Case Series of MRI and CT Assessment of Acquired Hepato-Biliary and Pancreatic Transdiaphragmatic Fistulae
Abstract
1. Introduction
1.1. Hepato-Thoracic Fistula
1.2. Pancreaticopleural Fistula
2. Materials and Methods
2.1. Patients
2.2. CT Imaging
2.3. MRI Imaging
3. Our Experience: Case Presentations
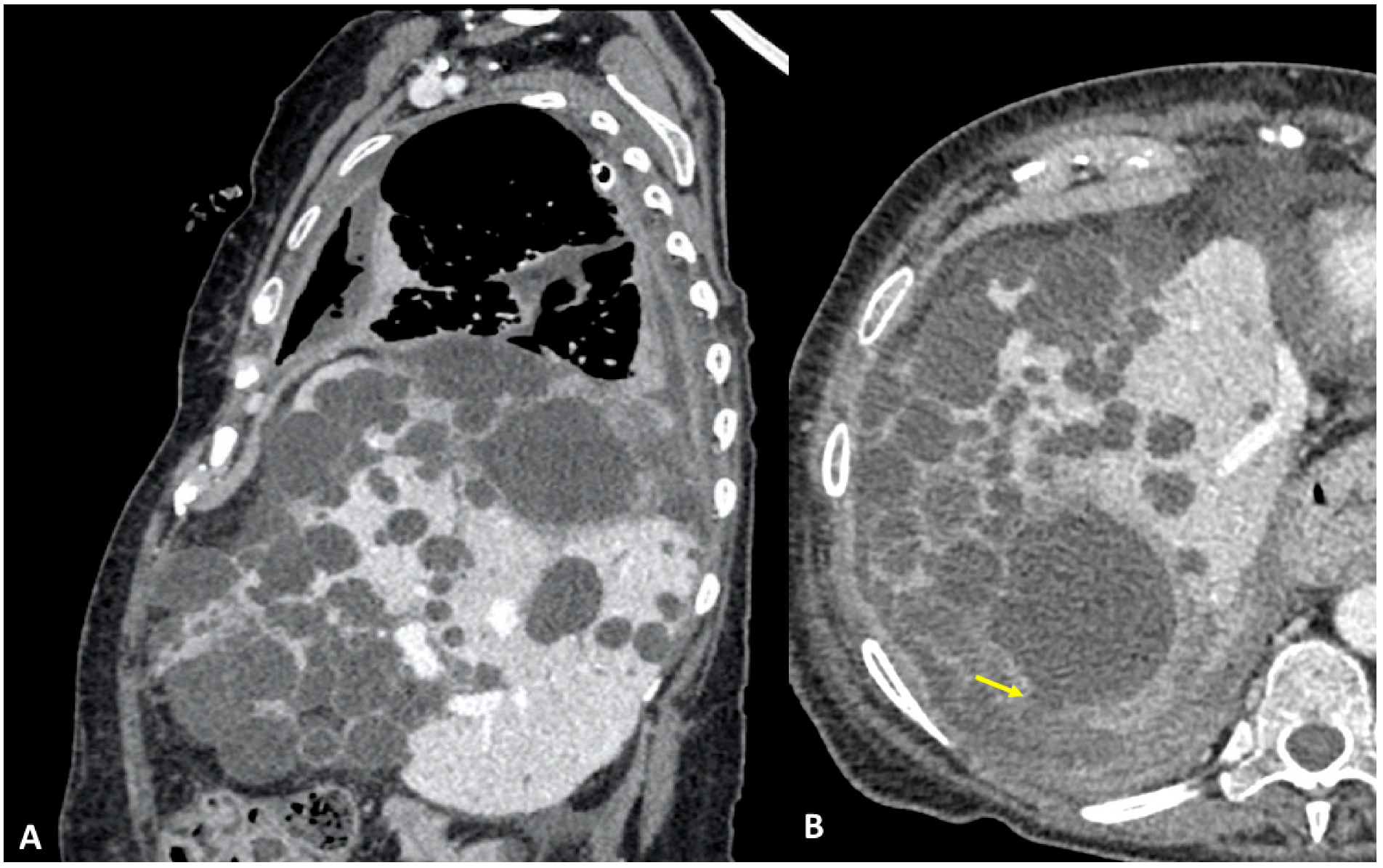
