Detection and Quantitative Assessment of Arthroscopically Proven Long Biceps Tendon Pathologies Using T2 Mapping
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Inclusion
2.2. Study Design
2.3. MRI Protocol and T2 Mapping
2.4. Image Analysis and Definition of LBT Pathologies
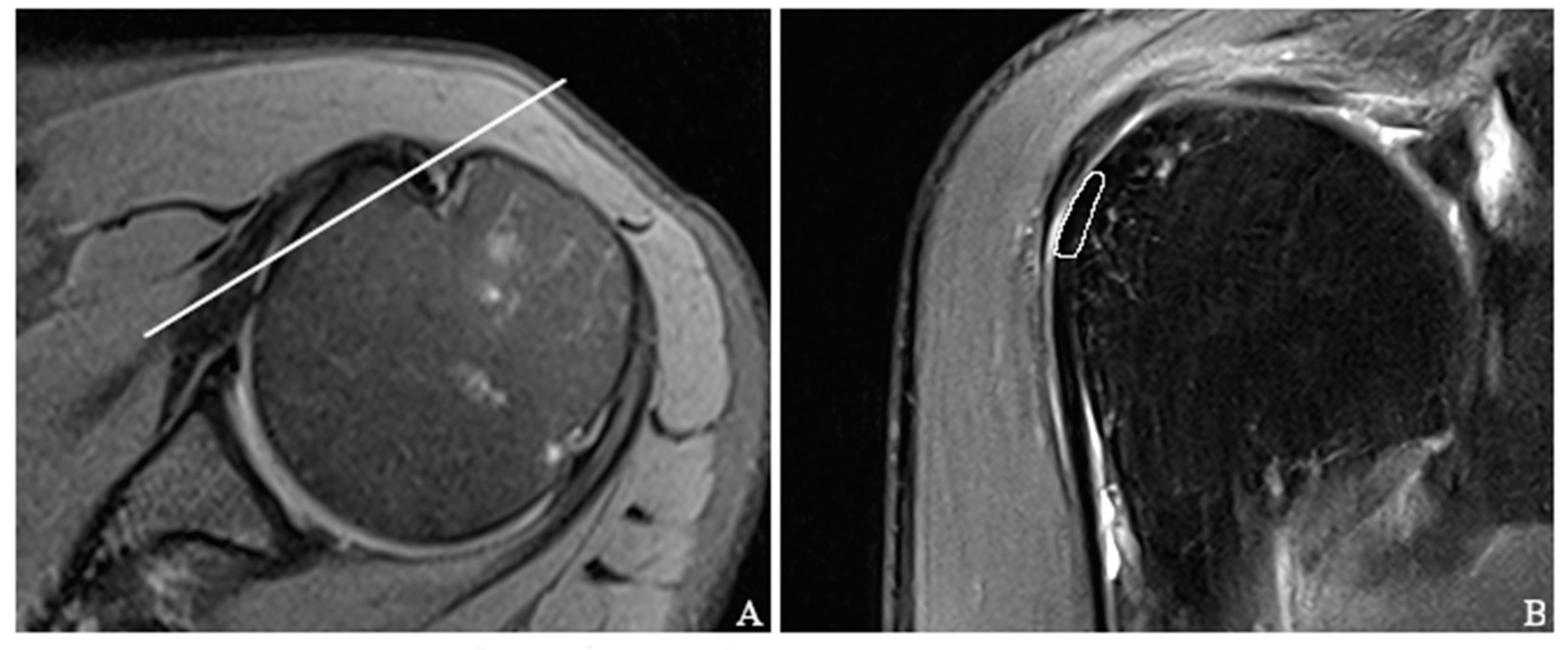
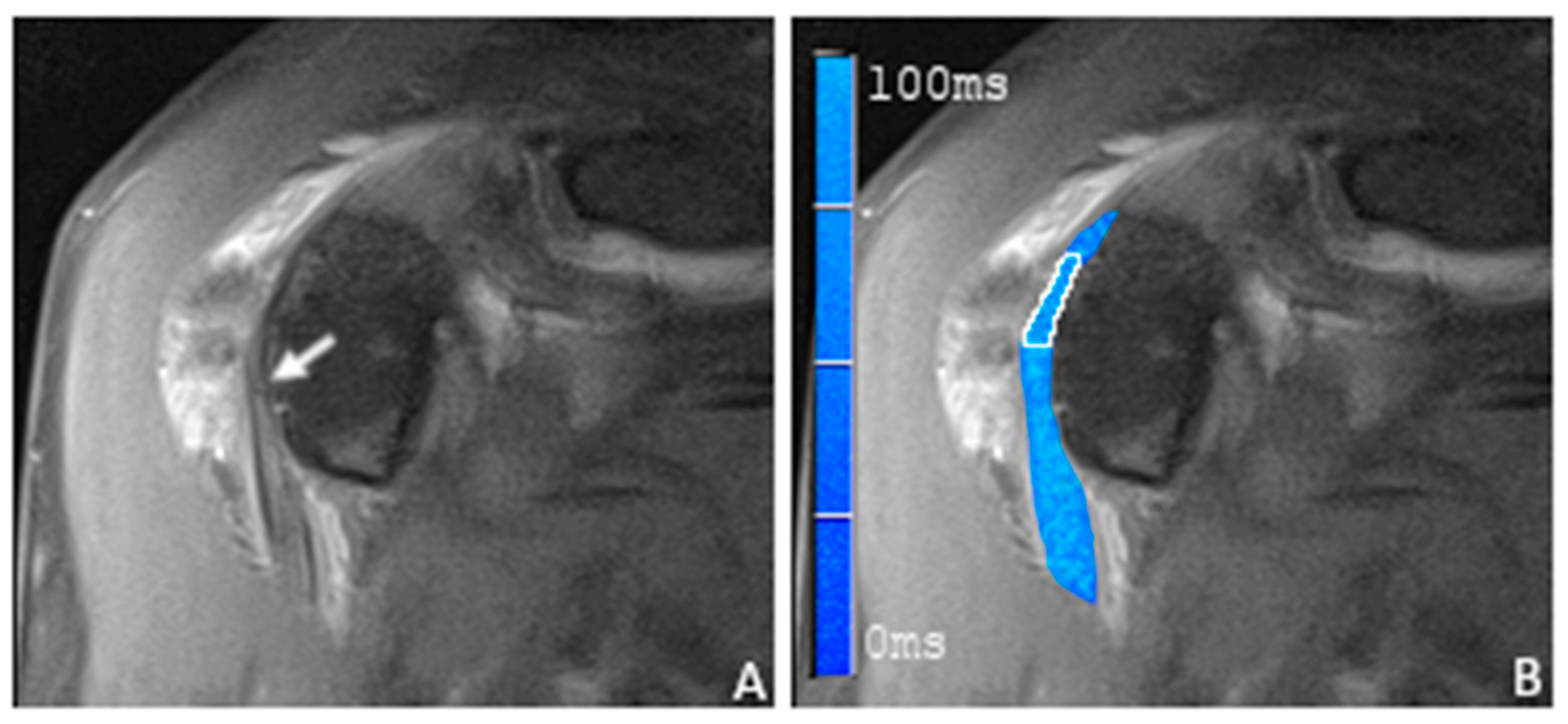
2.5. Placement of Regions of Interest
2.6. Arthroscopy
2.7. Statistical Analysis
3. Results
3.1. Demographics and Arthroscopic Evaluation of the Long Biceps Tendon
3.2. T2 Mapping of the Long Biceps Tendon
3.3. Inter- and Intra-Reader Agreement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gartsman, G.M.; Brinker, M.R.; Khan, M. Early Effectiveness of Arthroscopic Repair for Full-Thickness Tears of the Rotator Cuff: An Outcome Analysis. J. Bone Jt. Surg. Am. 1998, 80, 33–40. [Google Scholar] [CrossRef]
- Beaton, D.E.; Richards, R.R. Measuring Function of the Shoulder. A Cross-Sectional Comparison of Five Questionnaires. J. Bone Jt. Surg. Am. 1996, 78, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Raney, E.B.; Thankam, F.G.; Dilisio, M.F.; Agrawal, D.K. Pain and the Pathogenesis of Biceps Tendinopathy. Am. J. Transl. Res. 2017, 9, 2668–2683. [Google Scholar] [PubMed]
- Szabó, I.; Boileau, P.; Walch, G. The Proximal Biceps as a Pain Generator and Results of Tenotomy. Sport. Med. Arthrosc. Rev. 2008, 16, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.P.; Perkinson, S.G.; Ablove, R.H.; Tueting, J.L. Distal Biceps Tendon Ruptures. Am. J. Sport. Med. 2015, 43, 2012–2017. [Google Scholar] [CrossRef]
- Eakin, C.L.; Faber, K.J.; Hawkins, R.J.; Hovis, D.W. Biceps Tendon Disorders in Athletes. J. Am. Acad. Orthop. Surg. 1999, 7, 300–310. [Google Scholar] [CrossRef]
- Lee, R.W.; Choi, S.-J.; Lee, M.H.; Ahn, J.H.; Shin, D.R.; Kang, C.H.; Lee, K.W. Diagnostic Accuracy of 3T Conventional Shoulder MRI in the Detection of the Long Head of the Biceps Tendon Tears Associated with Rotator Cuff Tendon Tears. Skelet. Radiol. 2016, 45, 1705–1715. [Google Scholar] [CrossRef]
- Malavolta, E.A.; Assunção, J.H.; Guglielmetti, C.L.B.; de Souza, F.F.; Gracitelli, M.E.C.; Ferreira Neto, A.A. Accuracy of Preoperative MRI in the Diagnosis of Disorders of the Long Head of the Biceps Tendon. Eur. J. Radiol. 2015, 84, 2250–2254. [Google Scholar] [CrossRef]
