Abstract
Background. Colorectal cancer is a real public health issue, with high morbidity and severe impact on quality of life. Although mortality from this type of cancer is decreasing due to modern diagnostic and treatment methods, the understanding of its genetic and molecular mechanisms is important to develop a broader range of diagnostic and therapeutic approaches. Genetic therapy is an important strategy in cancer treatment, and the matrix Gla protein (MGP) gene expression has been described in numerous studies as increased in tumour pathology. In this article, we have summarized the currently available evidence on the connection between MGP and colorectal cancer. Materials and Methods. Following the PRISMA guidelines, we have searched the PubMed, ProQuest and ScienceDirect databases for relevant published works that studied the connection between colorectal cancer and MGP gene expression. Results: Three relevant works were included in this systematic review. Two of these studies have observed MGP gene overexpression in tumour cells, a result that contradicts the third study, where the MGP gene was underexpressed. Conclusions: The data provided by these articles is contradictory, and therefore more studies are needed on larger sets of subjects, to fully understand the connection between MGP and colorectal cancer.
Introduction
According to the WHO, colorectal cancer is the third most common type of cancer in men and the second most common type of cancer in women worldwide. Regarding mortality, colorectal cancer is the second leading cause of death due to cancer worldwide [1]. However, mortality related to this type of cancer is decreasing [2] due to the evolution of early diagnostic techniques such as the Guaiac-based fecal occult blood test, colonoscopy, barium enema, but also due to early polyp resection. Nevertheless, the understanding of the molecular mechanisms of this type of cancer could lead to new therapeutic targets, helping develop personalized therapies, especially for cancers diagnosed in advanced stages.
The matrix Gla protein (MGP) is a vitamin K-dependent protein (VKDP). However, it does not play a part in blood clotting and is secreted extrahepatically [3], especially by chondrocytes and vascular smooth muscle cells [4]. MGP is produced in an inactive form and requires vitamin K to be activated. Vitamin K, through the gamma-carboxylation reaction, transforms the glutamic acid (Glu) found in MGP into gamma-carboxyglutamic acid (Gla) and is thus a cofactor in activating MGP [5]. MGP is a protein with a molecular weight of 14.7 kDa and contains 84 amino acids (Figure 1). It contains nine glutamic acid residues [6]. Out of these, five undergo gamma-carboxylation, namely positions 2, 37, 41, 48 and 52. Two cysteine residues are in positions 54 and 60 and they form a disulfide bond. In addition, there are three serine residues in positions 3, 6 and 9, which are phosphorylated during the protein activation reactions.
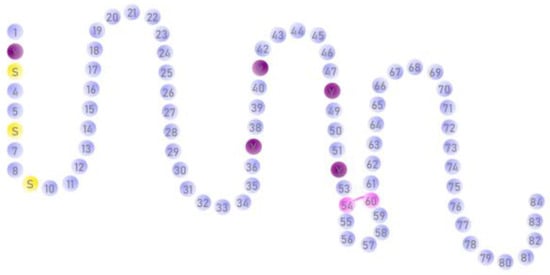
Figure 1.
Primary structure of the matrix Gla protein, which contains five gamma-carboxy glutamate (Gla) residues at positions 2, 37, 41, 48 and 52, three serine residues at position 3, 6 and 9, that are phosphorylated during the process of protein activation, and two cysteine residues at positions 54 and 60, forming a disulfide bond.
Due to the presence of the five Gla residues in its structure, MGP has a high affinity to Ca ions, which play an important part in mediating the activity of the various proteins and enzymes [5]. The most important role of MGP is the inhibition of vascular calcification [7]. However, numerous studies have outlined an aberrant expression of the MGP gene in tumour pathology. The role played by MGP in tumorigenesis is not entirely known, but some studies prove its role in tumor angiogenesis [8,9]. A study conducted by Chen et al. [10] in 1990 observed an overexpression of the MGP gene in malignant breast cells, compared to normal breast cells. Two years later, an increased expression of MGP in kidney, testicular and prostate cancer tumour cells was described in an article published by Levedakou et al. [11]. These two articles led to a series of studies that suggest there might be a connection between MGP gene expression and tumor pathology [4], which could significantly contribute to the development of personalized cancer treatment strategies.
