Systematic Surgical Approach to Juvenile Angiofibroma
Abstract
Introduction
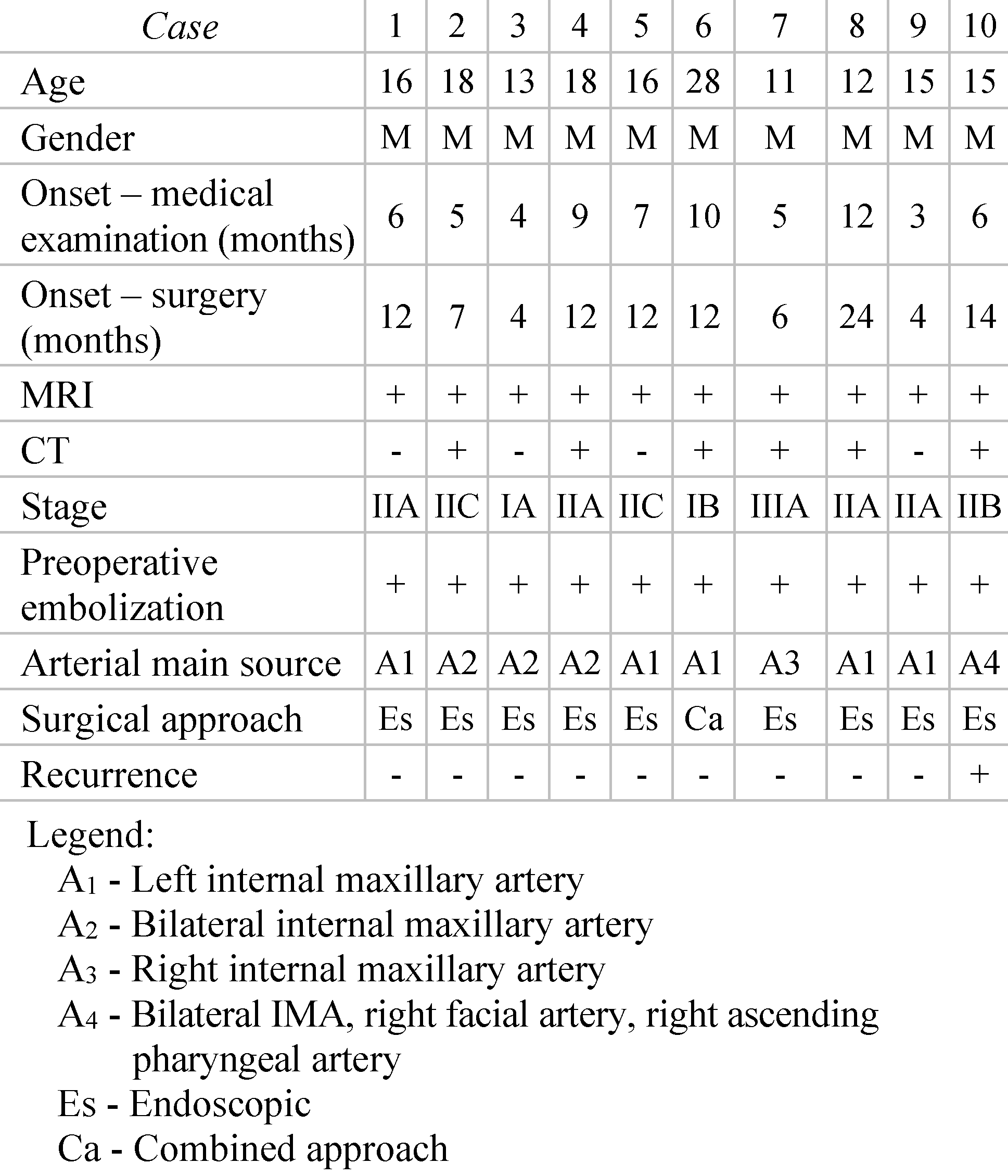
Materials and Methods
Results
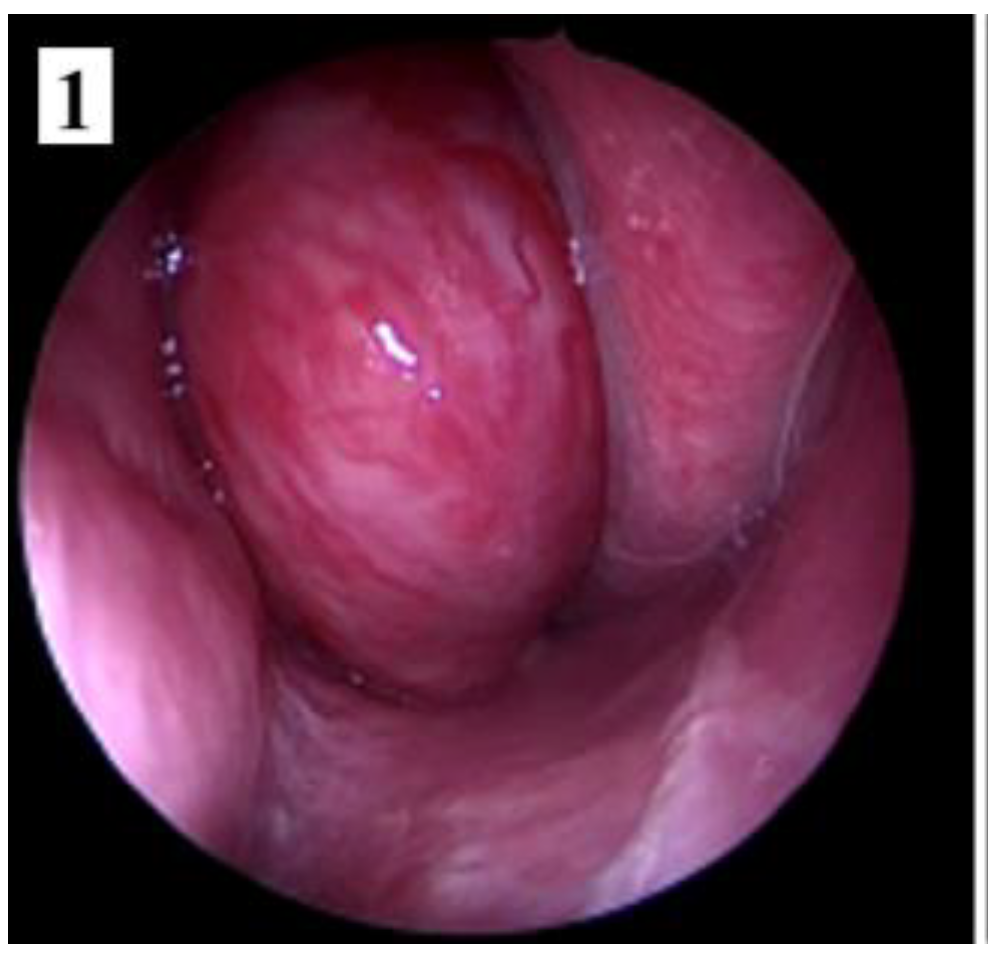
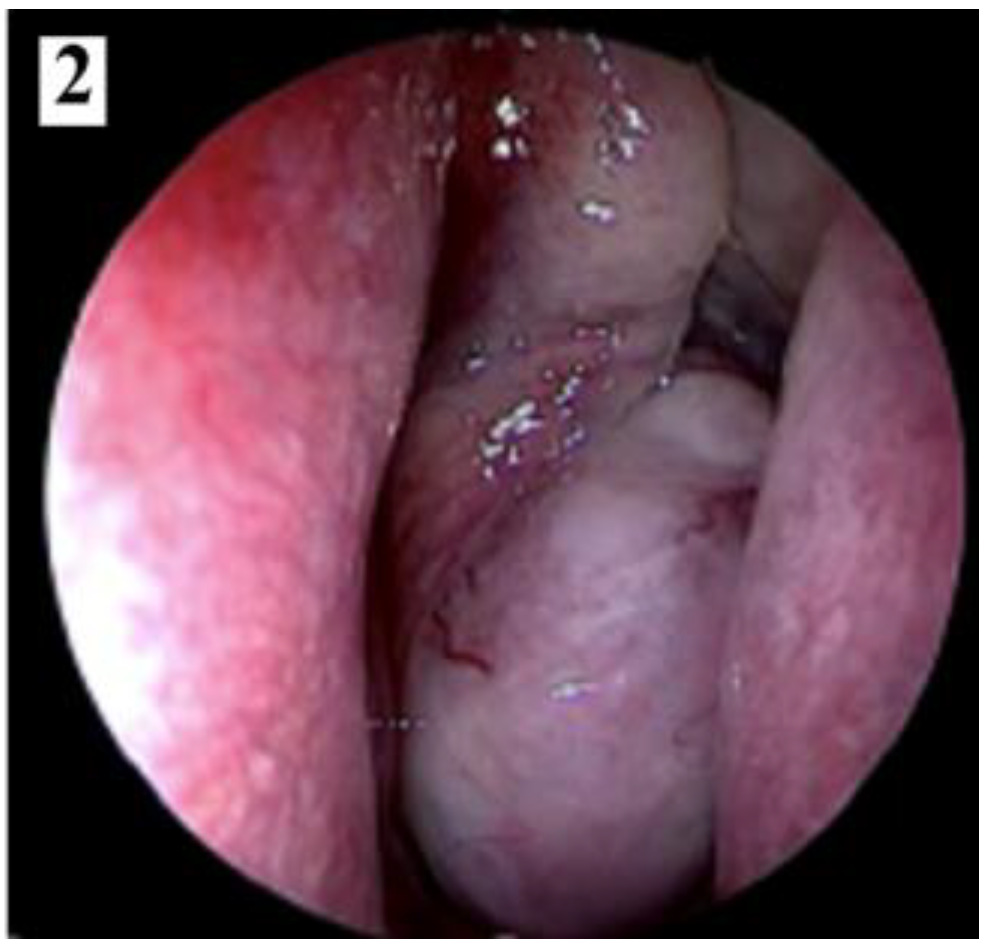


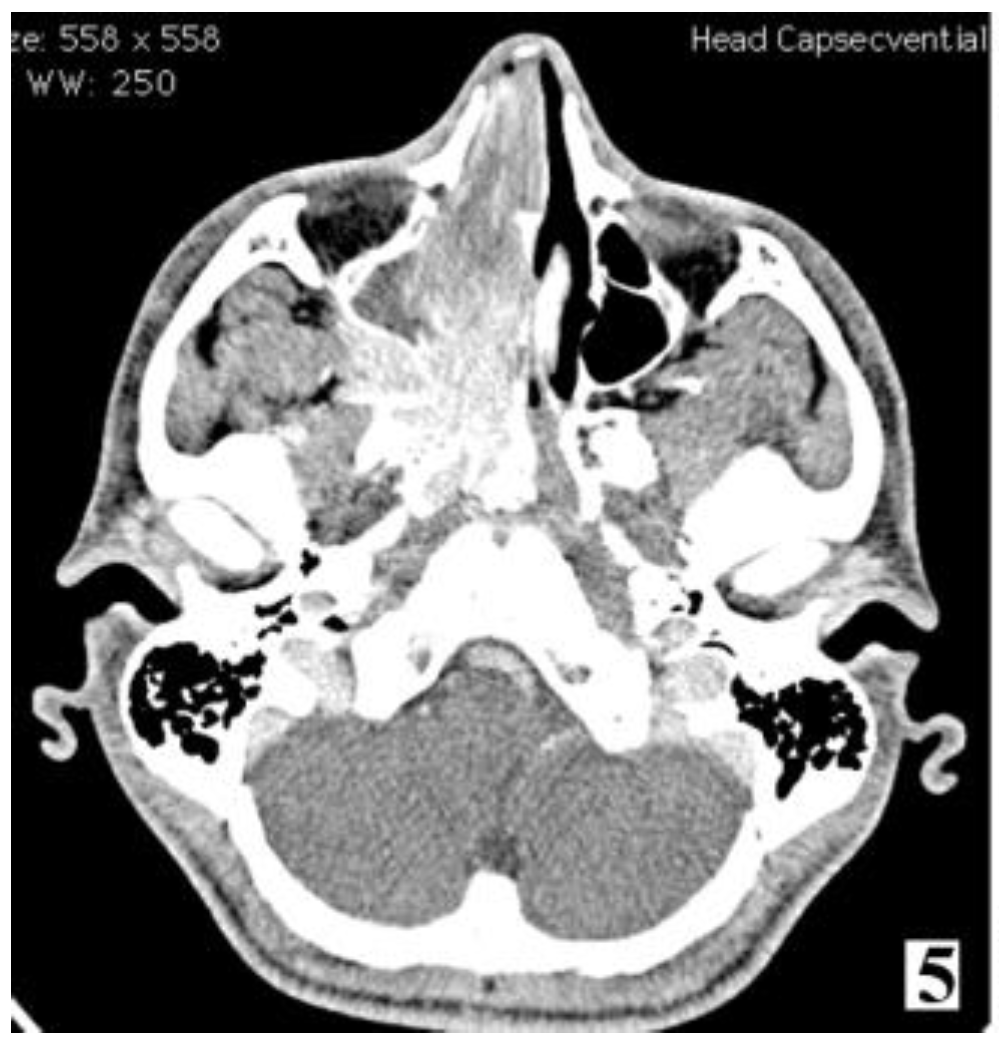
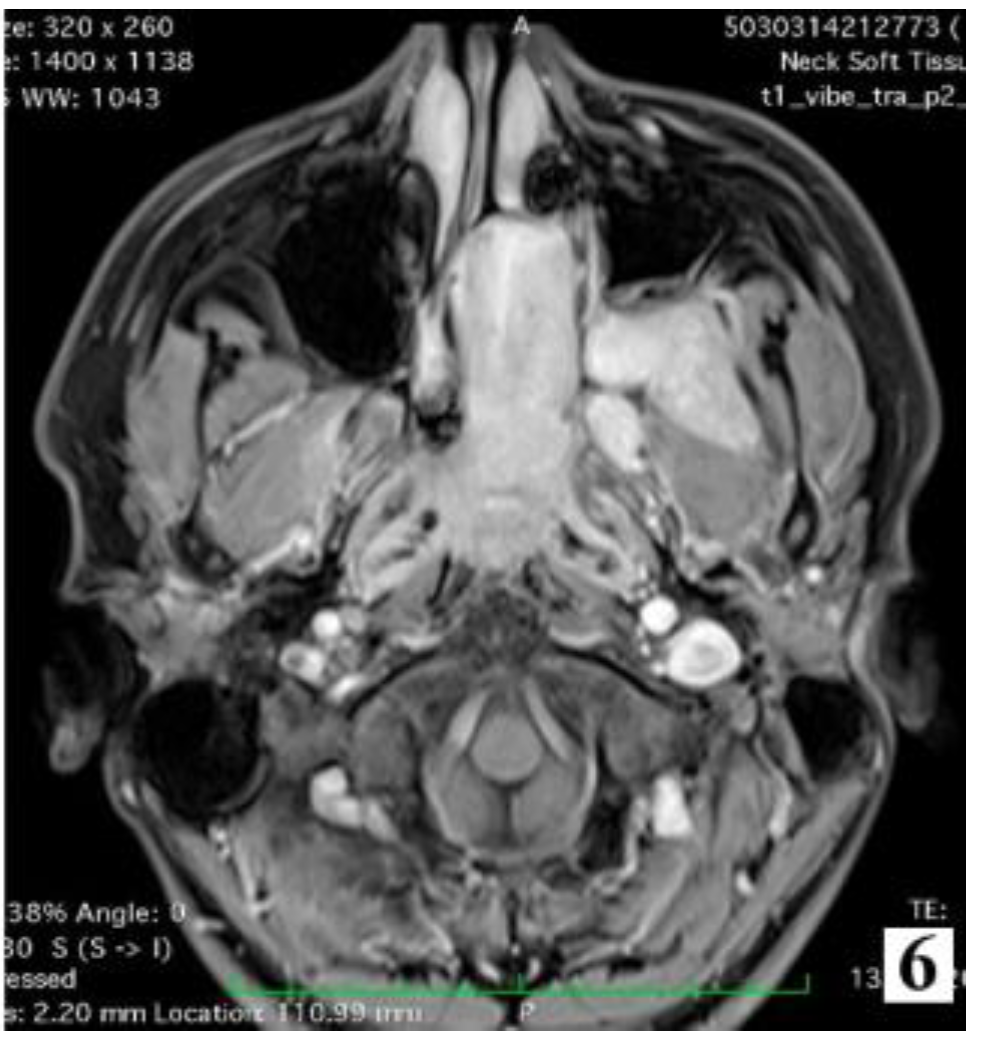
Discussions
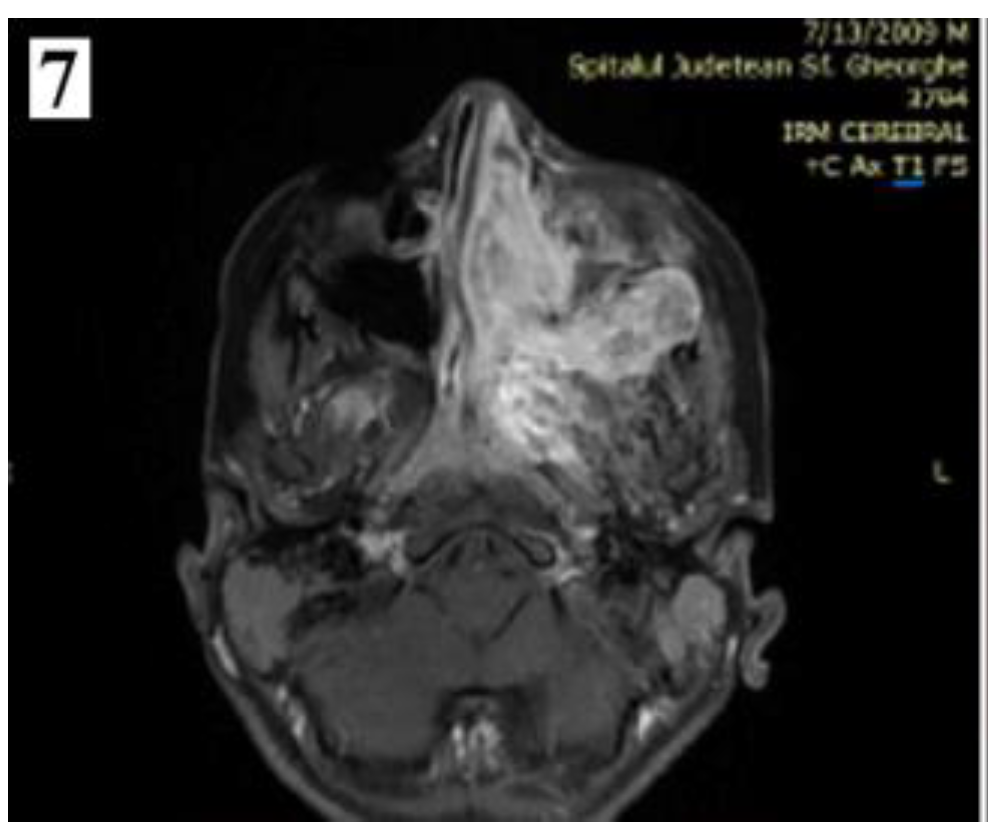
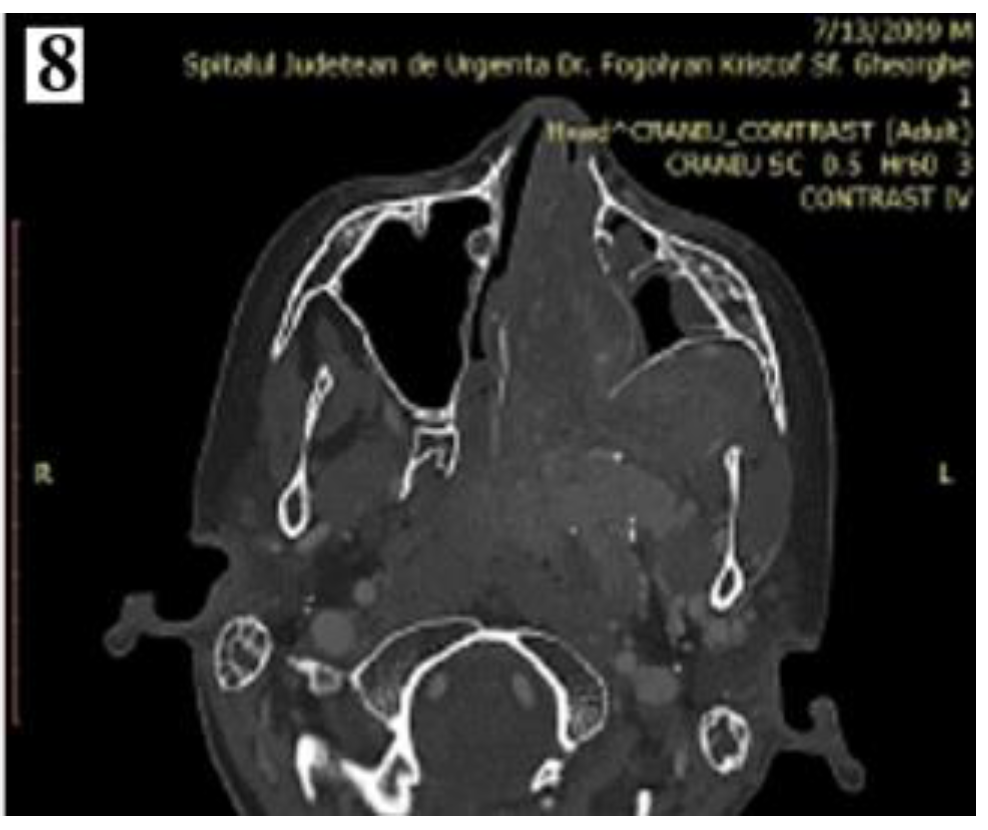
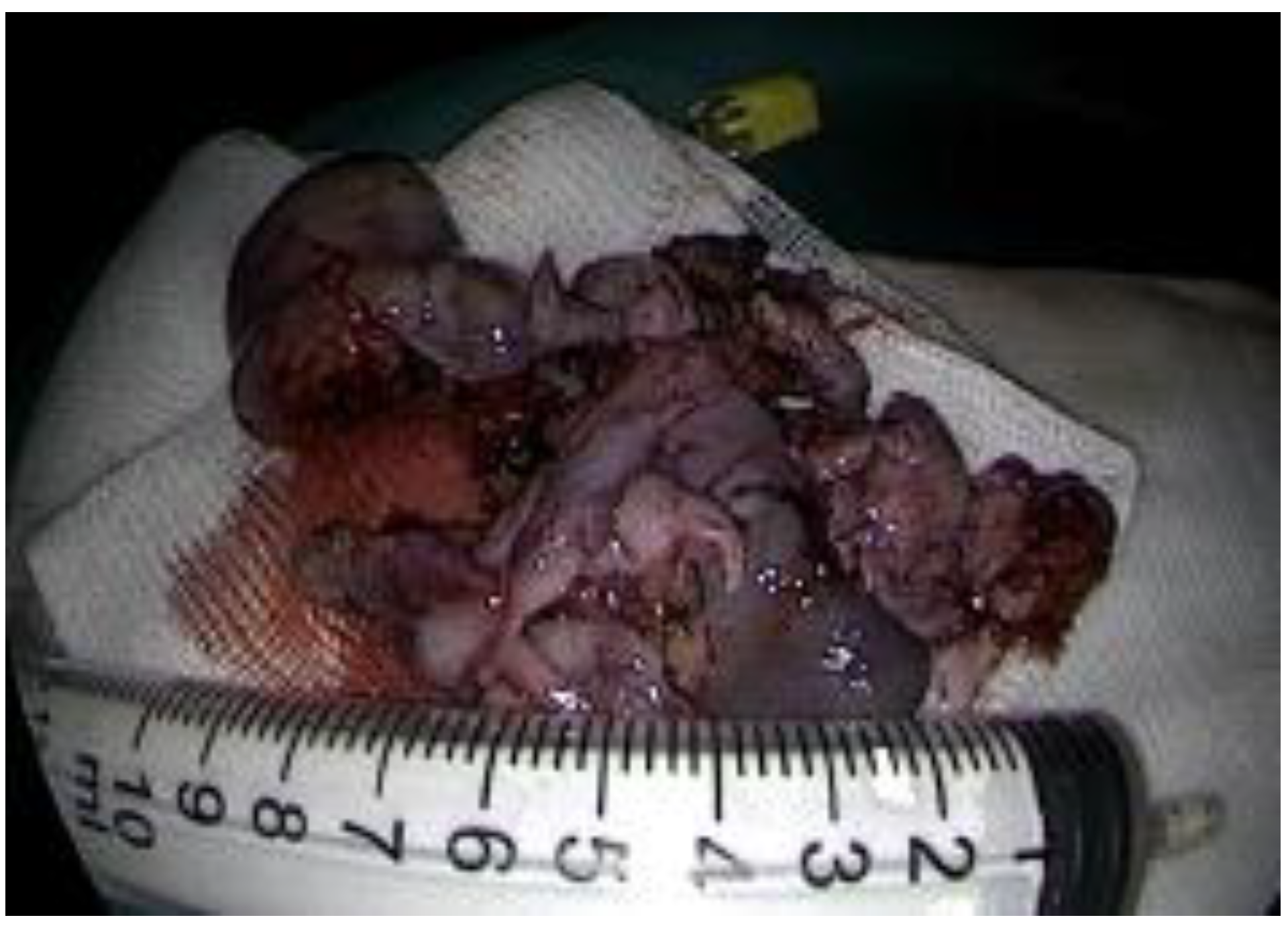
Staging
Management
Conclusions
Informed Consent Statement
Conflicts of Interest
References
- Gullane, P.J.; Davidson, J.; O’Dwyer, T.; Forte, V. Juvenile angiofibroma: A review of the literature and a case series report. Laryngoscope 1992, 102, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, P.; Schreiber, A.; Villaret, A.B. Juvenile Angiofibroma: Evolution of Management. Int. J. Pediatr. 2011, 2012, 412545. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, A.W.; Appenroth, E.; Kammen-Jolly, K.; Scholtz, L.U.; Thumfart, W.F. Juvenile Nasopharyngeal Angiofibroma: Management and Therapy. Laryngoscope 2001, 111, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.J.; Stammberger, H.; Nicolai, P.; Castelnuovo, P.; Beal, T.; Beham, A.; Bernal-Sprekelsen, M.; Braun, H.; Cappabianca, P.; Carrau, R.; et al. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol. Suppl. 2010, 22, 1–143. [Google Scholar]
- Lloyd, G.; Howard, D.; Phelps, P.; Cheesman, A. Juvenile angiofibroma: The lessons of 20 years of modern imaging. J. Laryngol. Otol. 1999, 113, 127–134. [Google Scholar] [CrossRef]