- Baptista, E.; Malavolta, E.A.; Gracitelli, M.E.C.; Alvarenga, D.; Bordalo-Rodrigues, M.; Ferreira Neto, A.A.; de Barros, N. Diagnostic Accuracy of MRI for Detection of Tears and Instability of Proximal Long Head of Biceps Tendon: An Evaluation of 100 Shoulders Compared with Arthroscopy. Skelet. Radiol. 2019, 48, 1723–1733. [Google Scholar] [CrossRef]
- Rol, M.; Favard, L.; Berhouet, J. Diagnosis of Long Head of Biceps Tendinopathy in Rotator Cuff Tear Patients: Correlation of Imaging and Arthroscopy Data. Int. Orthop. 2018, 42, 1347–1355. [Google Scholar] [CrossRef]
- Dubrow, S.A.; Streit, J.J.; Shishani, Y.; Robbin, M.R.; Gobezie, R. Diagnostic Accuracy in Detecting Tears in the Proximal Biceps Tendon Using Standard Nonenhancing Shoulder MRI. Open Access J. Sport. Med. 2014, 5, 81–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Maeseneer, M.; Boulet, C.; Pouliart, N.; Kichouh, M.; Buls, N.; Verhelle, F.; De Mey, J.; Shahabpour, M. Assessment of the Long Head of the Biceps Tendon of the Shoulder with 3T Magnetic Resonance Arthrography and CT Arthrography. Eur. J. Radiol. 2012, 81, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Tadros, A.S.; Huang, B.K.; Wymore, L.; Hoenecke, H.; Fronek, J.; Chang, E.Y. Long Head of the Biceps Brachii Tendon: Unenhanced MRI versus Direct MR Arthrography. Skelet. Radiol. 2015, 44, 1263–1272. [Google Scholar] [CrossRef]
- Cook, J.L.; Purdam, C.R. Is Tendon Pathology a Continuum? A Pathology Model to Explain the Clinical Presentation of Load-Induced Tendinopathy. Br. J. Sport. Med. 2009, 43, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Riley, G. Chronic Tendon Pathology: Molecular Basis and Therapeutic Implications. Expert. Rev. Mol. Med. 2005, 7, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Rehnitz, C.; Klaan, B.; Burkholder, I.; von Stillfried, F.; Kauczor, H.-U.; Weber, M.-A. Delayed Gadolinium-Enhanced MRI of Cartilage (DGEMRIC) and T2 Mapping at 3T MRI of the Wrist: Feasibility and Clinical Application. J. Magn. Reson. Imaging 2017, 45, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Park, H.-J.; Kwon, H.-J.; Kim, M.S.; Choi, S.H.; Choi, Y.J.; Kim, E. T2 Relaxation Times of the Glenohumeral Joint at 3.0 T MRI in Patients with and without Primary and Secondary Osteoarthritis. Acta Radiol. 2015, 56, 1388–1395. [Google Scholar] [CrossRef]