The aim of this article is to conduct a systematic review of current literature assessing the relationship between MGP and colorectal cancer, and whether MGP could be considered a biomarker of colorectal cancer.
Materials and Methods
This review was conducted following PRISMA guidelines [12].
Database search strategy
Searches were conducted in the PubMed, ScienceDirect and ProQuest databases using the following key words: MGP, Gla matrix protein, colon cancer/carcinoma, colorectal cancer/carcinoma. The exact search formula in the PubMed database was: ((MGP[Title/Abstract]) OR (Gla matrix [Title/Abstract])) AND ((colon cancer [Title/Abstract]) OR (colorectal cancer [Title/Abstract]) OR (colon carcinoma [Title/Abstract]) OR (colorectal carcinoma [Title/Abstract])) NOT (hepatic OR hepatocellular).
Equivalent formulas were used for the other databases, following the specific search criteria of each database. In addition, we have also checked the references of the articles selected for the study, in order to add any other relevant papers.
Inclusion and exclusion criteria
We included English language articles on studies conducted on human subjects that included the assessment of MGP expression in colorectal cancer.
We excluded studies conducted on animals, studies assessing MGP in relation to other types of digestive cancer, and those studying the relationship between other biomarkers or the expression of other genes and colorectal cancer.
Data collection and analysis
Two authors participated in data collection and analysis. Both authors worked independently and noted their reasons for including or excluding articles from the study. The data extracted and described from each eligible study was the type of study, characteristics of study participants, tumour characteristics and MGP expression in malignant cells compared to normal cells. Due to the small number of studies available, we were unable to compile them into a meta-analysis, and therefore a narrative description of each study was provided.
Results
Article search
Using the search strategy outlined above, we found 787 studies (Figure 2). After eliminating duplicates, we reviewed the title and abstract of each remaining article and found nine possibly eligible studies. Out of these, three met the eligibility criteria after reading the full study. After reviewing the references of these articles, we found no additional studies to complete this review.
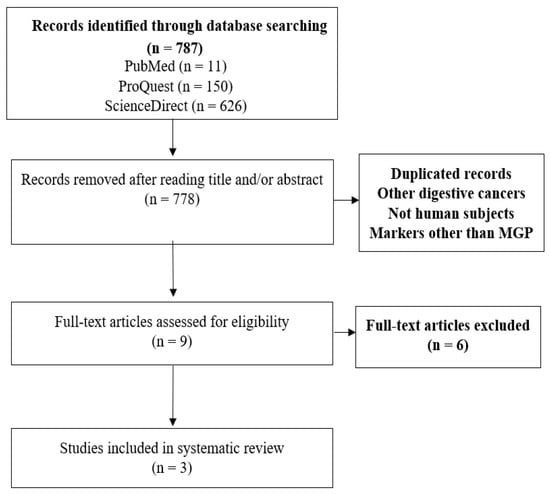
Figure 2.
Study selection diagram following PRISMA guidelines.
Characterization of studies
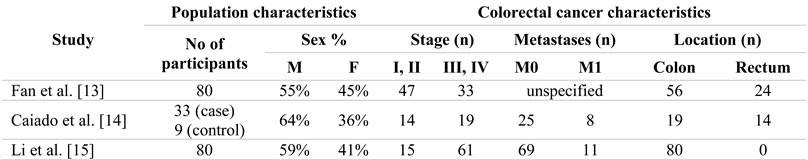
All three studies [13,14,15] are observational studies. Two of them are cross-sectional studies and one is a case-control study [14]. All the studies involved biopsies from the colon or rectal tumour tissue and healthy tissue located at a distance from the tumour, and the comparison of MGP expression between the resulting tissue pairs. For the case-control study, biopsies were also collected for nine healthy subjects, in order to assess MGP gene expression in the control group compared to the normal tissues of the case group. Table 1 summarizes the studies considered for this review.

