Introduction
Rectal cancer is a pathology that is currently of great interest to the medical community, primarily due to its high incidence but also the high mortality rate [
1,
2]. Worldwide, along with colon cancer, rectal neoplasm ranks third in incidence in men and second in women. According to the World Health Organization, 1.9 million new cases of colorectal cancer were diagnosed in 2020. In addition, there has been an alarming increase in the incidence of this cancer in recent decades, with the rates of this disease being estimated (for adults aged between 20 and 34 years) at 124.2% by the year 2030 [
3,
4]. It is estimated that 1 in 23 men have a lifetime risk of developing rectal cancer, with the risk being 1 in 25 for women [
5,
6].
The surgical management of rectal cancer has benefited from constant improvement over time, particularly due to the continuous development of medical technology, which has led to better outcomes in terms of local recurrence and mortality rates [
7]. The development of technology dates back to the year 1908 when surgical staplers were invented, the moment marking not only a progress in surgical techniques, but also the beginning of debates regarding the advantages and disadvantages of using mechanical anastomoses in rectal cancer surgery [
5].
The purpose of this study is to perform a retrospective, longitudinal, cohort analysis regarding the therapeutic options and postoperative complications of patients with rectal oncological pathology in the Surgery Department I of the Sibiu County Emergency Clinical Hospital.
The results obtained in this study are consistent with literature data, which generally support the performance of mechanical anastomoses in digestive neoplasia, especially in upper and middle rectal cancers. This means that surgeons must overcome any skepticism and consider it as a therapeutic option of choice, of course in patients who have the appropriate indication and when such a surgical technique is available.
Results
A number of 70 patients were enrolled in the study, most of them being male from urban environment. The mean age of the group of subjects is 66 years.
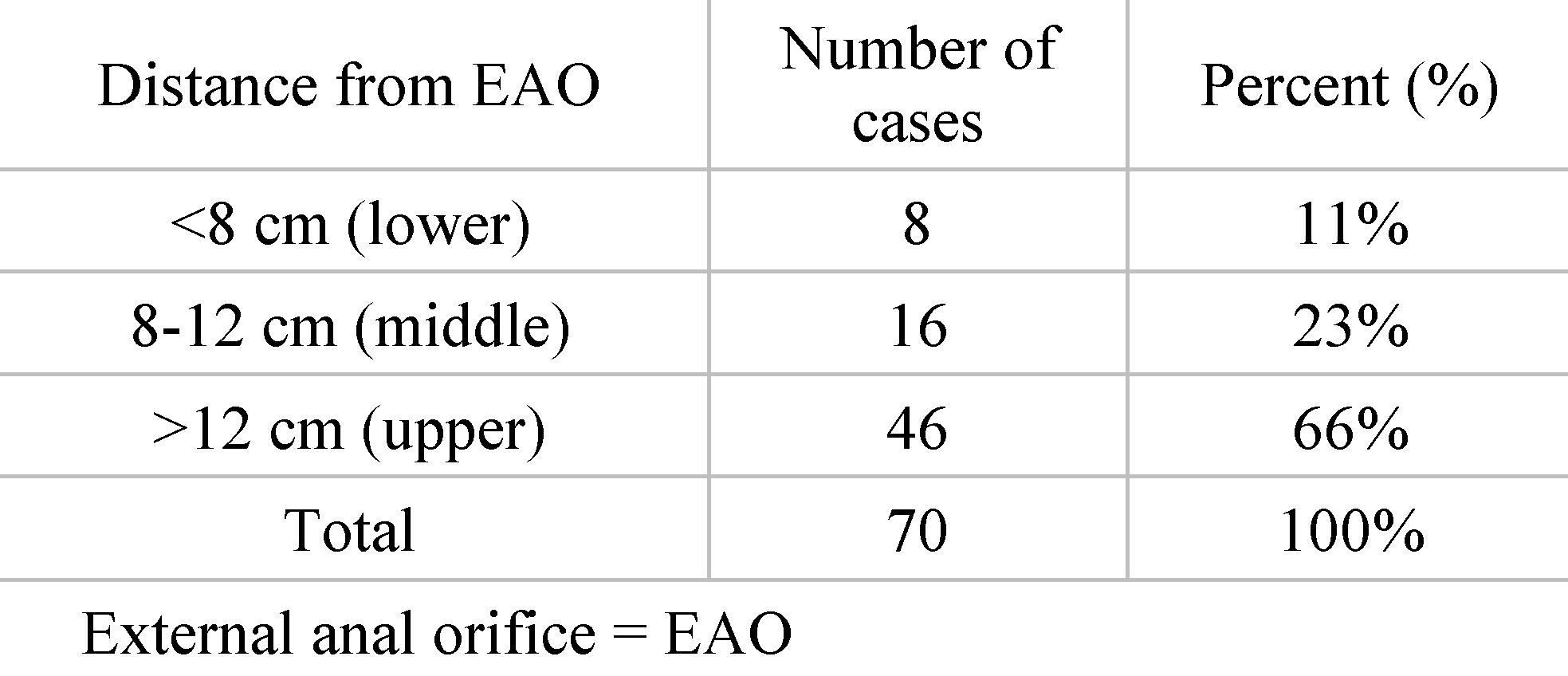
Distribution of patients according to the distance of the tumor location from the external anal orifice (EAO). Out of the total group of 70.8 of the patients had the rectal tumor located at a distance of less than 8 cm from the EAO, a situation found in 11% cases. 16 of the patients had the rectal tumor located at a distance of 8 to 12 cm from the EAO, which was found in 23% cases. 46 of the patients had the rectal tumor located at a distance greater than 12 cm from the EAO, representing 66% of the entire group (
Table 1).
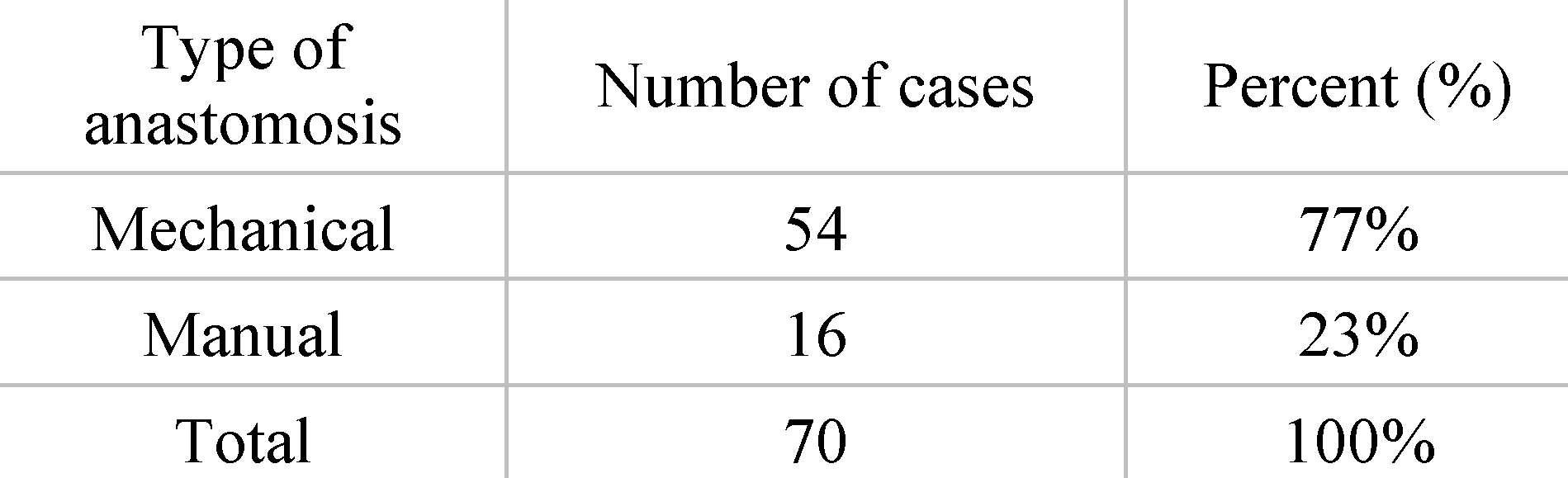
Distribution of patients by type of anastomosis. From the total group of 70 patients operated on for rectal cancer, 54 patients received mechanical anastomosis. This situation was present in 22% of cases. Manual anastomosis was performed in 16 of the patients, representing 12% of cases (
Table 2).
