods: between 2007 and 2014, when the main staging modalities were scarcely available, and between 2015-2019 when the percentage of complete preoperative staging increased significantly (chi-square test, p=0.004, contingency coefficient 0.236).
At the time of clinical diagnosis and/or after the performance of thoracotomy and/or laparotomy, 19 patients (19.58%) presented remote metastases. The metastasis topography was the liver in 11 cases, the lung and/or the pleura in 7 cases, the peritoneum in 3 cases, the supraclavicular lymph nodes in one case, the lumbar aortic ganglia in two cases, the left adrenal gland in one case and the abdominal wall in one case; multiple concomitant metastases were present in 6 cases.
Chest and abdomen computed tomography was available in 78 cases (
Table 1).
Chest and abdominal computed tomography has diagnosed the presence of metastases in 16 cases, of which 7 were false positive; diagnosing laparoscopy excluded 3 cases: two cases with peritoneal carcinomatosis (CT) suspicion and one suspected case of hepatic metastases, in reality being a hepatic hemangioma; in one case the CT scan suspicion of tomographic metastasis was excluded; in two cases the tomographic suspicion of hepatic and peritoneal metastases were excluded from the exploratory laparotomy; a case with a tomographic suspicion of ovarian metastasis, excluded from left ovariectomy, with the histological result of ovarian fibroblast. In 10 cases, computed tomography did not identify distant metastases:
- -
in one case, a 2 cm diameter tomographically unhighlighted tumor was identified and extirpated during thoracotomy for esophagectomy; the histopathological result confirmed the diagnosis of metastasis;
- -
a case of metastasis in the adrenal gland was identified by PET-CT, not being identified tomographically;
- -
lombo-aortic adenopathies have been identified in two cases where computed tomography has not identified them;
- -
in two cases, multiple small-scale liver metastases were diagnosed during laparotomy;
- -
in one case, liver metastases, also of small size, have been identified during diagnostic laparoscopy;
- -
also, during laparotomy or diagnostic laparoscopy, two cases of peritoneal carcinomatosis unidentified tomographically, were also identified;
- -
PET-CT has identified in one case metastases in superclavicular, hepatic and parietal abdominal nodes, not identified tomographically.
The accuracy, sensitivity and specificity, positive predictive value and negative predictive value of computed tomography for remote metastatic detection in esophageal cancer are shown in the
Table 1.
Five cases (5.15% incidence) of which preoperative imaging investigations detected two synchronous malignant lesions: colorectal adenocarcinoma and renal carcinoma were identified in patients in the study group. Renal urothelial carcinoma was asymptomatic, being incidentally discovered during the abdominal ultrasound examination, followed by computed tomography. Computed tomography failed in the identification of asymptomatic colon adenocarcinoma.
Following preoperative imaging investigations and patient field assessment, 74 patients underwent a potentially curative surgery (46 in group A and 28 in group B). Tumor resection was possible in 52 cases (29 resections in group A and 23 resections in group B).
Of the 52 cases where esophageal cancer resection was possible, 43 cases had preoperative computed tomography; therefore, the statistical analysis of the computed tomography value in the T and N stage of esophageal cancer is related to these 43 cases.
The histopathological analysis of the resection pieces belonging to 43 patients who also benefited from tumor resection and preoperative computed tomography, has diagnosed 8 T2 esophageal cancers, 30 T3 tumors and 5 T4 tumors (
Table 2).
In these cases, computed tomography has staged 31 esophageal tumors such as T2/T3 and 10 tumors in the T4 category, and in two cases computed tomography did not specify the T category (
Table 2).
Thus, computed tomography correctly diagnosed 35 cases of 52, respectively correctly, as non-resectable 15 out of 26 cases; according to the staging of the T category by computer tomography, in 17 cases the tumor would not have been resected, although resection was possible, while according to the T-stage staging of computer tomography, 11 cases were thoracotomized or laparotomized (
Table 3).