Table 1.
Summary of studies.
In two of the studies samples were collected post-surgery [13,15], while Caiado et al. [14] collected biopsies during colonoscopy, directly from the tumour tissue and from healthy tissue located at least 5 cm away from the tumour. The histological type of tumour was adenocarcinoma in all three studies. MGP gene expression was assessed by Caiado et al. [14] both through RNA extraction and through immunohistochemistry. Li et al. [15] used only the immunohistochemistry technique, while Fan et al. [13] conducted RNA extraction.
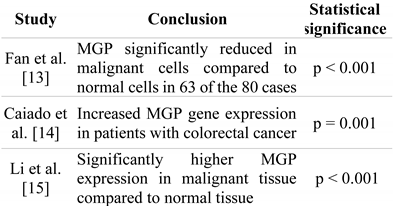
Conclusions of studies
The study conducted in 2001 [13] concluded that MGP gene expression was significantly reduced in the tumour tissue compared to the healthy tissue in 63 of the 80 cases (79%). The other two studies observed the opposite, namely that MGP gene expression is significantly increased in the neoplastic tissue, compared to the healthy tissue (Table 2). Moreover, in the case-control study, no statistically significant difference was found between MGP gene expression in the healthy tissue of patients in the case group compared to the colon tissue of patients in the control group.

Table 2.
Main conclusions of the studies regarding MGP gene expression in patients with colorectal cancer, in tumour tissues compared to normal tissues.
Two of the studies observed that MGP gene expression is not correlated with the stage of the disease nor with distance metastases. However, the most recent study [15] observed that the more advanced the disease, the more significant MGP gene expression is. Regarding prognosis, two of the studies [14,15] noted that a more important MGP gene expression points to a worse prognosis and decreases survival rates.
Discussions
Studies conducted in recent years concluded that the matrix Gla protein is involved in tumour pathology [4]. MGP gene expression has been studied in multiple types of cancer, but its role is not yet understood. Some studies have shown that in some types of cancer the MGP gene is underexpressed [13,16]. Other studies provide contradictory data showing that the MGP gene is overexpressed [17,18,19], and it has been hypothesized that this overexpression contributes to cell invasion and proliferation, as well as physiological angiogenesis, by inducing the expression of the vascular endothelial growth factor (VEGF) [20], and tumour angiogenesis, proving the direct correlation between MGP expression and tumour vascularization [8]. This study is the first systematic literature review studying the connection between MGP and colorectal cancer. The main limitation of this study is the small number of articles available to date. More studies are needed, on larger groups of participants, representing more histopathological tumour types, in various stages, to establish whether the matrix Gla protein could be considered a marker in colorectal cancer detection and prognosis, as well as to develop an efficient therapeutic target.
Another important limitation of this study is that all articles included studied MGP gene expression but not the serum concentration levels of this protein. One study [21] published in 2016, conducted on 22 pediatric patients with osteosarcoma, concluded that there is an important correlation between MGP serum levels and the development of pulmonary metastases. Therefore, MGP serum levels could be used as a prognosis marker, but also as a therapeutic target. Another aspect to consider for future studies could be MGP carboxylation. As previously mentioned, MGP is secreted in inactive form (noncarboxylated), and it is activated through the carboxylation of Glu amino acids, rendering it capable of binding Ca ions. It is, however, important to study the role of MGP in tumour pathology both in its active and inactive form.
It is also important to mention that the results obtained by Fan et al. [13] contradict the results of the other two studies. Factors that could explain this include the different techniques used to assess MGP gene expression, tumour location, stage, as well as the relatively small number of patients included in each study.