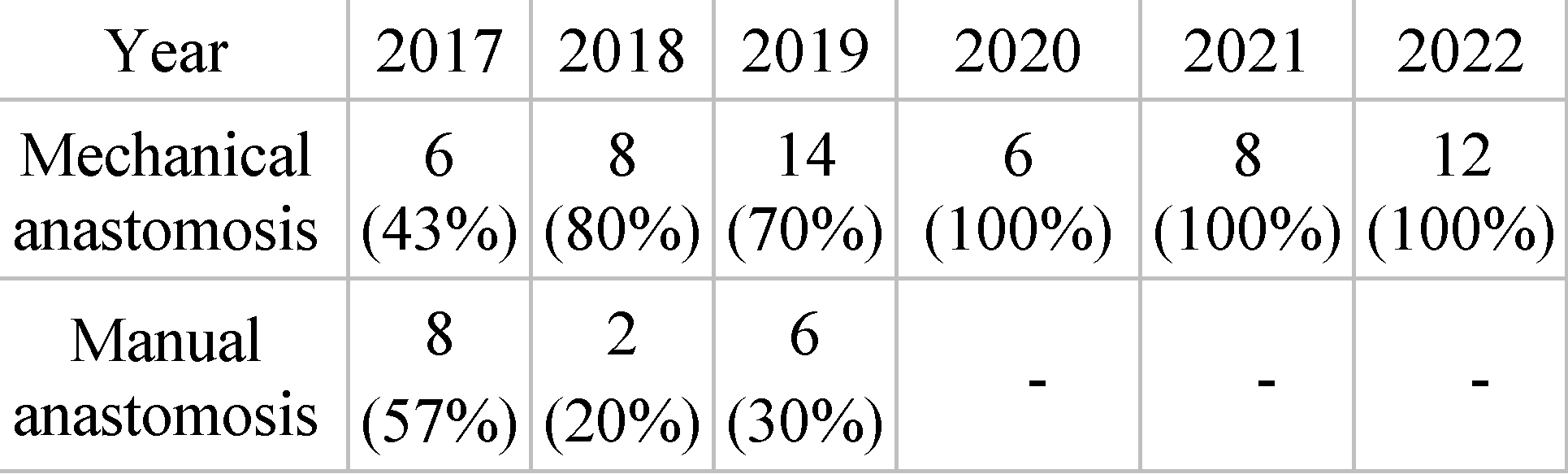
Incidence of mechanical anastomosis by year. From the total of 6 years analyzed, we want to highlight the use of mechanical anastomoses to the detriment of manual ones. In 2017, 14 anastomoses were performed, 6 being mechanical (43%), and 8 being manual (57%). In 2018, 10 anastomoses were performed, 8 being mechanical (80%), and 2 being manual (20%). Over the course of 2019, 20 anastomosis sutures were performed, 14 being mechanical (70%) and the remaining 6 being manual (30%). For the years 2020, 2021 and 2022, all anastomoses of the analyzed batch were mechanical. There were 6 mechanical anastomoses in 2020, 8 in 2021 and 12 in 2022 (
Table 3).
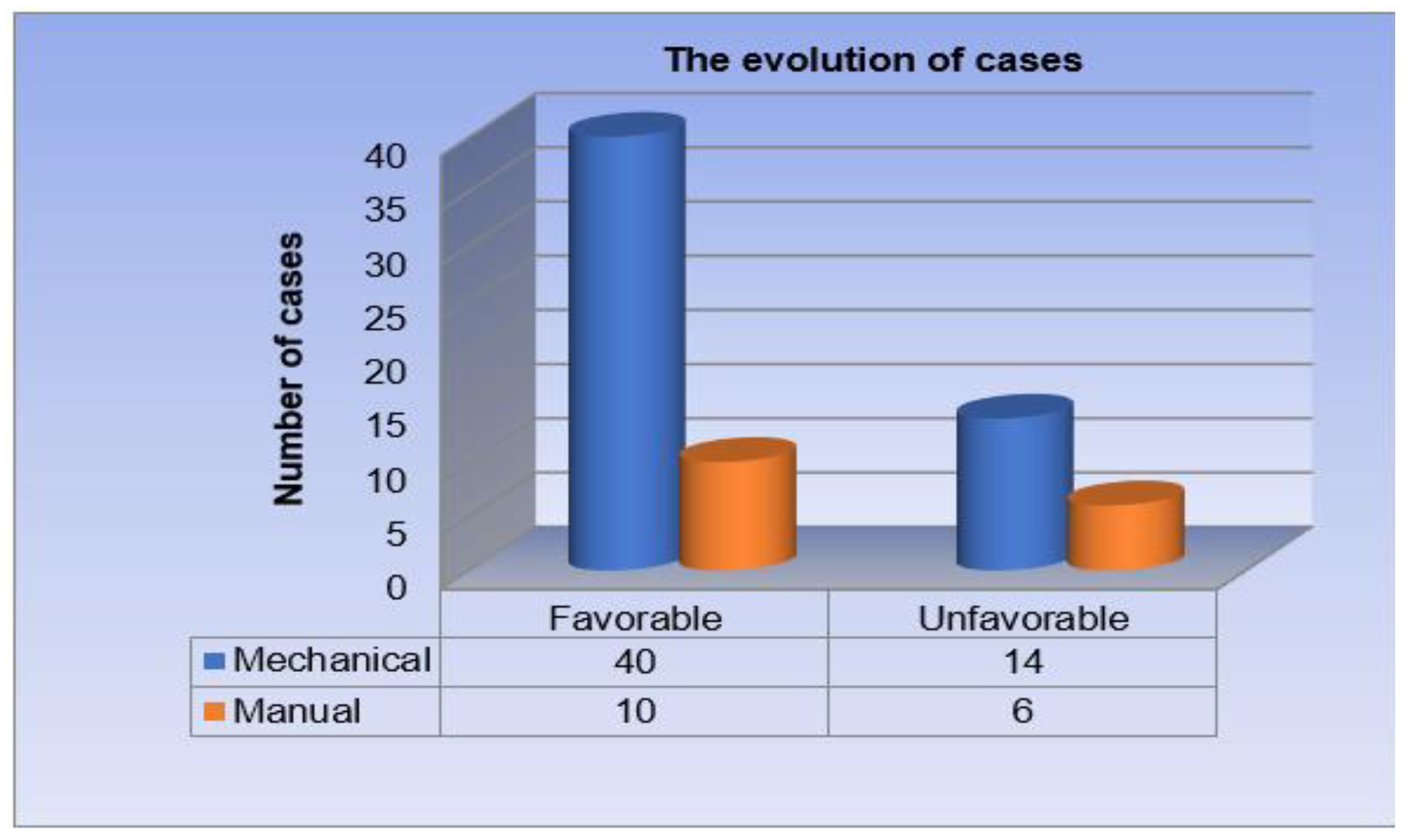
Distribution of patients by postoperative outcome. Out of a total of 70 patients operated on for rectal cancer, 50 patients had a favorable postoperative outcome (71.4% of cases). The remaining 20 patients had an unfavorable postoperative evolution, presenting complications (28.6% of cases) (
Table 4,
Figure 1).