Of the 38 T2 and T3 histologically tumors diagnosed in preoperative computed tomography group, 20 cases were preoperatively diagnosed as T2/T3 tumors, the 8 cases of T2 tumors not being correctly diagnosed; 8 cases were over-staged as T4; 3 cases of T4 tumors were sub-staged as T3 tumors (
Table 4).
Within T4 category, two cases were incorrectly diagnosed as T4b; in two cases the computed tomography did not specify the tumor stage. Therefore, from the 43 preoperative computed tomography cases that could be resected, the correct preoperative diagnosis of the T-category in the 7th Stage TNM staging was established in only 20 cases, resulting in a confidence level of computed tomography in the identification of baseline esophageal tumor status of only 46.51%. In the case of the T4 tumor group, only 5 cases were histologically diagnosed of the 10 cases tomographically diagnosed:
- -
two cases were correctly diagnosed as T4, but within the T4 subgroup a case was misdiagnosed as T4b, in fact, resection being possible;
- -
5 cases were over-staged tomographically, being in fact T3 tumors;
- -
3 cases were under-staged as T3, being in fact T4a tumors.
The statistical parameters characterizing the tomographic staging of T4 (a + b) in esophageal cancer, the results are presented in
Table 5.
However, considering the classification on the two categories T4a and T4b, the accuracy of computed tomography decreases with only one case correctly diagnosed (
Table 6).
On the other hand, the above statistical analysis refers strictly to cases where esophageal resection was possible. In addition, the cases outside the analyzed lot, in which computed tomography had erroneously diagnosed 6 tumors such as T3, when they were actually T4b, irremovable tumors, from the intraoperative point of view, the invasion of irremovable neighboring organs (trachea, left bronchus, aorta and/or spine) was evidently demonstrated. Therefore, the diagnostic accuracy, also extended for these cases, is even lower: out of the 11 cases diagnosed with T4, only 2 were diagnosed positively (
Table 7).
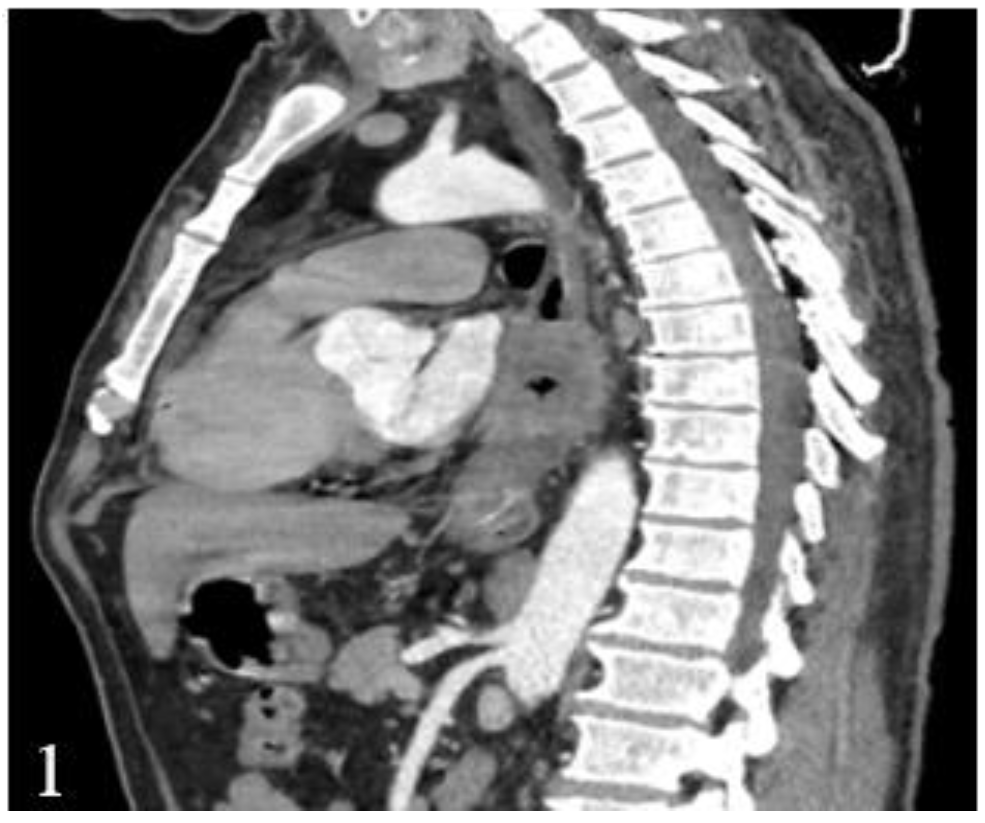
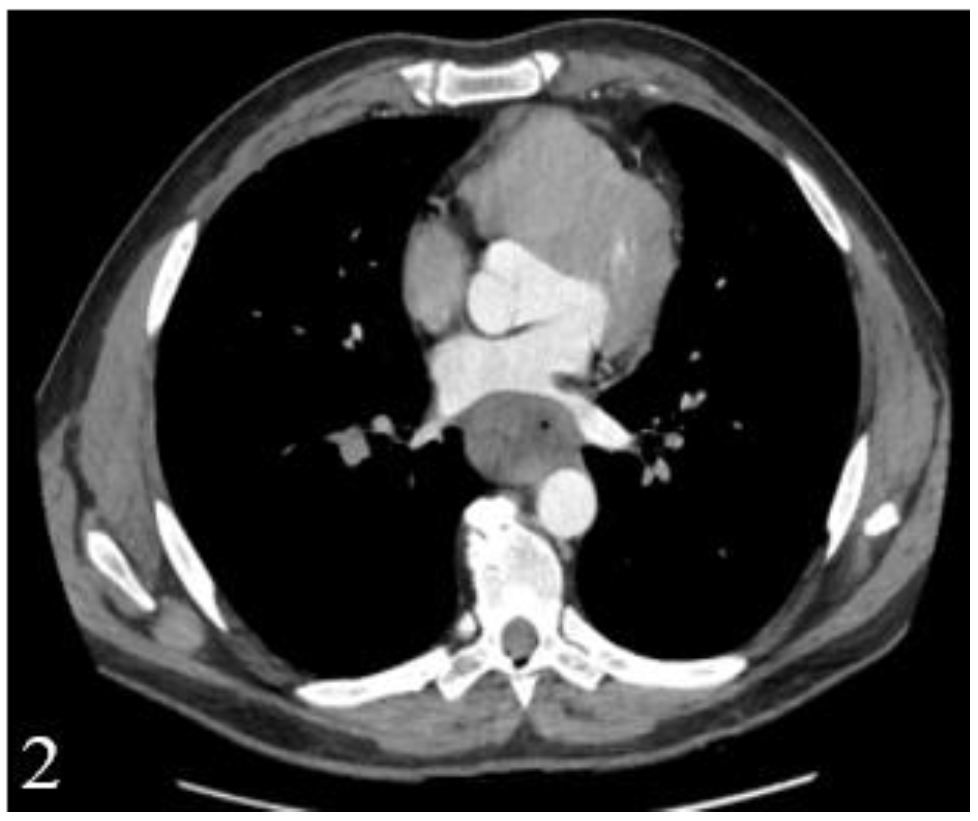
This finding has a major influence on the thoracotomy decision, which has to be taken not only based on computed tomography, due to the low diagnostic accuracy (
Figure 1 and
Figure 2).