- Nguyen, J.C.; Liu, F.; Blankenbaker, D.G.; Woo, K.M.; Kijowski, R. Juvenile Osteochondritis Dissecans: Cartilage T2 Mapping of Stable Medial Femoral Condyle Lesions. Radiology 2018, 288, 536–543. [Google Scholar] [CrossRef]
- Quatman, C.E.; Hettrich, C.M.; Schmitt, L.C.; Spindler, K.P. The Clinical Utility and Diagnostic Performance of Magnetic Resonance Imaging for Identification of Early and Advanced Knee Osteoarthritis. Am. J. Sport. Med. 2011, 39, 1557–1568. [Google Scholar] [CrossRef]
- Crema, M.D.; Roemer, F.W.; Marra, M.D.; Burstein, D.; Gold, G.E.; Eckstein, F.; Baum, T.; Mosher, T.J.; Carrino, J.A.; Guermazi, A. Articular Cartilage in the Knee: Current MR Imaging Techniques and Applications in Clinical Practice and Research. RadioGraphics 2011, 31, 37–61. [Google Scholar] [CrossRef]
- Eckstein, F.; Wirth, W.; Nevitt, M.C. Recent Advances in Osteoarthritis Imaging—The Osteoarthritis Initiative. Nat. Rev. Rheumatol. 2012, 8, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Kasar, S.; Ozturk, M.; Polat, A.V. Quantitative T2 Mapping of the Sacroiliac Joint Cartilage at 3T in Patients with Axial Spondyloarthropathies. Eur. Radiol. 2022, 32, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Buck, F.M.; Grehn, H.; Hilbe, M.; Pfirrmann, C.W.A.; Manzanell, S.; Hodler, J. Degeneration of the Long Biceps Tendon: Comparison of MRI with Gross Anatomy and Histology. AJR Am. J. Roentgenol. 2009, 193, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Anz, A.W.; Lucas, E.P.; Fitzcharles, E.K.; Surowiec, R.K.; Millett, P.J.; Ho, C.P. MRI T2 Mapping of the Asymptomatic Supraspinatus Tendon by Age and Imaging Plane Using Clinically Relevant Subregions. Eur. J. Radiol. 2014, 83, 801–805. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, S.; Qiao, Y.; Hu, Y.; Zhang, Y.; Qu, J.; Shen, Y.; Tao, H.; Chen, S. Quantitative T2 Mapping-Based Tendon Healing Is Related to the Clinical Outcomes during the First Year after Arthroscopic Rotator Cuff Repair. Knee Surg. Sport. Traumatol. Arthrosc. 2021, 29, 127–135. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W.; et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Radiology 2015, 277, 826–832. [Google Scholar] [CrossRef]