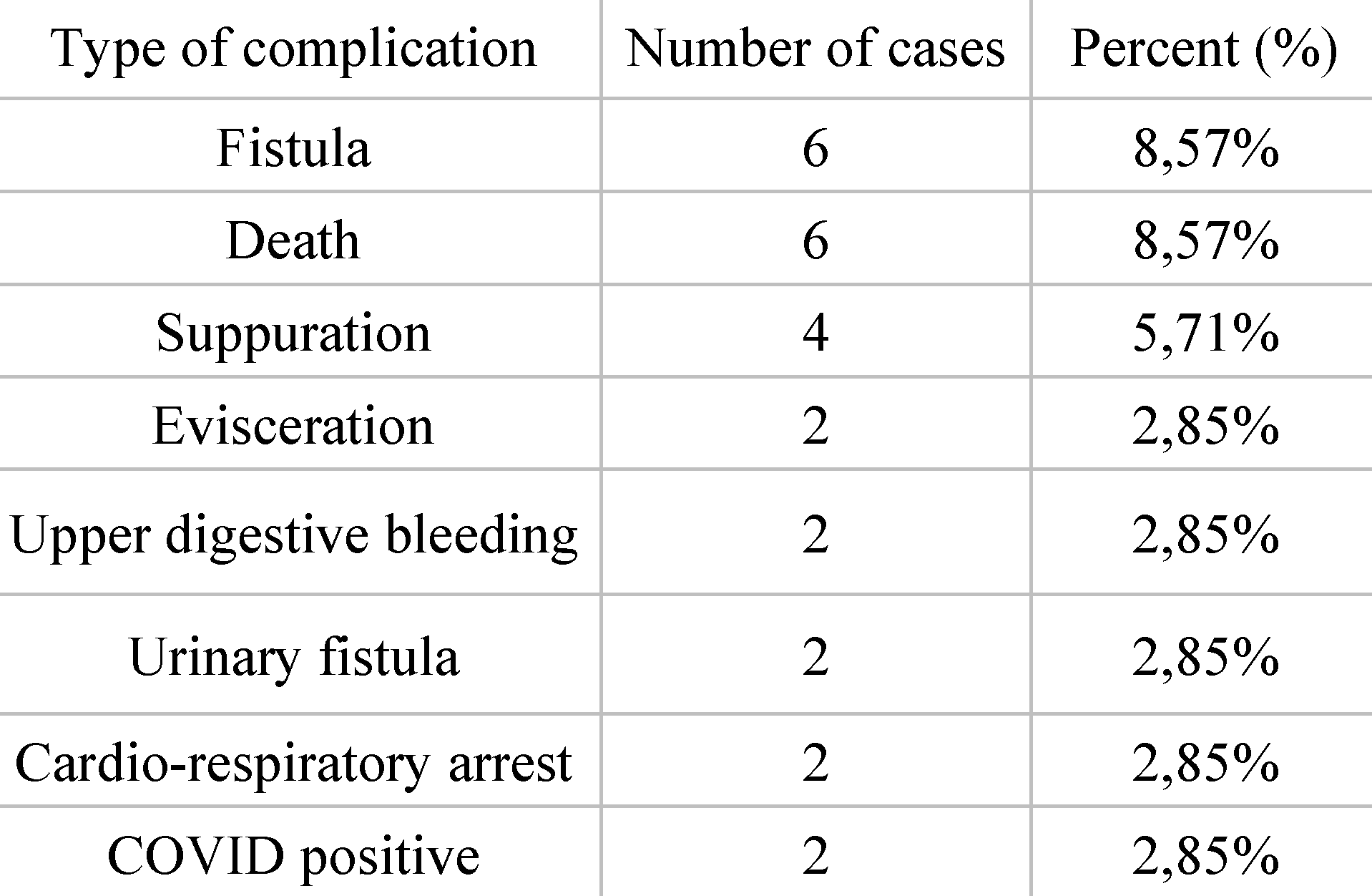
Distribution of patients according to complications. Out of the total of 70 patients, 20 of them had an unfavorable postoperative outcome, reporting the following complications: 6 cases were complicated by the occurrence of fistula (8.57%), 6 patients died (8.57%), 4 cases were complicated by the occurrence of suppuration (5.71%), 2 were complicated by the occurrence of evisceration (2.85%), 2 patients bled postoperatively from the upper digestive tract (2.85%), 2 patients developed urinary fistula (2.85%), 2 patients went into cardio-respiratory arrest (asystole) (2.85%), 2 patients tested positive for COVID during hospitalization (2.85%) (
Table 5).
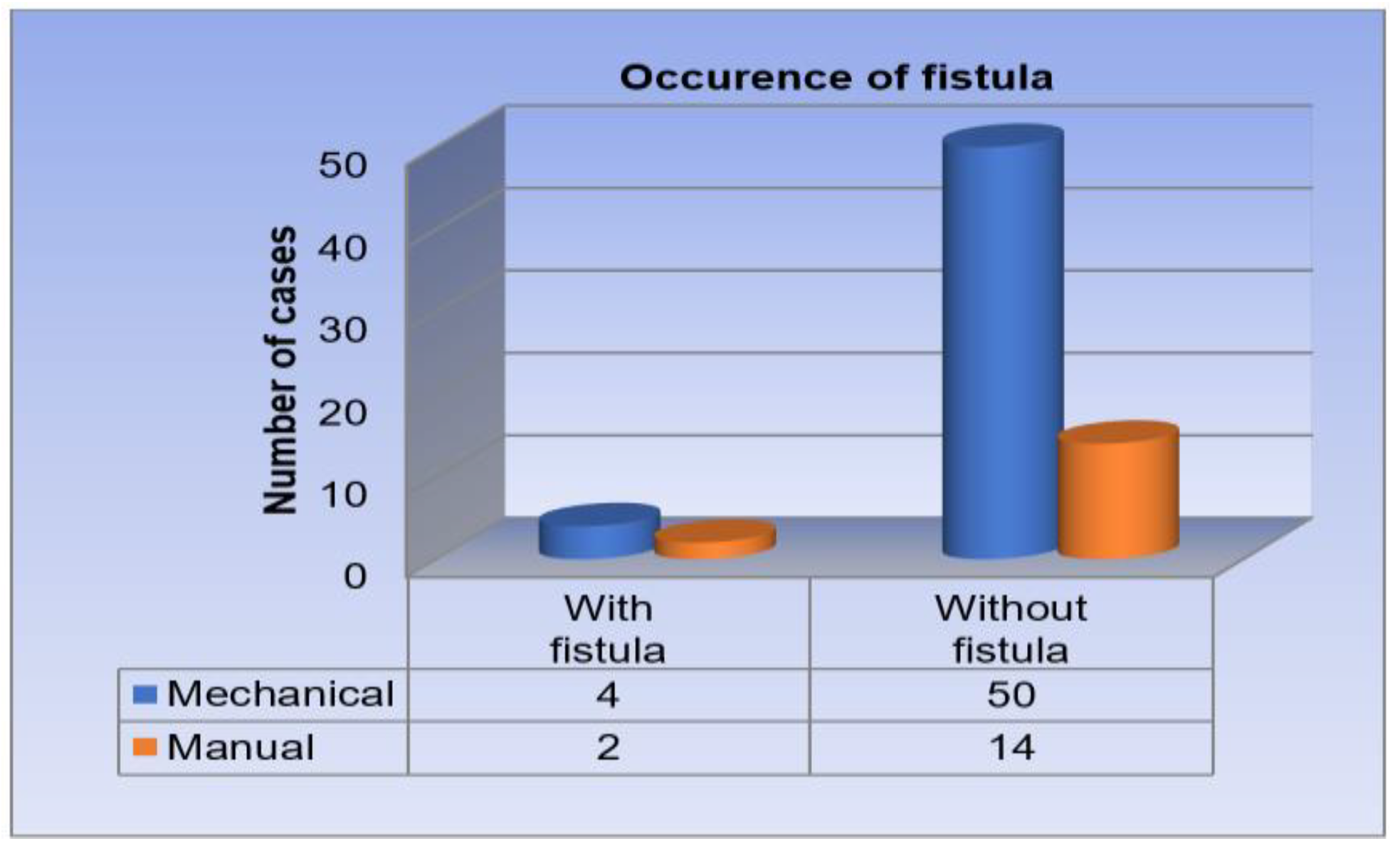
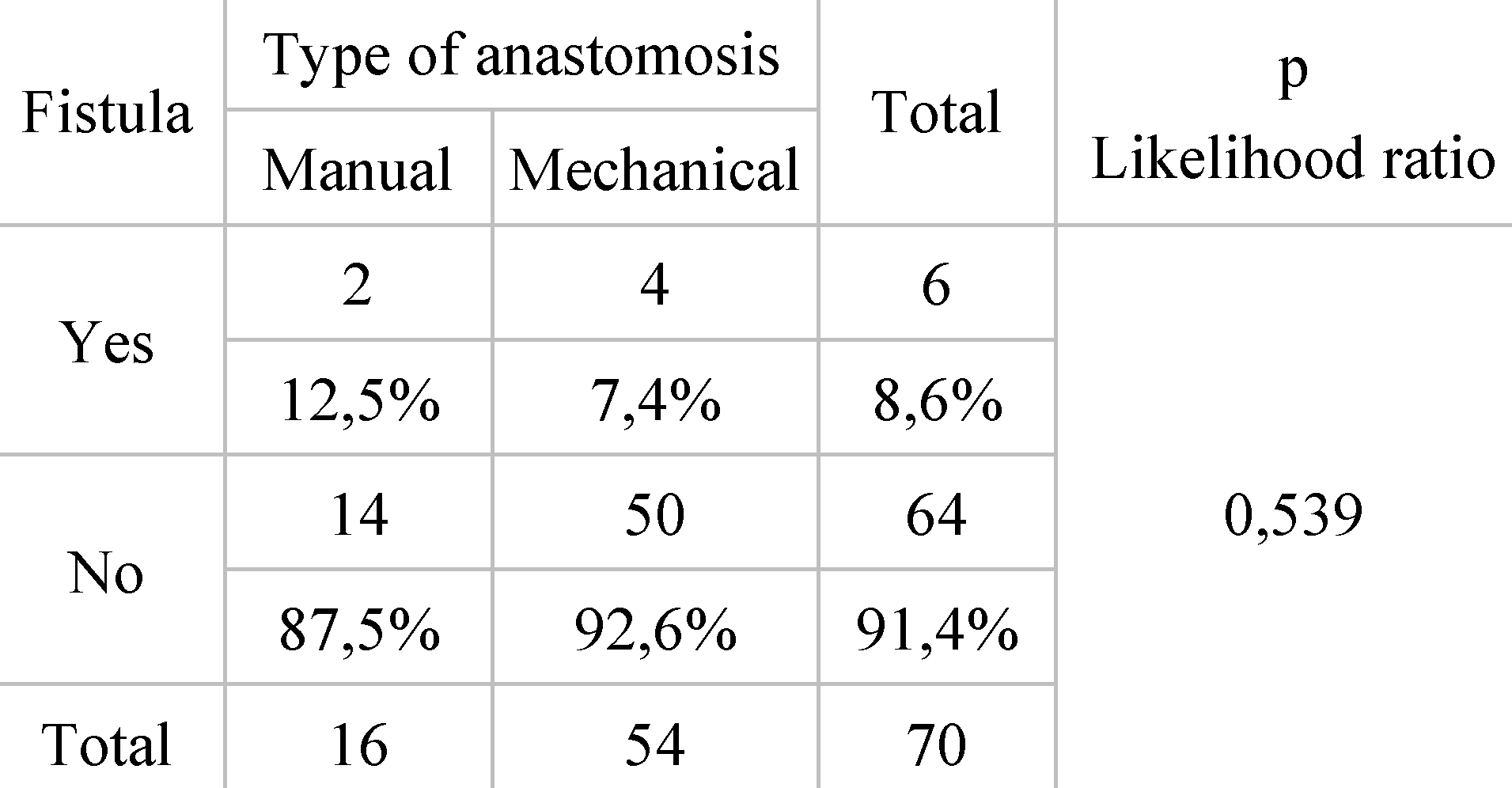