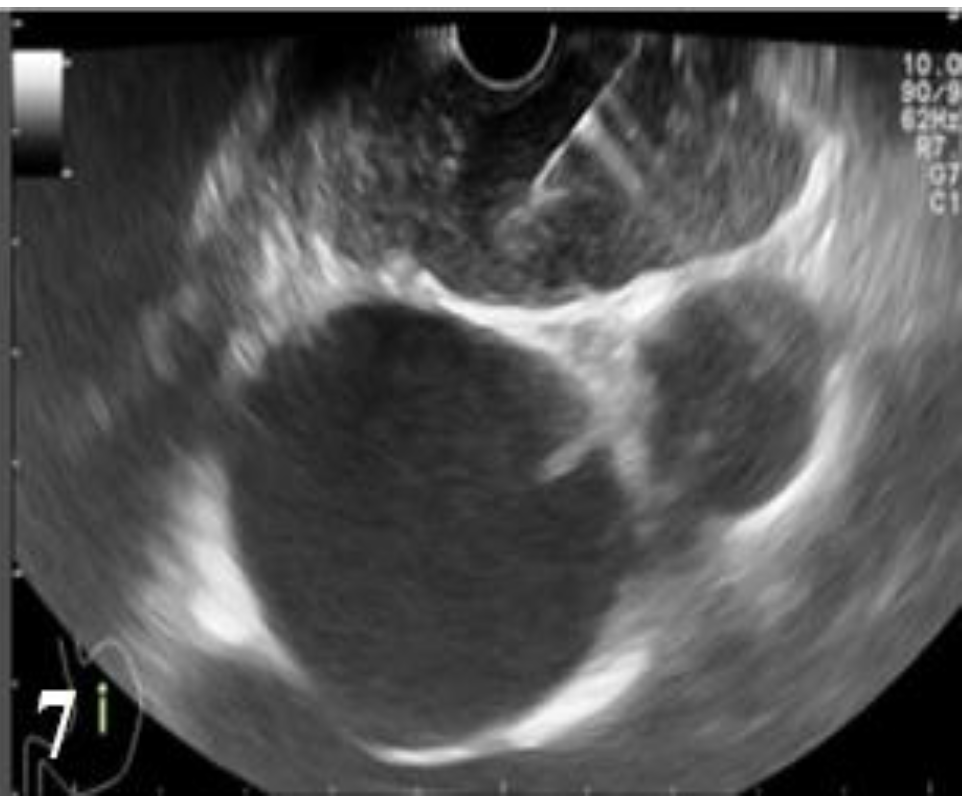
Endoscopic ultrasound had the primary role in the pre-operative setting of stage T, being used in 29 of the 52 cases where esophageal resection was possible (55.76% of resected cases). Endoscopic ultrasound staged a case as T1, 8 cases as T2, 14 cases as T3 and 6 cases as T4, while the histopathological examination staged only 6 tumors as T2, 19 cases as T3, 4 cases as T4 (
Table 8).
In order to establish the resection of esophageal cancer, it is necessary to distinguish T3/T4a tumors from T4b tumors (invasion of non-resectable structures). In the case of T3 tumors, 12 cases were correctly diagnosed by endoscopic ultrasound. In two cases, esophageal endoscopic ultrasound erroneously diagnosed T3 as T2 (one case) and T4 (one case), respectively (
Table 9).