- Stein, P.; Wuennemann, F.; Schneider, T.; Zeifang, F.; Burkholder, I.; Weber, M.-A.; Kauczor, H.-U.; Rehnitz, C. 3-Tesla T2 Mapping Magnetic Resonance Imaging for Evaluation of SLAP Lesions in Patients with Shoulder Pain: An Arthroscopy-Controlled Study. J. Clin. Med. 2023, 12, 3109. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- Renner, N.; Kleyer, A.; Krönke, G.; Simon, D.; Söllner, S.; Rech, J.; Uder, M.; Janka, R.; Schett, G.; Welsch, G.H.; et al. T2 Mapping as a New Method for Quantitative Assessment of Cartilage Damage in Rheumatoid Arthritis. J. Rheumatol. 2020, 47, 820–825. [Google Scholar] [CrossRef]
- Simon, M.J.K.; Yeoh, J.; Nevin, J.; Nimmo, M.; Regan, W.D. Histopathology of Long Head of Biceps Tendon Removed during Tenodesis Demonstrates Degenerative Histopathology and Not Inflammatory Changes. BMC Musculoskelet. Disord. 2022, 23, 185. [Google Scholar] [CrossRef]
- Golditz, T.; Steib, S.; Pfeifer, K.; Uder, M.; Gelse, K.; Janka, R.; Hennig, F.F.; Welsch, G.H. Functional Ankle Instability as a Risk Factor for Osteoarthritis: Using T2-Mapping to Analyze Early Cartilage Degeneration in the Ankle Joint of Young Athletes. Osteoarthr. Cartil. 2014, 22, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Zarins, Z.A.; Bolbos, R.I.; Pialat, J.B.; Link, T.M.; Li, X.; Souza, R.B.; Majumdar, S. Cartilage and Meniscus Assessment Using T1rho and T2 Measurements in Healthy Subjects and Patients with Osteoarthritis. Osteoarthr. Cartil. 2010, 18, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Baum, T.; Joseph, G.B.; Karampinos, D.C.; Jungmann, P.M.; Link, T.M.; Bauer, J.S. Cartilage and Meniscal T2 Relaxation Time as Non-Invasive Biomarker for Knee Osteoarthritis and Cartilage Repair Procedures. Osteoarthr. Cartil. 2013, 21, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, H.; Lu, Y.; Jiang, M.; Chen, Z.; Xi, X.; Ding, X.; Yan, F. Diagnostic Value of T1ρ and T2 Mapping Sequences of 3D Fat-Suppressed Spoiled Gradient (FS SPGR-3D) 3.0-T Magnetic Resonance Imaging for Osteoarthritis. Medicine 2019, 98, e13834. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Paavola, M.; Paakkala, T.; Parkkari, J.; Järvinen, T.; Järvinen, M. Pathophysiologie Des Sehnenüberlastungsschadens. Radiologe 2002, 42, 766–770. [Google Scholar] [CrossRef]
- Kannus, P.; Józsa, L. Histopathological Changes Preceding Spontaneous Rupture of a Tendon. A Controlled Study of 891 Patients. J. Bone Jt. Surg. 1991, 73, 1507–1525. [Google Scholar] [CrossRef]