- Schick, B.; Kahle, G. Radiological findings in angiofibroma. Acta Radiol. 2000, 41, 585–593. [Google Scholar] [CrossRef]
- Schuon, R.; Brieger, J.; Heinrich, U.R.; Roth, Y.; Szyfter, W.; Mann, W.J. Immunohistochemical analysis of growth mechanisms in juvenile nasopharyngeal angiofibroma. Eur. Arch. Otorhinolaryngol. 2006, 264, 389–394. [Google Scholar] [CrossRef]
- Stiller, D.; Küttner, K. Wachstumsmuster juveniler Nasenrachenfibrome. Eine histologische Analyse an Hand von 40 Fällen [Growth patterns of juvenile nasopharyngeal fibromas. A histological analysis on the basis of 40 cases]. Zentralbl. Allg. Pathol. 1988, 134, 409–422. [Google Scholar]
- Sternberg, S.S. Pathology of juvenile nasopharyngeal angiofibroma—A lesion of adolescent males. Cancer 1954, 7, 15–28. [Google Scholar] [CrossRef]
- Hubbard, E.M. Nasopharyngeal angiofibromas. AMA Arch. Pathol. 1958, 65, 192–204. [Google Scholar]
- Schiff, M. Juvenile nasopharyngeal angiofibroma. a theory of pathogenesis. Laryngoscope 1959, 69, 981–1016. [Google Scholar] [CrossRef] [PubMed]
- Beham, A.; Beham-Schmid, C.; Regauer, S.; Auböck, L.; Stammberger, H. Nasopharyngeal Angiofibroma: True Neoplasm or Vascular Malformation? Adv. Anat. Pathol. 1999, 7, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Schick, B.; Plinkert, P.K.; Prescher, A. Die vaskuläre Komponente: Gedanken zur Entstehung des Angiofibroms. Laryngo-Rhino-Otologie 2002, 81, 280–284. [Google Scholar] [CrossRef]