Distribution of patients by type of anastomosis and fistula occurrence. From the total group of 70 patients, 54 subjects benefited from mechanical anastomosis, while 16 from manual anastomosis. In the case of those with mechanical anastomosis, 4 cases were complicated by the appearance of fistula, the incidence being 7.40% of cases. Among the patients with manual anastomosis, 2 subjects developed postoperative fistula, the incidence being 12.5% (
Table 6,
Figure 2).
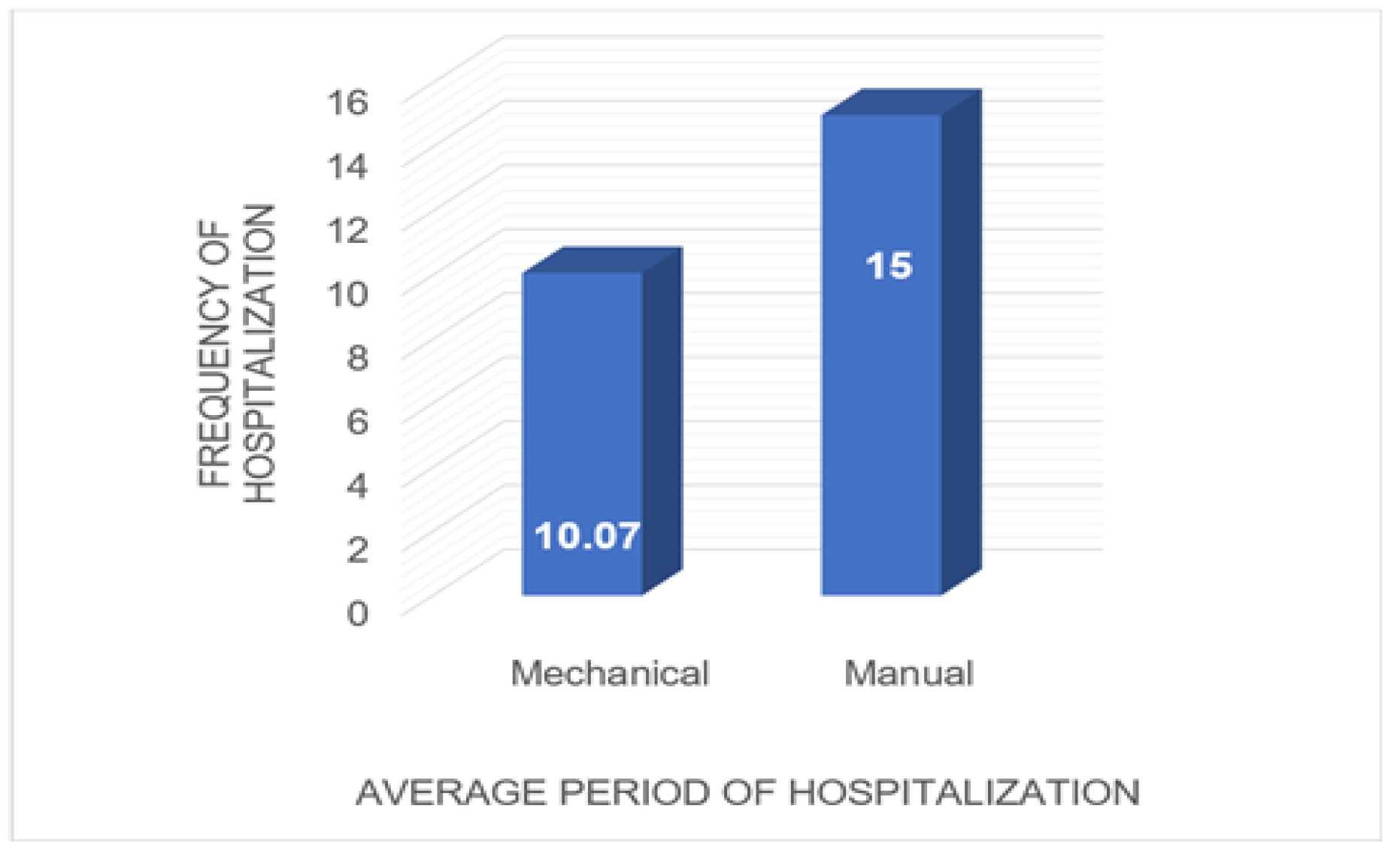
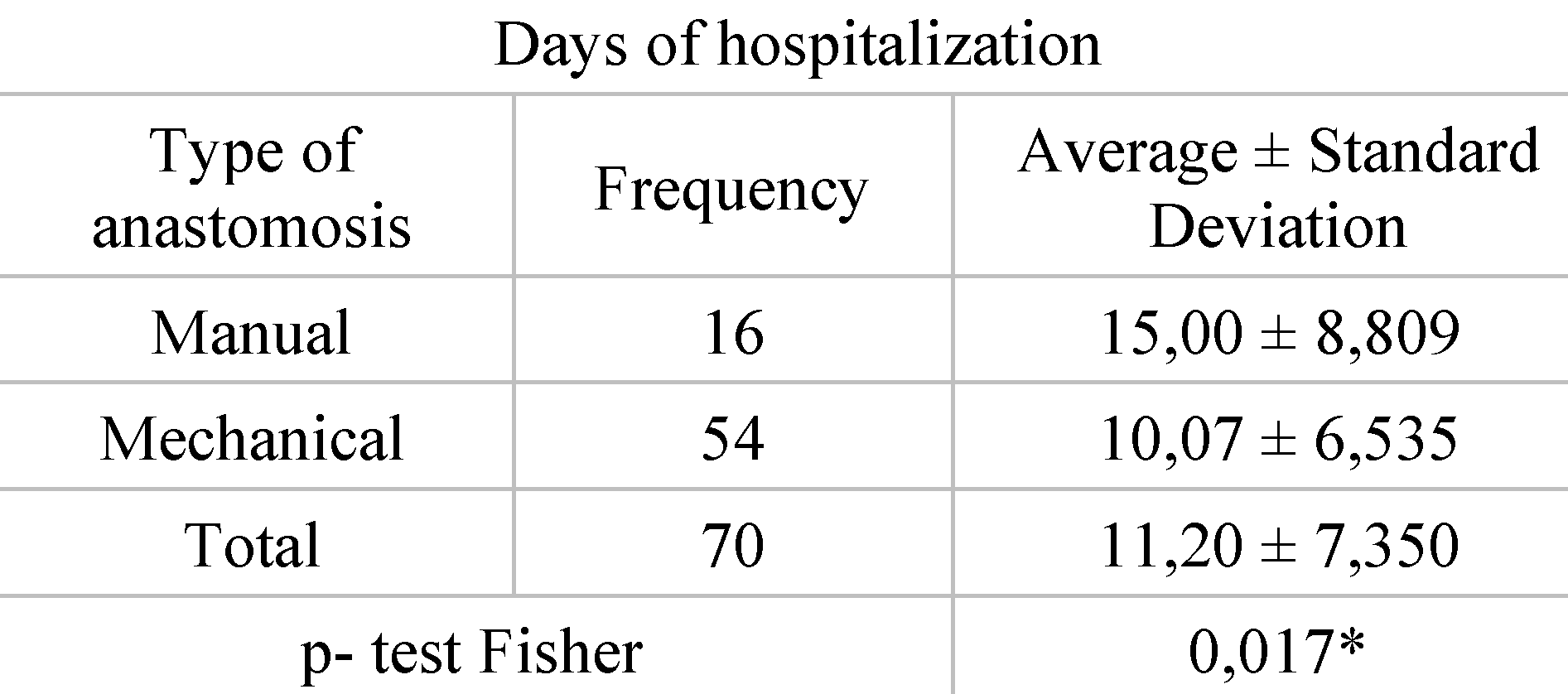
Distribution of patients according to type of anastomosis and length of hospitalization. Of the 54 patients who underwent mechanical anastomosis for rectal cancer, an average hospital stay of 10 days was registered in this