Regarding the mechanism through which the matrix Gla protein or its gene expression influences tumour pathology, it is still not fully understood, but some studies have proposed several theories. First, MGP promotes cellular proliferation of colon tumors through the direct and indirect activation of NG-kB [15], due to the increase in the intracellular concentration of Ca ions. Second, MGP interacts with fibronectin, vitronectin and elastin [22,23,24], all components of the extracellular matrix, modulating intracellular communication and promoting cellular proliferation. Although MGP does not modulate cellular binding, tumour cells attach and proliferate better in an environment containing fibronectin and MGP, compared to fibronectin on its own [22]. In addition, MGP binds integrins to fibronectin, which activates intracellular signaling pathways, resulting in the reorganization of the cytoskeleton, increased antiapoptotic molecule secretion and decreased proapoptotic molecule secretion. Therefore, MGP may be a factor that delays apoptosis in tumor cells [25,26].
Matrix Gla protein appears to also be involved in both physiological and tumour angiogenesis. MGP thus stimulates endothelial cell differentiation [25,26,27] and VEGF-A secretion, leading to increased proliferation and migration of endothelial cells as well as tube formation [20]. In addition, in a study published in 2010 [8], conducted on glioblastoma xenografts, it was observed that those tumour cell cultures that express high levels of inhibitor of differentiation-4 (ID-4) produce grafts that are better vascularized than the control group. Similarly, cultures expressing high levels of ID-4 also present high levels of MGP, and researchers have proposed the hypothesis that the MGP gene is a target for ID-4, and its overexpression promotes angiogenesis. It was concluded that high levels of ID-4 and MGP are directly involved in tumour angiogenesis and these levels could represent therapeutic targets for developing new treatments.
Matrix Gla protein can influence the metastatic dissemination of some lung tumors. In the case of osteosarcoma [21], MGP overexpression has been observed to lead to a significant increase in metastasis, by altering endothelial cell adhesion, facilitating trans- endothelial migration and the extravasation of lung tumour cells. This mechanism is independent of the carboxylation of the glutamic acid in MGP.
Moreover, MGP can contribute to tumour resistance to chemotherapy [28]. An increase in MGP expression was observed in Topotecan and Paclitaxel resistant ovarian tumors [24,25,26]. By modelling cell interaction with cellular matrix components, researchers have demonstrated that MGP is an important contributing factor in cancer resistance to chemotherapy. They therefore consider that MGP could be a new therapeutic target in chemotherapy resistant tumors.
Although the mechanisms through which MGP influences tumour pathology are not fully understood, it appears that it could influence tumour development in all stages (Figure 3).
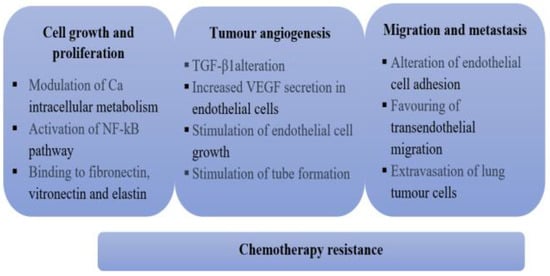
Figure 3.
Mechanisms through which MGP is involved with tumour pathology.
Conclusions
Based on the numerous mechanisms by which matrix Gla protein seems to be involved in tumorigenesis and cancer progression, it could be a new marker for colorectal cancer. However, data in the literature published to date is contradictory and inconsistent, and therefore more studies on larger groups of subjects are needed to evaluate this protein as a potential marker for colorectal cancer diagnosis and prognosis.
Matrix Gla protein gene expression has been studied in multiple tumour pathologies. However, the serum level of the MGP has not been studied sufficiently. There are some studies that explain the possible mechanisms through which the MGP influences tumour evolution, but these mechanisms are not fully understood.