- Starlinger, V.; Wendler, O.; Gramann, M.; Schick, B. Laminin expression in juvenile angiofibroma indicates vessel’s early developmental stage. Acta Oto-Laryngologica 2007, 127, 1310–1315. [Google Scholar] [CrossRef]
- Tiu, V.E.; Popescu, B.O.; Enache, I.I.; Tiu, C.; Terecoasa, E.; Panea, C.A. Serum and CSF Biomarkers Predict Active Early Cognitive Decline Rather Than Established Cognitive Impairment at the Moment of RRMS Diagnosis. Diagnostics 2022, 12, 2571. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.M.; Ghanimah, S.E.; Ragaie, A.; Saleem, T.H. Hormonal receptors in juvenile nasopharyngeal angiofibroma. Laryngoscope 1987, 97, 208–211. [Google Scholar] [CrossRef]
- Hwang, H.C.; E Mills, S.; Patterson, K.; Gown, A.M. Expression of androgen receptors in nasopharyngeal angiofibroma: An immunohistochemical study of 24 cases. Mod. Pathol. 1998, 11, 1122–1126. [Google Scholar]
- Saylam, G.; Yücel, O.T.; Sungur, A.; Önerci, M. Proliferation, angiogenesis and hormonal markers in juvenile nasopharyngeal angiofibroma. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 227–234. [Google Scholar] [CrossRef]
- Safadi, A.; Schreiber, A.; Fliss, D.M.; Nicolai, P. Juvenile Angiofibroma: Current Management Strategies. J. Neurol. Surg. Part B Skull Base 2018, 79, 021–030. [Google Scholar] [CrossRef]
- Lloyd, G.; Howard, D.; Lund, V.; Savy, L. Imaging for juvenile angiofibroma. J. Laryngol. Otol. 2000, 114, 727–730. [Google Scholar] [CrossRef]