study. At opposite end, for the 16 patients who underwent manual anastomosis for rectal cancer, the average length of hospitalization was approximately 15 days (
Table 7,
Figure 3).
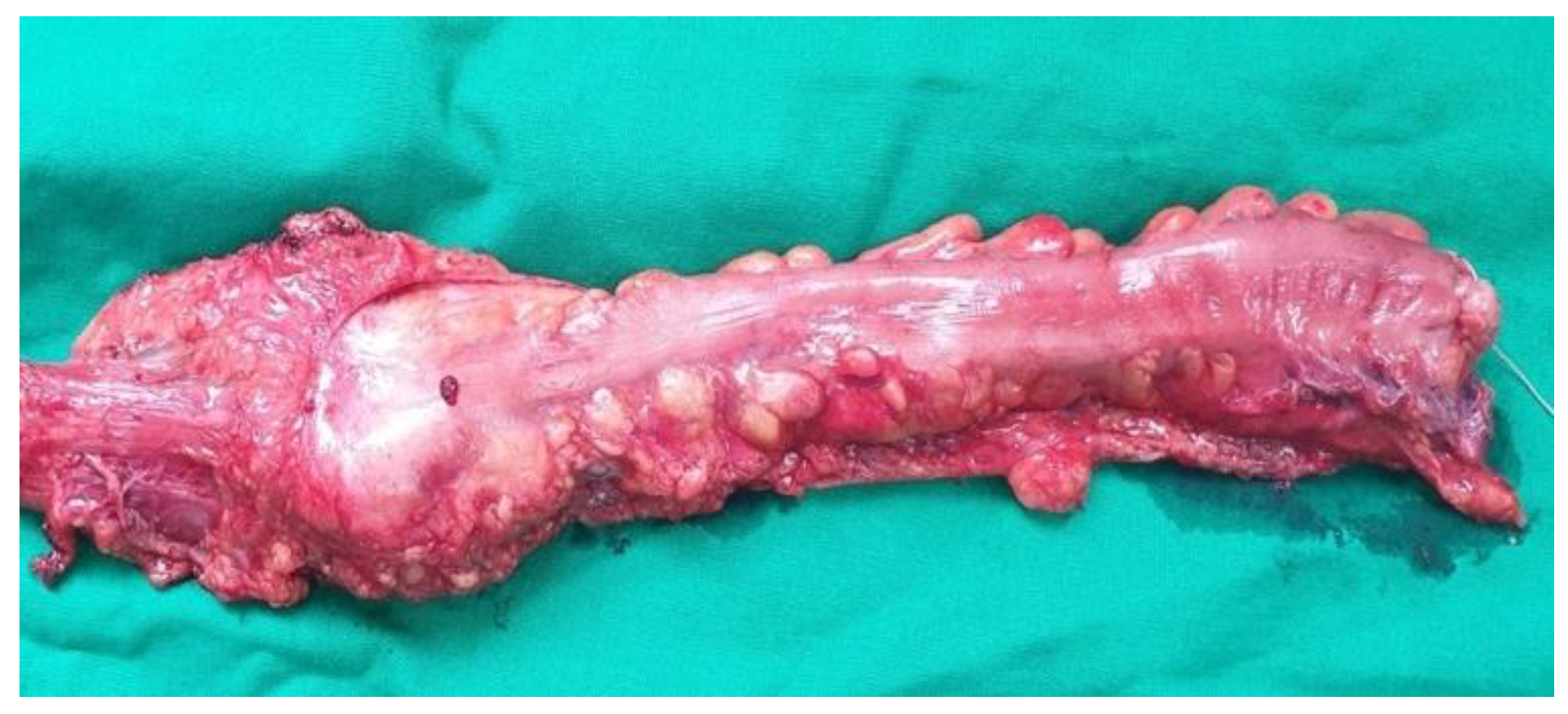
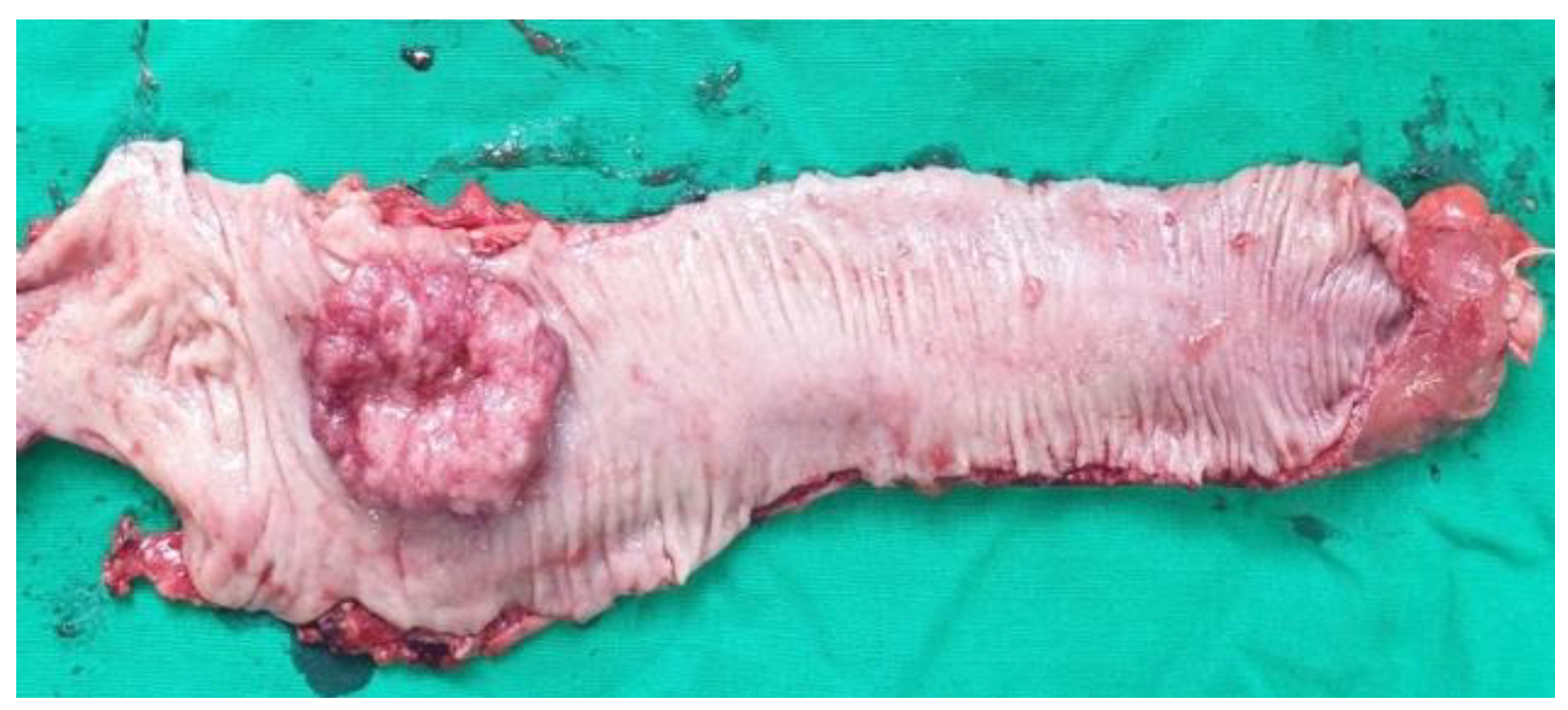
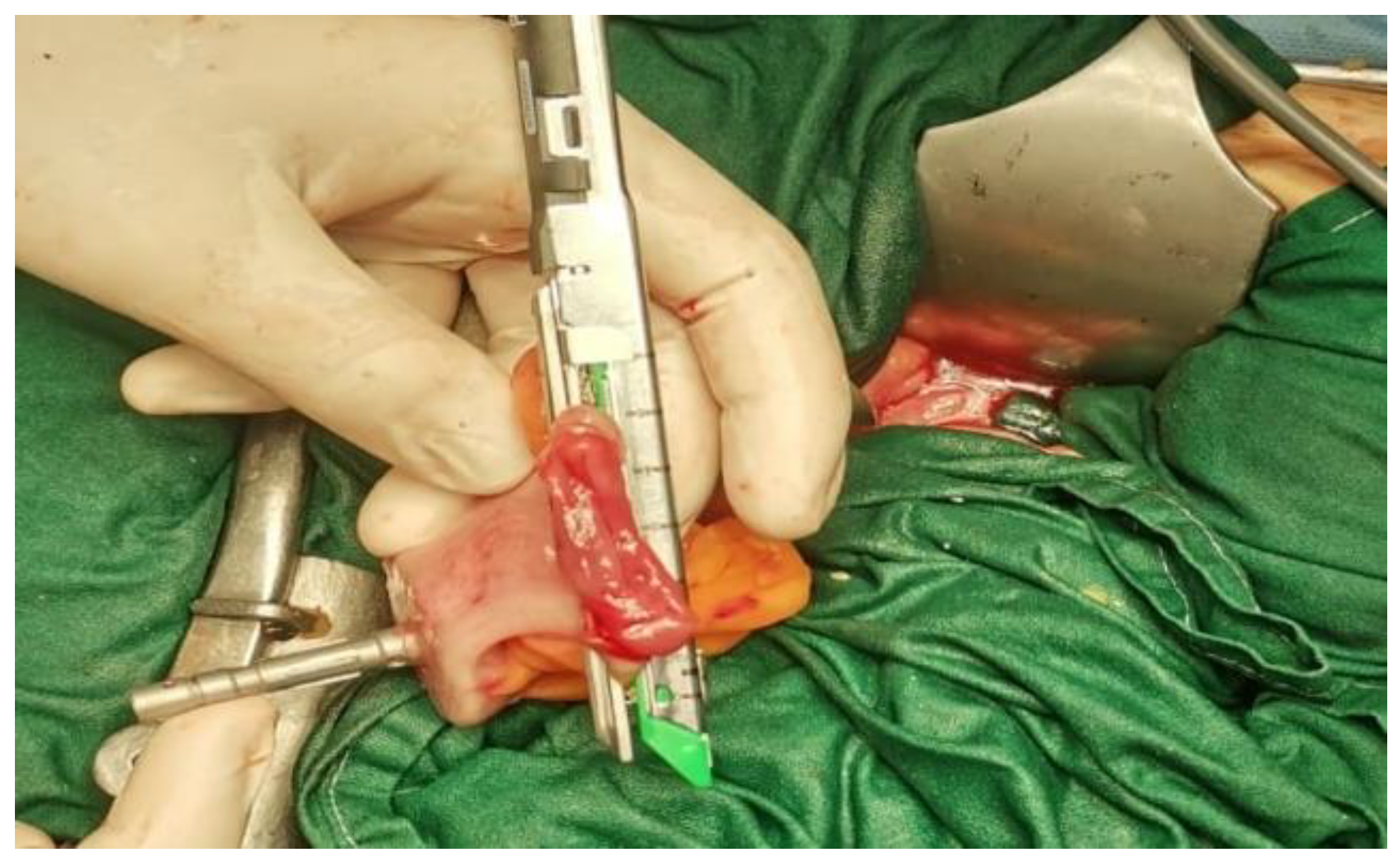
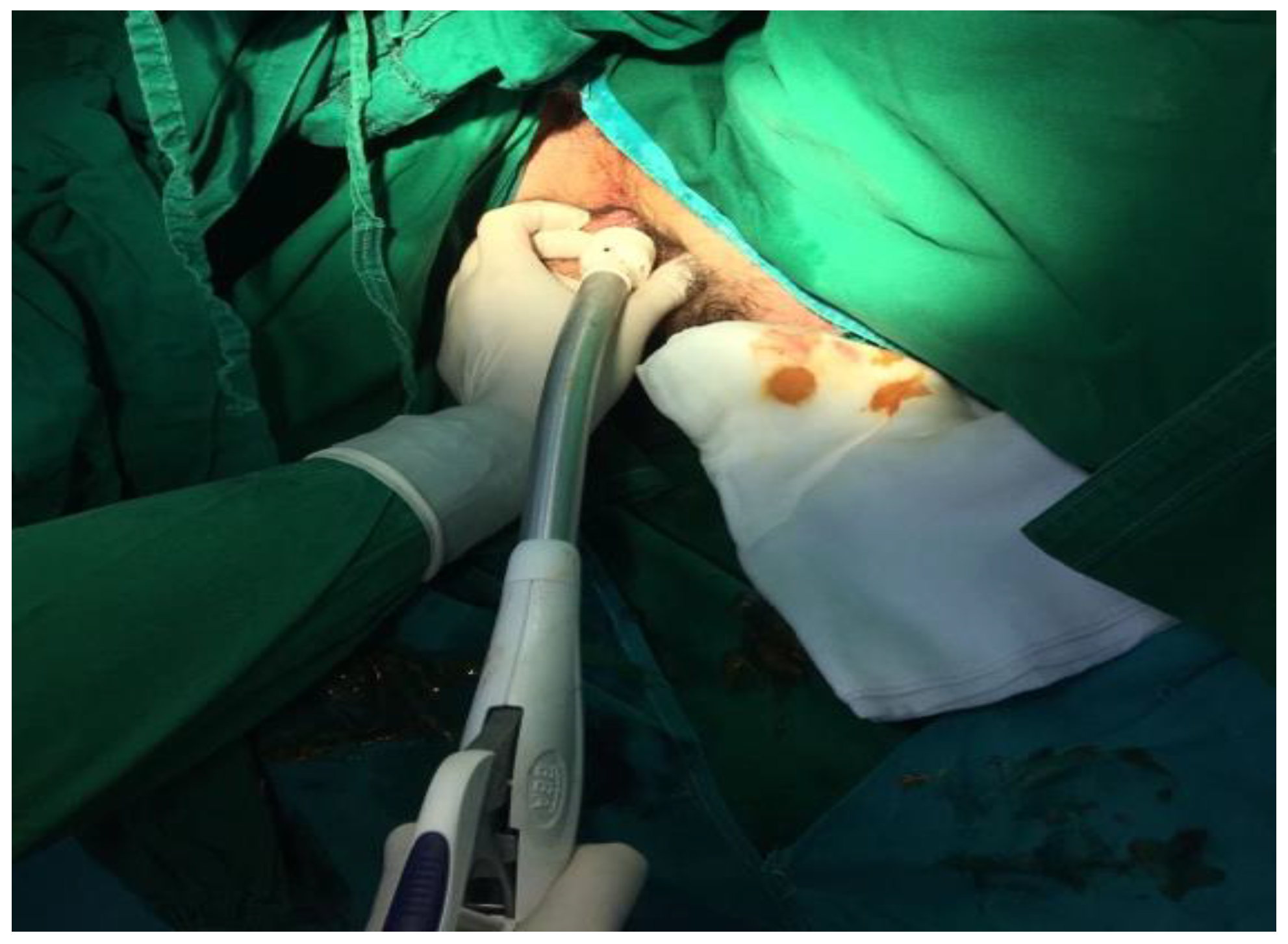
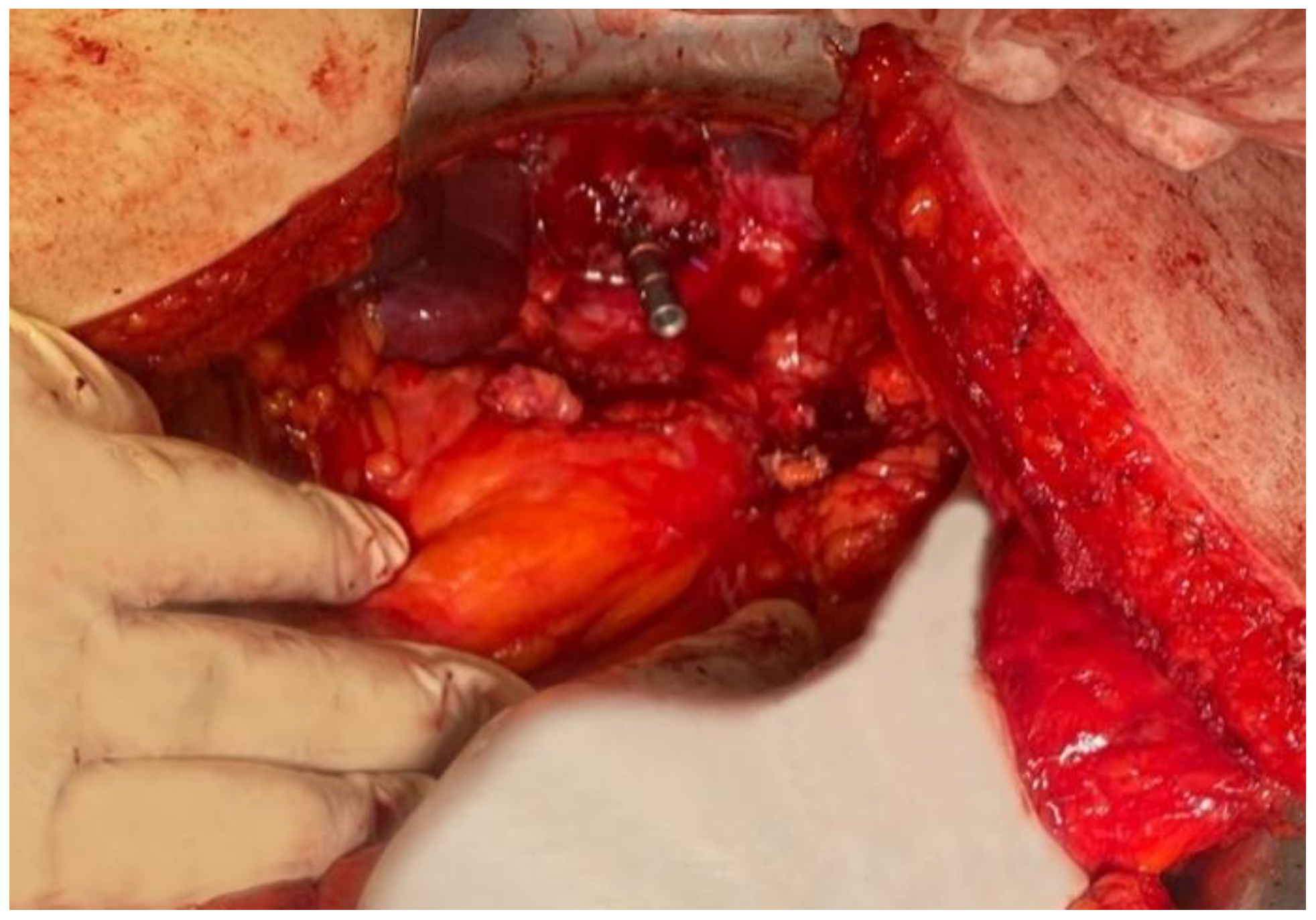
The data presented above are illustrated by several
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8, representing personal imaging data of Dr. Tănăsescu Ciprian from the Surgery Clinic I of the Sibiu County Emergency Clinical Hospital.
Such intervention is generally recommended for patients with upper and middle rectum pathologies. The main objectives of this procedure are the removal of the tumor with preservation of the anal sphincter (
Figure 6), when patients do not have pre-existing sphincter lesions or tumor extension in the pelvis [
8,
9,
10].
Descending-rectal anastomoses were performed for both mechanical and manual sutures. In the case of mechanical anastomoses, latero-terminal anastomoses and linear stapler blunt anastomoses were performed, while in the case of manual anastomoses only termino-terminal anastomoses were performed (
Figure 7 and
Figure 8).
Discussions
In this study, 70 patients with rectal oncological pathology were selected, being admitted to the Surgery I ward of the Sibiu County Emergency Hospital from 2017–2022.
According to international treatment protocols, therapeutic possibilities in rectal cancer are largely influenced by the TNM stage. Thus, for stage 0 the treatment of choice is polyp removal or local excision.
For stage I, if the formation presents itself as a polyp, it is recommended to remove it through colonoscopy; if the histopathological examination shows elements of aggression (for example a high grade or positive margins), a more extensive surgical intervention is recommended. If the formation is not a polyp, then a partial colectomy is recommended [
11,
12].
Regarding stage II, international guidelines support the effectiveness of partial colectomy together with removal of nearby lymph nodes. In most cases, this intervention is sufficient, but in the presence of risk factors, the patient together with the physician evaluate the risks and benefits of adjuvant treatment [
11].