- Joseph, G.B.; Baum, T.; Alizai, H.; Carballido-Gamio, J.; Nardo, L.; Virayavanich, W.; Lynch, J.A.; Nevitt, M.C.; McCulloch, C.E.; Majumdar, S.; et al. Baseline Mean and Heterogeneity of MR Cartilage T2 Are Associated with Morphologic Degeneration of Cartilage, Meniscus, and Bone Marrow over 3years—Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2012, 20, 727–735. [Google Scholar] [CrossRef]
- Prasad, A.P.; Nardo, L.; Schooler, J.; Joseph, G.B.; Link, T.M. T1ρ and T2 Relaxation Times Predict Progression of Knee Osteoarthritis. Osteoarthr. Cartil. 2013, 21, 69–76. [Google Scholar] [CrossRef]
- Joseph, G.B.; Baum, T.; Carballido-Gamio, J.; Nardo, L.; Virayavanich, W.; Alizai, H.; Lynch, J.A.; McCulloch, C.E.; Majumdar, S.; Link, T.M. Texture Analysis of Cartilage T2 Maps: Individuals with Risk Factors for OA Have Higher and More Heterogeneous Knee Cartilage MR T2 Compared to Normal Controls—Data from the Osteoarthritis Initiative. Arthritis Res. Ther. 2011, 13, R153. [Google Scholar] [CrossRef]
- Baum, T.; Stehling, C.; Joseph, G.B.; Carballido-Gamio, J.; Schwaiger, B.J.; Müller-Höcker, C.; Nevitt, M.C.; Lynch, J.; McCulloch, C.E.; Link, T.M. Changes in Knee Cartilage T2 Values over 24 Months in Subjects with and without Risk Factors for Knee Osteoarthritis and Their Association with Focal Knee Lesions at Baseline: Data from the Osteoarthritis Initiative. J. Magn. Reson. Imaging 2012, 35, 370–378. [Google Scholar] [CrossRef]
- Wessling, D.; Herrmann, J.; Afat, S.; Nickel, D.; Othman, A.E.; Almansour, H.; Gassenmaier, S. Reduction in Acquisition Time and Improvement in Image Quality in T2-Weighted MR Imaging of Musculoskeletal Tumors of the Extremities Using a Novel Deep Learning-Based Reconstruction Technique in a Turbo Spin Echo (TSE) Sequence. Tomography 2022, 8, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Biology of Tendon Injury: Healing, Modeling and Remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar] [PubMed]