- Longacre, M.M.; Seshadri, S.C.; Adil, E.; Baird, L.C.; Goobie, S.M. Perioperative management of pediatric patients undergoing juvenile angiofibroma resection. A case series and educational review highlighting patient blood management. Pediatr. Anesthesia 2023, 33, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.R.; Cappiello, J.; Di Lorenzo, D.; Donajo, C.A.; Nicolai, P.; Orlandini, A. Diagnosis, staging, and treatment of juvenile nasopharyngeal angiofibroma (JNA). Laryngoscope 1987, 97, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, A.; Szymański, M.; Czekajska-Chehab, E.; Szczerbo-Trojanowska, M. Two types of lateral extension in juvenile nasopharyngeal angiofibroma: Diagnostic and therapeutic management. Eur. Arch. Oto-Rhino-Laryngology 2014, 272, 159–166. [Google Scholar] [CrossRef]
- Zanation, A.M.; Mitchell, C.A.; Rose, A.S. Endoscopic Skull Base Techniques for Juvenile Nasopharyngeal Angiofibroma. Otolaryngol. Clin. N. Am. 2012, 45, 711–730. [Google Scholar] [CrossRef]
- Sessions, R.B.; Bryan, R.N.; Naclerio, R.M.; Alford, B.R. Radiographic staging of juvenile angiofibroma. Head Neck Surg. 1981, 3, 279–283. [Google Scholar] [CrossRef]
- Radkowski, D.; McGill, T.; Healy, G.B.; Ohlms, L.; Jones, D.T. Angiofibroma: Changes in Staging and Treatment. Arch. Otolaryngol. Neck Surg. 1996, 122, 122–129. [Google Scholar] [CrossRef]
- Andrews, J.C.; Fisch, U.; Aeppli, U.; Valavanis, A.; Makek, M.S. The surgical management of extensive nasopharyngeal angiofibromas with the infratemporal fossa approach. Laryngoscope 1989, 99, 429–437. [Google Scholar] [CrossRef] [PubMed]