Therefore, more studies are needed to establish whether this protein can be considered a new prognosis marker in tumour pathology.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Conflict of interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Przegla̜d Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Cancela, M.L.; Laizé, V.; Conceição, N. Matrix Gla protein and osteocalcin: From gene duplication to neofunctionalization. Arch. Biochem. Biophys. 2014, 561, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, S.R.; Crăciun, A.M. Matrix Gla protein in tumoral pathology. Med. Pharm. Rep. 2016, 89, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Urist, M.R.; Otawara, Y. Matrix Gla protein, a new γ-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem. Biophys. Res. Commun. 1983, 117, 765–771. [Google Scholar] [CrossRef]
- Wei, F.-F.; Trenson, S.; Verhamme, P.; Vermeer, C.; Staessen, J.A. Vitamin K–Dependent Matrix Gla Protein as Multifaceted Protector of Vascular and Tissue Integrity. Hypertension 2019, 73, 1160–1169. [Google Scholar] [CrossRef]
- Theuwissen, E.; Smit, E.; Vermeer, C. The Role of Vitamin K in Soft-Tissue Calcification. Adv. Nutr. Int. Rev. J. 2012, 3, 166–173. [Google Scholar] [CrossRef]
- Kuzontkoski, P.M.; Mulligan-Kehoe, M.J.; Harris, B.T.; A Israel, M. Inhibitor of DNA binding-4 promotes angiogenesis and growth of glioblastoma multiforme by elevating matrix GLA levels. Oncogene 2010, 29, 3793–3802. [Google Scholar] [CrossRef]
- Sharma, B.; Albig, A.R. Matrix Gla protein reinforces angiogenic resolution. Microvasc. Res. 2012, 85, 24–33. [Google Scholar] [CrossRef]
- Chen, L.; Obryan, J.; Smith, H.; Liu, E. Overexpression of matrix gla protein messenger-rna in malignant human breast cells - isolation by differential cdna hybridization. Oncogene 1990, 5, 1391–1395. [Google Scholar]
- Levedakou, E.N.; Strohmeyer, T.G.; Effert, P.J.; Liu, E.T. Expression of the matrix Gla protein in urogenital malignancies. Int. J. Cancer 1992, 52, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-W.; Sheu, D.-L.; Fan, H.-A.; Hsu, K.-C.; Chang, C.A.; Chan, E.-C. Down-regulation of matrix Gla protein messenger RNA in human colorectal adenocarcinomas. Cancer Lett. 2001, 165, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Caiado, H.; Conceição, N.; Tiago, D.; Marreiros, A.; Vicente, S.; Enriquez, J.L.; Vaz, A.M.; Antunes, A.; Guerreiro, H.; Caldeira, P.; et al. Evaluation of MGP gene expression in colorectal cancer. Gene 2020, 723, 144120. [Google Scholar] [CrossRef]
- Li, X.; Wei, R.; Wang, M.; Ma, L.; Zhang, Z.; Chen, L.; Guo, Q.; Guo, S.; Zhu, S.; Zhang, S.; et al. MGP Promotes Colon Cancer Proliferation by Activating the NF-κB Pathway through Upregulation of the Calcium Signaling Pathway. Mol. Ther. - Oncolytics 2020, 17, 371–383. [Google Scholar] [CrossRef]
- Tiago, D.M.; Conceição, N.; Caiado, H.; Laizé, V.; Cancela, M.L. Matrix Gla protein repression by miR-155 promotes oncogenic signals in breast cancer MCF-7 cells. FEBS Lett. 2016, 590, 1234–1241. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Chen, Y.; Wei, R.; Guo, Q.; Zhu, S.; Guo, S.; Zhu, S.; Zhang, S.; Min, L. Intracellular matrix Gla protein promotes tumor progression by activating JAK2/STAT5 signaling in gastric cancer. Mol. Oncol. 2020, 14, 1045–1058. [Google Scholar] [CrossRef]