- Park, S.-Y.; Lee, S.H.; Lee, M.H.; Chung, H.W.; Shin, M.J. Changes in the T2 Value of Cartilage after Meniscus Transplantation over 1 Year. Eur. Radiol. 2017, 27, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Liess, C.; Lüsse, S.; Karger, N.; Heller, M.; Glüer, C.-C. Detection of Changes in Cartilage Water Content Using MRI T2-Mapping in Vivo. Osteoarthr. Cartil. 2002, 10, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Rehnitz, C.; Kupfer, J.; Streich, N.A.; Burkholder, I.; Schmitt, B.; Lauer, L.; Kauczor, H.-U.; Weber, M.-A. Comparison of Biochemical Cartilage Imaging Techniques at 3 T MRI. Osteoarthr. Cartil. 2014, 22, 1732–1742. [Google Scholar] [CrossRef]
- Mosher, T.J.; Smith, H.; Dardzinski, B.J.; Schmithorst, V.J.; Smith, M.B. MR Imaging and T2 Mapping of Femoral Cartilage. Am. J. Roentgenol. 2001, 177, 665–669. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nozaki, T.; Yu, H.; Chang, A.; Kaneshiro, K.; Schwarzkopf, R.; Hara, T.; Yoshioka, H. Normal T2 Map Profile of the Entire Femoral Cartilage Using an Angle/Layer-Dependent Approach. J. Magn. Reson. Imaging 2015, 42, 1507–1516. [Google Scholar] [CrossRef]
- Lockard, C.A.; Wilson, K.J.; Ho, C.P.; Shin, R.C.; Katthagen, J.C.; Millett, P.J. Quantitative Mapping of Glenohumeral Cartilage in Asymptomatic Subjects Using 3 T Magnetic Resonance Imaging. Skelet. Radiol. 2018, 47, 671–682. [Google Scholar] [CrossRef]
- Kang, Y.; Choi, J.-A. T2 Mapping of Articular Cartilage of the Glenohumeral Joint at 3.0 T in Healthy Volunteers: A Feasibility Study. Skelet. Radiol. 2016, 45, 915–920. [Google Scholar] [CrossRef]
- Baeßler, B.; Schaarschmidt, F.; Stehning, C.; Schnackenburg, B.; Maintz, D.; Bunck, A.C. A Systematic Evaluation of Three Different Cardiac T2-Mapping Sequences at 1.5 and 3T in Healthy Volunteers. Eur. J. Radiol. 2015, 84, 2161–2170. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, W.; Wang, F.; Wang, Y.; Wu, P.-Y.; Zhou, J.; Liu, H. Study of T2 Mapping in Quantifying and Discriminating Uterine Lesions under Different Magnetic Field Strengths: 1.5 T vs. 3.0 T. BMC Med. Imaging 2023, 23, 1. [Google Scholar] [CrossRef]



| No. | Sequence | Orientation | Repetition Time (TR; ms) | EchoTime (TE; ms) | Acquisition Matrix | Flip Angle | Echo Train Length | No. of Slices | TA (min) | Slices (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PD FS TSE | axial | 3660 | 24 | 384 × 346 | 176 | 7 | 27 | 04:32 | 3 |
| 2 | PD FS TSE | oblique coronal | 2490 | 24 | 384 × 307 | 160 | 7 | 19 | 03:37 | 3 |
| 3 | PD FS TSE | oblique sagittal | 3950 | 23 | 320 × 256 | 140 | 7 | 29 | 04:49 | 3 |