- El Sharkawy, A.A. Endonasal endoscopic management of juvenile nasopharyngeal angiofibroma without angiographic embolization. Eur. Arch. Otorhinolaryngol. 2012, 270, 2051–2055. [Google Scholar] [CrossRef]
- El-Banhawy, O.A.; Ragab, A.; El-Sharnoby, M.M. Surgical resection of type III juvenile angiofibroma without preoperative embolization. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1715–1723. [Google Scholar] [CrossRef]
- Nicolai, P.; Villaret, A.B.; Farina, D.; Nadeau, S.; Yakirevitch, A.; Berlucchi, M.; Galtelli, C. Endoscopic Surgery for Juvenile Angiofibroma: A Critical Review of Indications after 46 Cases. Am. J. Rhinol. Allergy 2010, 24, e67–e72. [Google Scholar] [CrossRef]
- Hackman, T.; Snyderman, C.H.; Carrau, R.; Vescan, A.; Kassam, A. Juvenile Nasopharyngeal Angiofibroma: The Expanded Endonasal Approach. Am. J. Rhinol. Allergy 2009, 23, 95–99. [Google Scholar] [CrossRef]
- Roger, G.; Huy, P.T.B.; Froehlich, P.; Abbeele, T.V.D.; Klossek, J.-M.; Serrano, E.; Garabedian, E.-N.; Herman, P. Exclusively Endoscopic Removal of Juvenile Nasopharyngeal Angiofibroma. Arch. Otolaryngol. Neck Surg. 2002, 128, 928–935. [Google Scholar] [CrossRef]
- Santos-Franco, J.A.; Lee, A.; Campos-Navarro, L.A.; Tenorio-Sánchez, J.; Zenteno, M.; Osorio-Alvarado, A.R. Bilateral Non-Superselective Embolization With Particles Under Transient Occlusion of the Internal Carotid Artery in