Therefore, of 19 cases of histologically diagnosed tumors as T3, 7 cases were incorrectly diagnosed: 4 cases were under-staged as T2 (21.05%) and 3 cases over-staged as T4 (15.79%). In case of T4 tumors, endoscopic ultrasound correctly diagnosed 3 cases, while in 3 cases endoscopic ultrasound erroneously considered T3 tumors as T4. A T4 tumor case was under-staged (25%) (
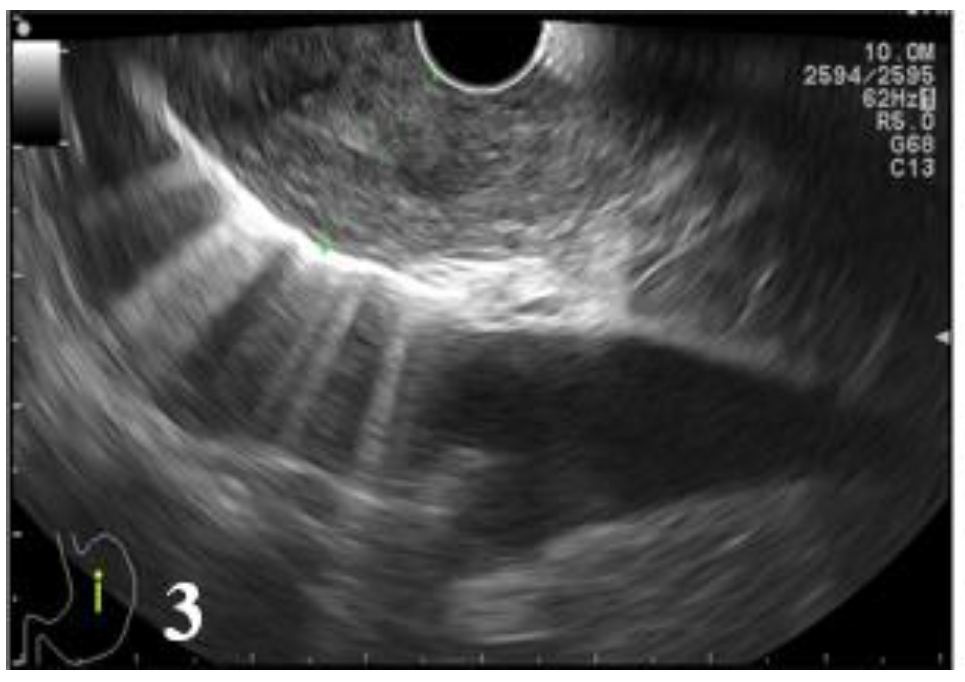
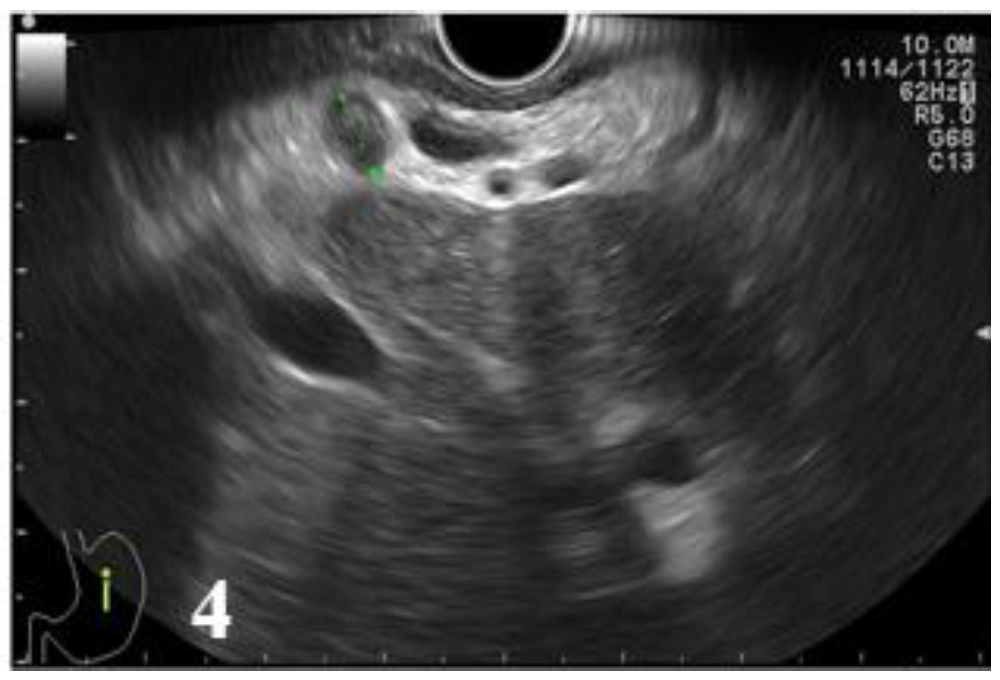
Figure 3 and
Figure 4,
Table 10).