For stage III, partial colectomy, lymph node removal and adjuvant chemotherapy represent the standard. The most used techniques for stage II and III are the invasive procedures, namely low anterior resection and abdomino-perineal resection [
12]. Within these procedures, total mesorectal excision can be performed, leading to beneficial outcome for the oncological patient [
13].
In the case of patients with stage IV rectal cancer, curative surgical intervention is unlikely. In selected cases, for example in the case of oligometastatic patients, surgical removal of the main tumor and metastasectomy can be performed simultaneously. Other indications for surgery at this stage are with palliative intentions, for example for the desobstruction of the digestive tract. In most cases systemic therapy is the standard approach [
11,
12].
In the Dukes scale, TNM stage II includes stages A and B, and TNM stage III includes C, C1, and C2. For the modified Astler-Coller (MAC) classification method, TNM stage II comprises MAC stages B1, B2 and B3, and TNM stage III in the MAC scale means C1-C2 [
14].
The surgical technique used for the study patients is the Dixon operation. The Dixon technique or low anterior resection (LAR) became increasingly used with the publication in 1948 of a retrospective study conducted by Dixon [
15,
16]. The technique involves restoring the continuity of the digestive tube after the resection of the tumor portion, but situations are described in which the formation of a temporary colostomy or ileostomy is necessary [
17,
18].
Of particular importance in structuring a surgical therapeutic plan is the distance between the external anal orifice (EAO) and the location of the rectal tumor. Representing more than half of the cases, 46 patients (i.e., 66%), suffer from a superior localization of the rectal tumor, which is more than 12 cm away from the EAO. From the point of view of incidence, in second place is the subgroup of patients in whom the rectal tumor was diagnosed at a distance of 8 to 12 cm from the external anal opening. These data were recorded in 16 patients, representing 23%. The fewest interventions were performed for inpatients whose tumor, according to paraclinical data, was described as being located less than 8 cm from the EAO (lower rectum). Here we are talking about 8 patients, who represent the remaining 11% of the study group. Looking at the data in the literature, we note that our results do not coincide with them. The cited article, led by Cheng et al., describes the differences between upper, middle and lower rectal cancer. According to this publication, the incidence of rectal cancer localization in the upper third is the lowest (32.5%), being higher is in the rest the middle and lower thirds (67.5%) [
19]. Referring to the study published by Chiang et al. that analyzed the pattern of rectal cancer according to its location, we observe similar data to that of our work, namely the first-place incidence of upper rectal tumors [
20]. Another study on the upper location of rectal neoplasm, published by Gao et al. reports a higher incidence of lower location [
21]. Being a widely discussed topic, we decided to consult another source, this time referring to a study on complications following rectal cancer surgery conducted by Popa et al., which showed the lowest incidence among tumors with upper location, opposite results to the results of our work [
22].
As a result of the surgical possibilities, given the situation of each patient, out of the total of 70 cases operated on, 54 of them benefited from mechanical anastomoses, thus representing the major percentage, 77%. In about 23% of cases (16 patients), manual anastomoses were used. Also, regarding the choice of anastomosis type, we note that in 5 of the 6 years studied, mechanical anastomosis is preferred by surgeons over manual, reporting its incidence of 100% for the years 2020, 2021 and 2022 respectively. Such approach of the Surgery I ward is similar to that of the study published by Sciumè et al. When the anastomosis is possible, we observe in the study that took place in Palermo, a situation similar to the one presented by us. In both cases, the number of mechanical anastomoses predominates, these being almost twice as often used as the manual ones [
23]. The publication by Tudosie et al., focused on the differences between manual and mechanical anastomoses, highlights the preference of surgeons in favor of stapled sutures [
24].
Postoperatively, the 70 patients had different evolutions. The majority (71.4%, meaning 50 patients) had a favorable evolution, intestinal transit being resumed in a few days, the wound healing quickly and without the appearance of other complications. The remaining 20 patients, representing a percentage of 28.6%, had an unfavorable postoperative evolution, presenting various complications that we will return to in detail in the following lines. In a paper from China analyzing the discharge of patients with colorectal pathology undergoing surgery, published by Yang et al., we find results similar to those of the Surgery I department. In the mentioned article we find a percentage relatively close to ours, that is 80% of patients following surgery for rectal oncological pathology with a favorable evolution [
25]. It is mentioned that the percentage of complications is also influenced by the pandemic context, the batch having two cases in which patients became COVID positive in the postoperative period, a situation also reported in a study that analyzes the implications of post-operative coronavirus positivity, published by Prasad et al. [
26]. Due to the growing interest for COVID positive patients, various studies have been made on the management of these cases for oncological patients. The studies lead by Boicean et al. state that fecal microbiota transplantation on patients co-infected with COVID and Clostridioides difficile could be a choice for treatment and even have a positive impact on the response to chemotherapy in rectal cancer [
27,
28].
As mentioned above, 20 patients had some complications following surgery. The most frequent, with an incidence of 8.57%, was the development of postoperative fistula. Regarding the most common complication—fistula, a dreaded complication, our results are satisfactory. According to the paper on postoperative fistula, led by Elghamrini et al., we note that fistula is the most common complication [
29]. Referring to a study on mechanical sutures used in rectal cancer surgery, published by Cheregi et al., the occurrence of fistula is noted in about 22% of patients who underwent such surgery, more than double percentage being obtained in our study [
30]. In a study on fistula after sphincter-preserving surgery in rectal cancer, led by Zhou et al., the prevalence of postoperative fistula is 9.2%, a comparable percentage, even if it is slightly higher than ours [
31]. Of the total group studied, 6 patients died postoperatively, this complication representing 8.57% of cases. Comparing these results with the literature, that is the study on postoperative mortality following rectal cancer surgery coordinated by van Eeghen et al. (in which a 12% of patients died is reported), we observe more optimistic results in our study [
32]. In the paper on colectomy published by Visser, et al., they report the incidence of postoperative death as high as 15.8% [
33]. Another study, focusing on the impact of age on the success of surgery for rectal adenocarcinoma, led by Widdison et al., states that postoperative death has an incidence of up to 11% [
34]. Suppuration was present in 4 cases out of the whole group analyzed (5.71%). In comparison with a publication by Zhang et al. on the incidence and risk factors related to wound infection in colorectal surgery, which included 1046 patients, it shows the occurrence of suppuration in 7.1% of the cases [
35]. Suppuration has a lower incidence in our study. Evisceration was present in 2 cases, with a percentage representation of 2.85%. Comparing the data of our study with that of medical literature conducted by Bayar et al., we observe a lower incidence of evisceration among the patients of our study. The publication reports an incidence of evisceration of 11.5% [
36].
Correlating the type of anastomosis and the incidence of fistula, it shows an incidence of 7.40% for those who had mechanical anastomosis and 12.5% for those who had manual anastomosis. Referring to the study on mechanical sutures in rectal neoplasm surgery published by Cheregi et al., we observe similar data, fistula having an incidence of 8.16% for mechanical anastomosis and 13.8% for manual anastomosis [
30]. Another study on the benefits of using mechanical sutures, led by Coroș et al., shows low incidence of fistula among mechanical anastomoses, reporting a rate of 2.56% [
37].
In terms of length of hospitalization, it is shown that mechanical anastomoses shorten the period of hospitalization, which is on average 10 days versus a 15-day interval for patients with mechanical anastomoses. Looking at the most significant publication, we found that Rogers et al. describe a study from several countries in Europe that looked at length of hospitalization for patients undergoing surgery for rectal neoplasm. This study reported at the European level a hospital stays of more than 10 days for most patients [
38]. Comparing the data of the current study with those of the mentioned publication, we observe a beneficial contrast, namely the fact that the patients of this paper, most of them benefiting from mechanical anastomoses, required a shorter hospitalization than that reported in the specialized literature. Referring to the publication by Liu et al. on the differences between mechanical and manual sutures used in operations for gastrointestinal tumors, we observe results identical to ours, namely that mechanical anastomosis is associated with a shorter hospital stay [
39]. The study dedicated to analyzing the length of hospital stay following colorectal resection cancer, published by Kelly et al., notes a length of hospital stay (between 14 and 21 days) [
40] with longer periods than those obtained in our study card.