the Management of Juvenile Nasopharyngeal Angiofibroma. Vasc. Endovasc. Surg. 2012, 46, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.W.; Mowry, S.E.; Vinuela, F.; Abemayor, E.; Wang, M.B. Bilateral vascular supply in juvenile nasopharyngeal angiofibromas. Laryngoscope 2010, 121, 639–643. [Google Scholar] [CrossRef]
- Glad, H.; Vainer, B.; Buchwald, C.; et al. Juvenile nasopharyngeal angiofibromas in Denmark 1981–2003: Diagnosis, incidence, and treatment. Acta Otolaryngol. 2007, 127, 292–299. [Google Scholar] [CrossRef] [PubMed]
- McCombe, A.; Lund, V.J.; Howard, D.J. Recurrence in juvenile angiofibroma. Rhinology 1990, 28, 97–102. [Google Scholar]
- Alius, R.O.; Zainea, V.; Voiosu, C.; Ionita, I.G.; Rusescu, A.; Balalau, O.D.; Alius, C.; Pulpa, R.O.; Hainarosie, R. Nasal surgery versus pharyngeal surgery in the treatment of obstructive sleep apnea. J. Mind Med. Sci. 2022, 9, 318–323. [Google Scholar] [CrossRef]
- Liu, Q.; Xia, Z.; Hong, R.; Pan, Y.; Xue, K.; Liu, Q.; Sun, X.; Li, H.; Sha, Y.; Yu, H.; et al. Preoperative Embolization of Primary Juvenile Nasopharyngeal Angiofibroma: Is Embolization of Internal Carotid Artery Branches Necessary? Cardiovasc. Interv. Radiol. 2023, 46, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Motofei, I.G. Biology of cancer; from cellular and molecular mechanisms to developmental processes and adaptation. Semin. Cancer Biol. 2021, 86, 600–615. [Google Scholar] [CrossRef]