Globally, EUS had the ability to accurately diagnose 19 of the 29 cases (65.51% accuracy); In 6 cases (20.68%) the tumors were evaluated as having a lower category than in reality (under-staged), and in 4 cases (13.79%) the endoscopic ultrasound considered that the tumoral lesions are more advanced (
Table 11).
Regarding the prediction of tumor resection in the six cases EUS diagnosed, two cases were over-staged from T3 to T4 (non-resectable), therefore 33.33% (one third) of cases would miss the optimal therapeutic sequence (surgical resection). Consequently, in spite of the small number of cases analyzed, the predictive value of esophageal tumor resection by EUS remains low (
Figure 5 and
Figure 6).
The imaging predictability of the cTNM clinical stage, in particular of the T and M class, plays an essential role in establishing the therapeutic strategy appropriate to the case, and in particular to avoid extensive but unnecessary surgical procedures (thoracotomy and/or laparotomy).
In the studied group, the imaging methods used (computed tomography and esophageal EUS) detected 23 non-resectable cases: 4 cases with remote metastases and 11 cases with major loco-regional extension, with an irremovable esophageal tumor. The other 74 cases were potentially curative, but resection was possible in only 52 cases (53.6% overall resectability). Therefore, preoperative exploration was followed by 18 (24.32%) unnecessary thoracotomy and 4 (5.4%) laparotomies overly practiced. In the 18 unnecessary intraoperative thoracotomies, the tumor was much more locally advanced than diagnosed preoperative imaging explorations and therefore non-resectable. Also, in two cases where esophageal resection was technically possible, intraoperative exploration identified pulmonary metastasis, imagistic unidentified, respectively a non-resected celiac adenopathic block, although the primary tumor was extirpated.
Of the 4 unnecessary laparotomies, in two cases were detected intraoperative metastases, not identified by computed tomography. On the other hand, in two other cases where pre-operative imaging of remote metastases was suspected, intraoperative exploration has denied their presence, and therefore resection was possible. Therefore, the value of the preoperative imaging study is presented in
Table 12.
In conclusion, when the preoperative imaging exploration was poor (the first period of the study), the number of unnecessary surgical interventions (thoracotomy or laparotomy) was 3 times higher than in the second study period, where computed tomography and EUS were available in a significantly higher number of patients: 33.33% vs 10.86% (p = 0.034, contingency coefficient 0.189). Although the number of esophageal resections did not vary significantly between the two groups of patients (p = 0.7), however, there was a significant difference between non-resected and non-operated cases between the two study periods.
There was also a statistically significant difference between the two periods of the study in terms of the number of cases not operated: 5 out of 51 cases in the first group, respectively 18 out of 46 cases in the second group - p = 0.008 contingency coefficient 0.235).
Discussions
The main purpose of the imaging investigations involved in the staged assessment of esophageal cancer is the identification of incipient cases that can benefit from endoscopic therapy (T1N0 cases) and, on the other hand, the identification of cases (local or metastatic advanced tumors) in which surgical treatment is not only useless but also very aggressive for the patient, resulting in significant postoperative morbidity and mortality. On the other hand, the detection of regional lymph node invasion, although not influencing the surgical resection decision, is essential for the indication of neoadjuvant therapy [
13,
14,
15].
In accomplishing these two major objectives, computed tomography and preoperative esophageal EUS play a different role, including the impossibility of assessing the local extension, as a rule the two of them being complementary, due to different accuracy, sensitivity and specificity. Even the complementary use of these imaging investigations does not provide absolute certainty, and there is basically no 100% accuracy, sensitivity and specificity for assessing the esophageal cancer extension and establishing resectability [
16,
17].
After establishing the positive diagnosis of esophageal cancer, the first step in patient assessment is to determine the presence of diseases, including synchronic malignancies, that contravene the potentially curative surgery, as well as detecting the presence of remote metastases. In the next diagnostic step, the logical step is to determine the loco-regional extension and to establish tumor resectability [
18].