| 4 | PD TSE | oblique coronal | 1670 | 23 | 384 × 307 | 160 | 5 | 19 | 03:24 | 3 |
| 5 | T1 SE | oblique coronal | 787 | 10 | 384 × 346 | 90 | 1 | 19 | 04:51 | 3 |
| 6 | T2 TSE | oblique sagittal | 5640 | 88 | 384 × 307 | 150 | 15 | 29 | 02:33 | 3 |
| 7 | T2 MapIt | oblique coronal | 2140 | 13.8, 27.6, 41.4, 55.2, 69 | 320 × 320 | 180 | 1 | 16 | 06:50 | 3 |
| LBS Tendinopathy | ||||
|---|---|---|---|---|
| No (N = 10) | Yes (N = 8) | p-Value | ||
| Sex | Male | 5 (50.0%) | 7 (87.5%) | |
| Female | 5 (50.0%) | 1 (12.5%) | ||
| Age (years) | n | 10 | 8 | 0.1274 |
| Mean | 46.3 | 60.0 | ||
| SD | 17.24 | 5.01 | ||
| Median | 54.0 | 59.5 | ||
| Min | 22.0 | 52.0 | ||
| Max | 64.0 | 67.0 | ||
| Overall | Population without Lesion | Population with Lesion | p-Value (t Value) | ||
|---|---|---|---|---|---|
| n | 18 | 10 | 8 | ||
| T2 values (ms) | Mean | 34.2 | 23.3 | 47.9 | <0.001 (−8.33) |
| SD | 13.97 | 4.61 | 7.84 | ||
| Median | 29.0 | 24.2 | 48.2 | ||
| Min | 15.8 | 15.8 | 33.8 | ||
| Max | 61.4 | 29.6 | 61.4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stein, P.; Wuennemann, F.; Schneider, T.; Zeifang, F.; Burkholder, I.; Weber, M.-A.; Kauczor, H.-U.; Rehnitz, C. Detection and Quantitative Assessment of Arthroscopically Proven Long Biceps Tendon Pathologies Using T2 Mapping. Tomography 2023, 9, 1577-1591. https://doi.org/10.3390/tomography9050126
Stein P, Wuennemann F, Schneider T, Zeifang F, Burkholder I, Weber M-A, Kauczor H-U, Rehnitz C. Detection and Quantitative Assessment of Arthroscopically Proven Long Biceps Tendon Pathologies Using T2 Mapping. Tomography. 2023; 9(5):1577-1591. https://doi.org/10.3390/tomography9050126
Chicago/Turabian StyleStein, Patrick, Felix Wuennemann, Thomas Schneider, Felix Zeifang, Iris Burkholder, Marc-André Weber, Hans-Ulrich Kauczor, and Christoph Rehnitz. 2023. "Detection and Quantitative Assessment of Arthroscopically Proven Long Biceps Tendon Pathologies Using T2 Mapping" Tomography 9, no. 5: 1577-1591. https://doi.org/10.3390/tomography9050126
APA StyleStein, P., Wuennemann, F., Schneider, T., Zeifang, F., Burkholder, I., Weber, M.-A., Kauczor, H.-U., & Rehnitz, C. (2023). Detection and Quantitative Assessment of Arthroscopically Proven Long Biceps Tendon Pathologies Using T2 Mapping. Tomography, 9(5), 1577-1591. https://doi.org/10.3390/tomography9050126