- Baba, A.; Kurokawa, R.; Kurokawa, M.; Srinivasan, A. MRI features of sinonasal tract angiofibroma/juvenile nasopharyngeal angiofibroma: Case series and systematic review. J. Neuroimaging 2023, 33, 675–687. [Google Scholar] [CrossRef]
- Midilli, R.; Karci, B.; Akyildiz, S. Juvenile nasopharyngeal angiofibroma: Analysis of 42 cases and important aspects of endoscopic approach. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Hoeltgen, L.; Tessonnier, T.; Meixner, E.; Hoegen, P.; Kim, J.-Y.; Deng, M.; Seidensaal, K.; Held, T.; Herfarth, K.; Debus, J.; et al. Proton Therapy for Advanced Juvenile Nasopharyngeal Angiofibroma. Cancers 2023, 15, 5022. [Google Scholar] [CrossRef] [PubMed]
- Yiotakis, I.; Eleftheriadou, A.; Davilis, D.; Giotakis, E.; Ferekidou, E.; Korres, S.; Kandiloros, D. Juvenile nasopharyngeal angiofibroma stages I and II: A comparative study of surgical approaches. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 793–800. [Google Scholar] [CrossRef]
- Carrau, R.L.; Snyderman, C.H.; Kassam, A.B.; Jungreis, C.A. Endoscopic and Endoscopic-Assisted Surgery for Juvenile Angiofibroma. Laryngoscope 2001, 111, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, P.; Berlucchi, M.; Tomenzoli, D.; Cappiello, J.; Trimarchi, M.; Maroldi, R.; Battaglia, G.; Antonelli, A.R. Endoscopic Surgery for Juvenile Angiofibroma: When and How. Laryngoscope 2003, 113, 775–782. [Google Scholar] [CrossRef]