High aggressiveness and extremely low survival of patients with distant metastases (134 ± 45 days, compared with 470 ± 84 days for patients who were diagnosed with the resection) [
19], determined that all oncological therapeutic guidelines contraindicate esophageal resection in patients with distant metastases [
20,
21,
22].
The incidence of distant metastases at the time of diagnosis in esophageal cancer varies between 18-38% [
2,
20]. In the studied group, the incidence of distant metastases at the time of diagnosis was 19.58%, lower than in the previously mentioned statistics; this is determined by the collection of data from a surgical clinic in which not all patients diagnosed with metastases arrive, the presence of metastases transforming esophageal cancer into a “non-surgical” disease. Pulmonary radiography and abdominal ultrasound have the advantage of simplicity, accessibility and reduced cost but their accuracy, sensitivity and specificity make them insufficient for the diagnosis of metastases [
23]. The two main non-invasive imaging methods currently used in the diagnosis of esophageal cancer metastases are computed tomography and positron emission tomography.
Most studies recognize
computed thoracic and abdominal tomography as an investigation of choice in the staging diagnosis of esophageal cancer, providing information on primary tumor, regional lymph nodes, and on remote malignant dissemination [
24,
25,
26]. The accuracy of computed tomography in remote metastasis detection is 74%, with a sensitivity of 14-83% and a specificity of 75-97% [
27,
28].
Although in the analyzed group the accuracy of computed tomography in the detection of distant metastases was relatively good (78.2%), the sensitivity of the method was reduced (47.36%), the tomography being incapable to detect mainly peritoneal metastases, but also liver metastases, lung, adrenal gland or lymph nodes. Also, there were many CT images mimicking distant metastases, later disproved by laparotomy or exploratory thoracotomy. The relatively small number of cases, however, influence the outcome of the study. FDG-PET-CT provides better diagnostic performance than computed tomography for distant metastasis detection with a 74% accuracy, 71% sensitivity (with variations between 43-88%) and a specificity of 93% (with variations between 89-99%) [
23,
24,
25], but it is still an imaging method that is difficult to access.
On the other hand, there are numerous publications attesting to the ability of esophageal squamous cell carcinoma associated with multiple synchronic malignancies, mainly cervical, of the head region, but also pulmonary, renal or digestive malignancies, which can occur with an incidence of up to 7% [
29,
30,
31]. Such an association raises the question of excluding metastases of one of the associated cancers in the organ with synchronous malignancy, which can be established with certainty only by the histopathological examination of the resection pieces. The essential role in the detection of primary sinus malignancies in esophageal squamous carcinoma rests with imaging investigations, many of which are asymptomatic at the time of diagnosis [
32,
33].
Computed tomography plays an important role in the diagnosis of synchronous esophageal cancer malignancies, but cannot detect small malignant lesions of the organs of the digestive tract [
34,
35,
36].
PET-CT can detect lesions that have escaped other imaging methods. With a sensitivity in the detection of primary synchronous cancers of 88.2%, compared with only 52.9% for the other imaging methods [
15]. In conclusion, it appears that PET-CT is the investigation of choice for the detection of primary synchronous malignancies of esophageal carcinoma [
31,
32,
33].
After excluding distant metastases and other curatively synchronous malignant lesions, it is important to determine the degree of local tumor invasion in the determination of resection and mainly in the differentiation of T4/T4a tumors from the T4b tumors in the patient for esophagectomy.
The accuracy of tomographic staging of the T3 category is around 74%, ranging from 59 to 82%, with a sensitivity of 67% and a specificity of 56% [
37,
38,
39,
40]. In the current study, the accuracy, sensitivity and specificity of computed tomography in T3 category diagnosis were 32.55%, 40% and 15.38%, below the values reported by the authors, directly related to the quality of the CT scan (16 slice, compared to 64 slides).