- Mertsch, S.; Schurgers, L.J.; Weber, K.; Paulus, W.; Senner, V. Matrix gla protein (MGP): an overexpressed and migration-promoting mesenchymal component in glioblastoma. BMC Cancer 2009, 9, 302–302. [Google Scholar] [CrossRef]
- Fu, M.-H.; Wang, C.-Y.; Hsieh, Y.-T.; Fang, K.-M.; Tzeng, S.-F. Functional Role of Matrix gla Protein in Glioma Cell Migration. Mol. Neurobiol. 2017, 55, 4624–4636. [Google Scholar] [CrossRef]
- Boström, K.; Zebboudj, A.F.; Yao, Y.; Lin, T.S.; Torres, A. Matrix GLA Protein Stimulates VEGF Expression through Increased Transforming Growth Factor-β1 Activity in Endothelial Cells. J. Biol. Chem. 2004, 279, 52904–52913. [Google Scholar] [CrossRef]
- Zandueta, C.; Ormazábal, C.; Perurena, N.; Martínez-Canarias, S.; Zalacaín, M.; Julián, M.S.; Grigoriadis, A.E.; Valencia, K.; Campos-Laborie, F.J.; Rivas, J.D.L.; et al. Matrix-Gla protein promotes osteosarcoma lung metastasis and associates with poor prognosis. J. Pathol. 2016, 239, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.K.; Nishimoto, M. Matrix Gla Protein Binds to Fibronectin and Enhances Cell Attachment and Spreading on Fibronectin. Int. J. Cell Biol. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tudosie, M.; Pauna, A.; Stefani, C.; Staicu, I. Diet and Food chemicals increasing the risk of colorectal cancer – literature review. J. Mind Med Sci. 2022, 9, 118–124. [Google Scholar] [CrossRef]
- Nishimoto, S.; Nishimoto, M. Matrix Gla protein C-terminal region binds to vitronectin. Co-localization suggests binding occurs during tissue development. Matrix Biol. 2005, 24, 353–361. [Google Scholar] [CrossRef]
- Güngören, F.; Erol, C.; Bilici, A.; Dayangaç, M.; Şeker, M.; Ölmez, Ö.; Yaprak, O.; Yıldız, Ö.; Öncel, M. The comparison of local tumor control after microwave ablation, surgical resection and combined treatment for colorectal liver metastases. J. Mind Med Sci. 2022, 9, 125–132. [Google Scholar] [CrossRef]
- Sterzyńska, K.; Klejewski, A.; Wojtowicz, K.; Świerczewska, M.; Andrzejewska, M.; Rusek, D.; Sobkowski, M.; Kędzia, W.; Brązert, J.; Nowicki, M.; et al. The Role of Matrix Gla Protein (MGP) Expression in Paclitaxel and Topotecan Resistant Ovarian Cancer Cell Lines. Int. J. Mol. Sci. 2018, 19, 2901. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Guihard, P.J.; Blazquez-Medela, A.M.; Guo, Y.; Liu, T.; Boström, K.I.; Yao, Y. Matrix Gla protein regulates differentiation of endothelial cells derived from mouse embryonic stem cells. Angiogenesis 2015, 19, 1–7. [Google Scholar] [CrossRef]
- Januchowski, R.; Zawierucha, P.; Ruciński, M.; Nowicki, M.; Zabel, M. Extracellular Matrix Proteins Expression Profiling in Chemoresistant Variants of the A2780 Ovarian Cancer Cell Line. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
© 2023 by the author. 2023 Mirela-Georgiana Perné, Lorena Ciumărnean, Olga-Hilda Orășan, Vasile Negrean, Teodora-Gabriela Alexescu, Mircea Vasile Milaciu, Ioana Roșca, Răzvan Dan Togănel, Gabriel Emil Petre, Lucia Procopcoiuc, Cristina Drugan, Alexandra Crăciun.