- Ardehali, M.M.; Ardestani, S.-H.S.; Yazdani, N.; Goodarzi, H.; Bastaninejad, S. Endoscopic approach for excision of juvenile nasopharyngeal angiofibroma: Complications and outcomes. Am. J. Otolaryngol. 2010, 31, 343–349. [Google Scholar] [CrossRef]
 |
© 2024 by the author. 2024 Raluca Oana Pulpă, Viorel Zainea, Cătălina Voiosu, Ruxandra Oana Aliuș, Andreea Rusescu, Irina-Gabriela Ioniță, Veronica Epure, Dragoș Octavian Palade, Răzvan Hainăroșie
Share and Cite
Pulpă, R.O.; Zainea, V.; Voiosu, C.; Aliuș, R.O.; Rusescu, A.; Ioniță, I.-G.; Epure, V.; Palade, D.O.; Hainăroșie, R. Systematic Surgical Approach to Juvenile Angiofibroma. J. Mind Med. Sci. 2024, 11, 218-224. https://doi.org/10.22543/2392-7674.1471
Pulpă RO, Zainea V, Voiosu C, Aliuș RO, Rusescu A, Ioniță I-G, Epure V, Palade DO, Hainăroșie R. Systematic Surgical Approach to Juvenile Angiofibroma. Journal of Mind and Medical Sciences. 2024; 11(1):218-224. https://doi.org/10.22543/2392-7674.1471
Chicago/Turabian StylePulpă, Raluca Oana, Viorel Zainea, Cătălina Voiosu, Ruxandra Oana Aliuș, Andreea Rusescu, Irina-Gabriela Ioniță, Veronica Epure, Dragoș Octavian Palade, and Răzvan Hainăroșie. 2024. "Systematic Surgical Approach to Juvenile Angiofibroma" Journal of Mind and Medical Sciences 11, no. 1: 218-224. https://doi.org/10.22543/2392-7674.1471
APA StylePulpă, R. O., Zainea, V., Voiosu, C., Aliuș, R. O., Rusescu, A., Ioniță, I.-G., Epure, V., Palade, D. O., & Hainăroșie, R. (2024). Systematic Surgical Approach to Juvenile Angiofibroma. Journal of Mind and Medical Sciences, 11(1), 218-224. https://doi.org/10.22543/2392-7674.1471