Most authors agree that regarding the role of computed tomography in establishing T stage, it is more important to define the T4 category (invasion of organs and adjacent structures), which often turns the case into inoperable ones [
39,
40]. The accuracy of tomography in the assessment of invasion of mediastinal structures (T4 category) varies from 47% to 100%, depending on the invasive structure: a sensitivity of only 6% in prediction of aortic invasion and 31% in the assessment of airway invasion in the study of Hölscher et al. [
39,
41]. In the studied group, the accuracy of the T4-grade tomography is included in the literature, being 74.41, but with a sensitivity of only 40%. However, problems occur when reference is made to cases that have not been resected and have been given an inoperative preoperative tomography, the accuracy decreasing to 61.22%, with a sensitivity of only 9.09%. Also, the accuracy of tomography decreases when differentiation between T4a and T4b stages, but due to the small number of cases, we could not make any assessment of differences in the invading organs. In predicting tumor resection, computed tomography had an accuracy of 64.1% and a sensitivity of 67.3%, with a specificity of 57.69%.
Esophageal endoscopic ultrasound is the only imaging method that can differentiate the esophageal wall tunics; it is essential for determining the resection of esophageal cancer (both for the T1 category) if the endoscopic resection is to be applied (not in our cases), but especially for the differentiation of T3-T4 tumors [
40,
41,
42,
43]. For T3 tumors, EUS was superior to computed tomography: 68.96% vs. 32.55% accuracy, 63.15% vs. 40% sensitivity and 80% vs. 15.38% specificity. The data is similar to those in the literature, which report an accuracy of 80-89%, a sensitivity of 78-90% and a specificity of 80-87% [
39]. The under-staging rate of the T3 category was 21.05%, probably due to the inability of EUS to visualize the esophageal adventitia invasion [
44], while the over-staging rate was 15.79%. The latter may have repercussions on the indication of esophagectomy, with the risk that resected tumors are EUS diagnosed as non-resectable.
In setting the T4 category, the accuracy of the method is diminished by tumor esophageal stenosis, plus the risk of under-staging for tumors that exceed the penetration limit of EUS [
40]. However, for the T4 stage in the studied group we recorded an EUS accuracy of 86.2%, with a sensitivity of 75% and a specificity of 88%, values that can however be statistically influenced by the small number of cases of such tumors examined by EUS. Similar values were published by Hölscher et al., with 88% accuracy, 80% sensitivity and 98% specificity for EUS in T4 category evaluation [
39]. In the preoperative setting of resection, EUS can correctly assess the invasion of the aorta and the airways, being superior to computed tomography [
39,
45]. It should be noted that in the analyzed group, EUS has under-staged T4 tumors as T3 in 25% of cases, and the results should be considered with caution because the studied group only includes cases of esophageal cancer that could be resected. On the other hand, the study was able to distinguish between tumors T4a and T4b, basically the value of EUS in these cases being limited to the differentiation of T4 tumors resected by T2/T3 tumors.
The accuracy of EUS in primary tumor diagnosis in esophageal cancer can be increased by the use of miniaturized probes, but the overall accuracy is 84%, with a sensitivity of 82% and a specificity of 85%, which falls within the limits published for the usual EUS [
46]. Basically, in the studied group, with regard to the possibility of predicting tumor resection, EUS (despite good accuracy, sensitivity and specificity) remains of low value due to the significant over-and under-staging rates responsible for some unnecessary thoracotomy, but, more severely, in other situations of missing the main therapeutic indication - esophageal resection. Basically, one third of the analyzed cases would not benefit from esophageal resection due to EUS over-staging. The results are similar to those published by Zoonen et al., which found a similar T4 EUS under-staging of 33% of cases and a diagnostic accuracy of only 57% for T4 tumors in predicted resectability EUS [
47]. Although the prediction of resectability by ultrasonography can achieve an accuracy of 83%, it differs between histological patterns, being higher for adenocarcinoma and much lower for squamous carcinoma (82% vs. 64%) [
39]. Taking into account the net predominance of esophageal squamous carcinomas in the studied group, this could explain the low possibility of predictability of resection in the analyzed group.