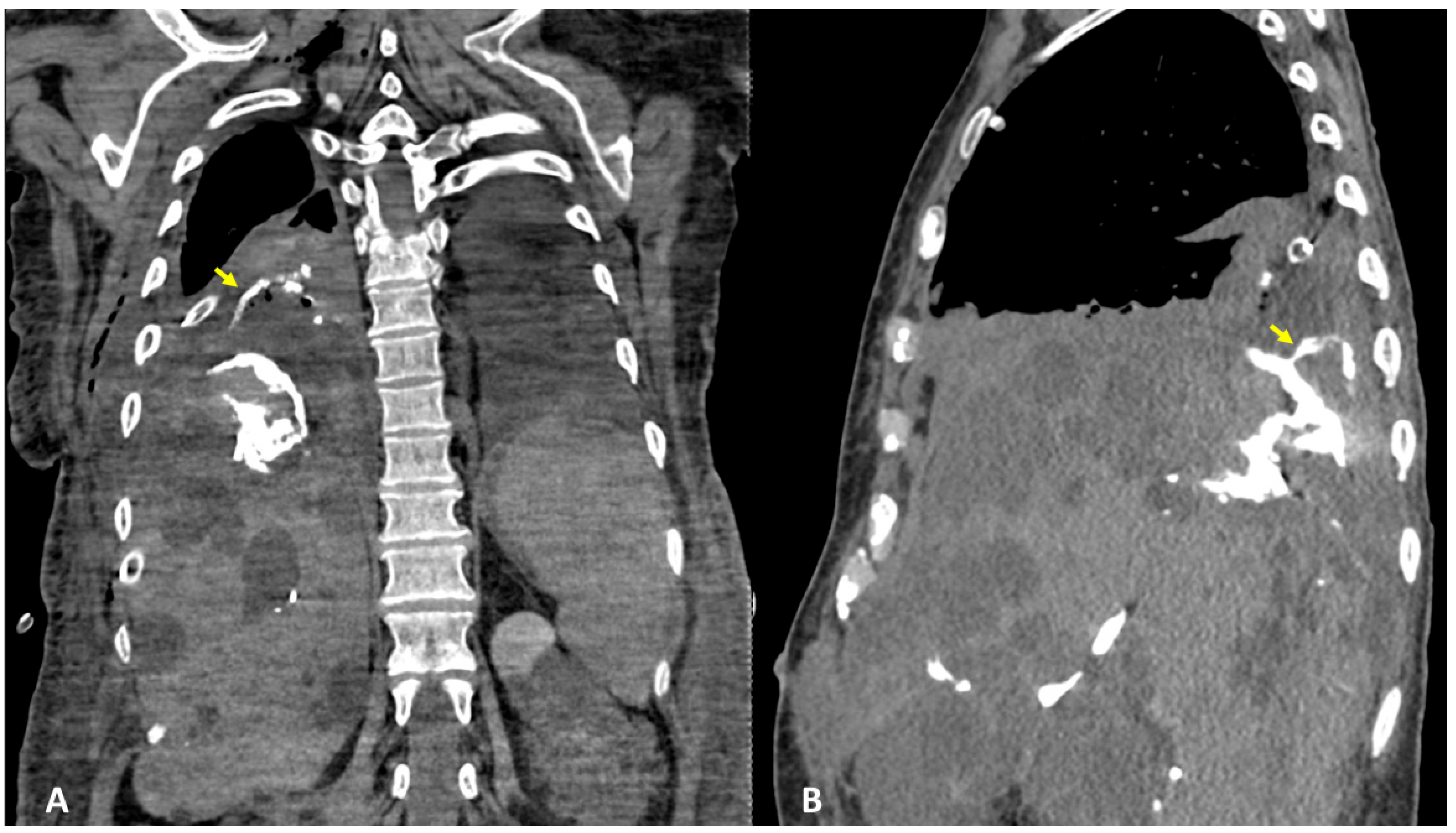
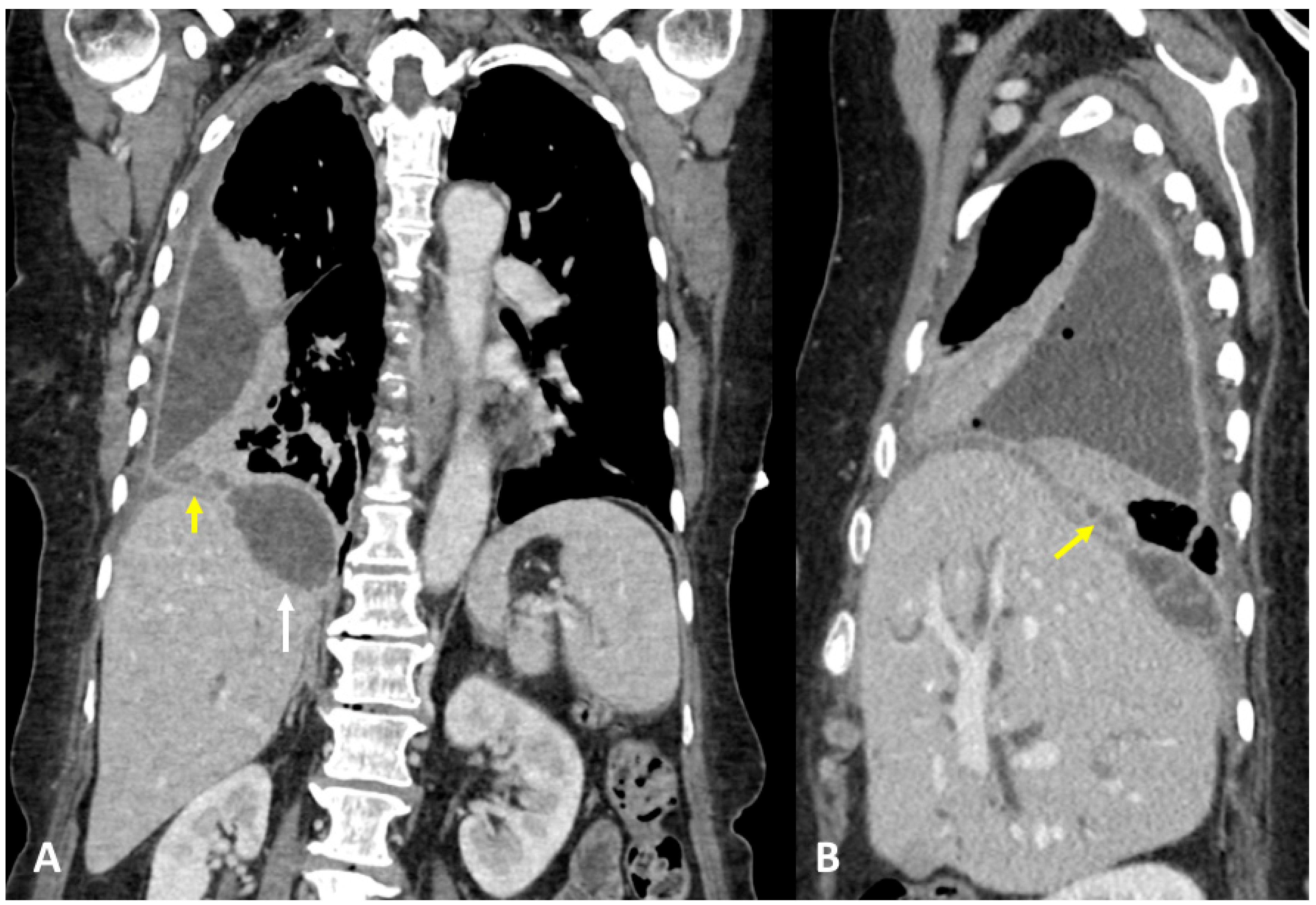
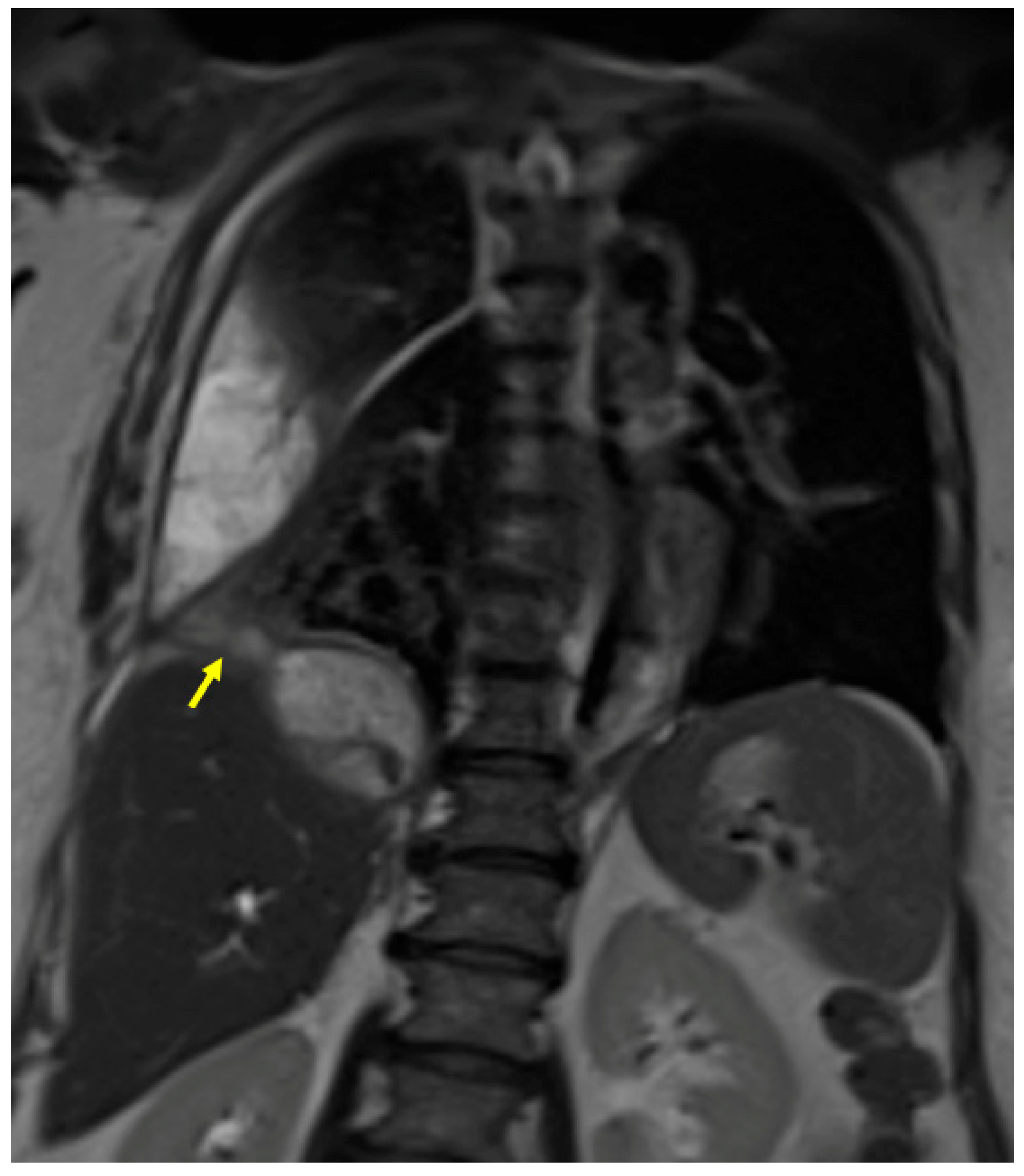
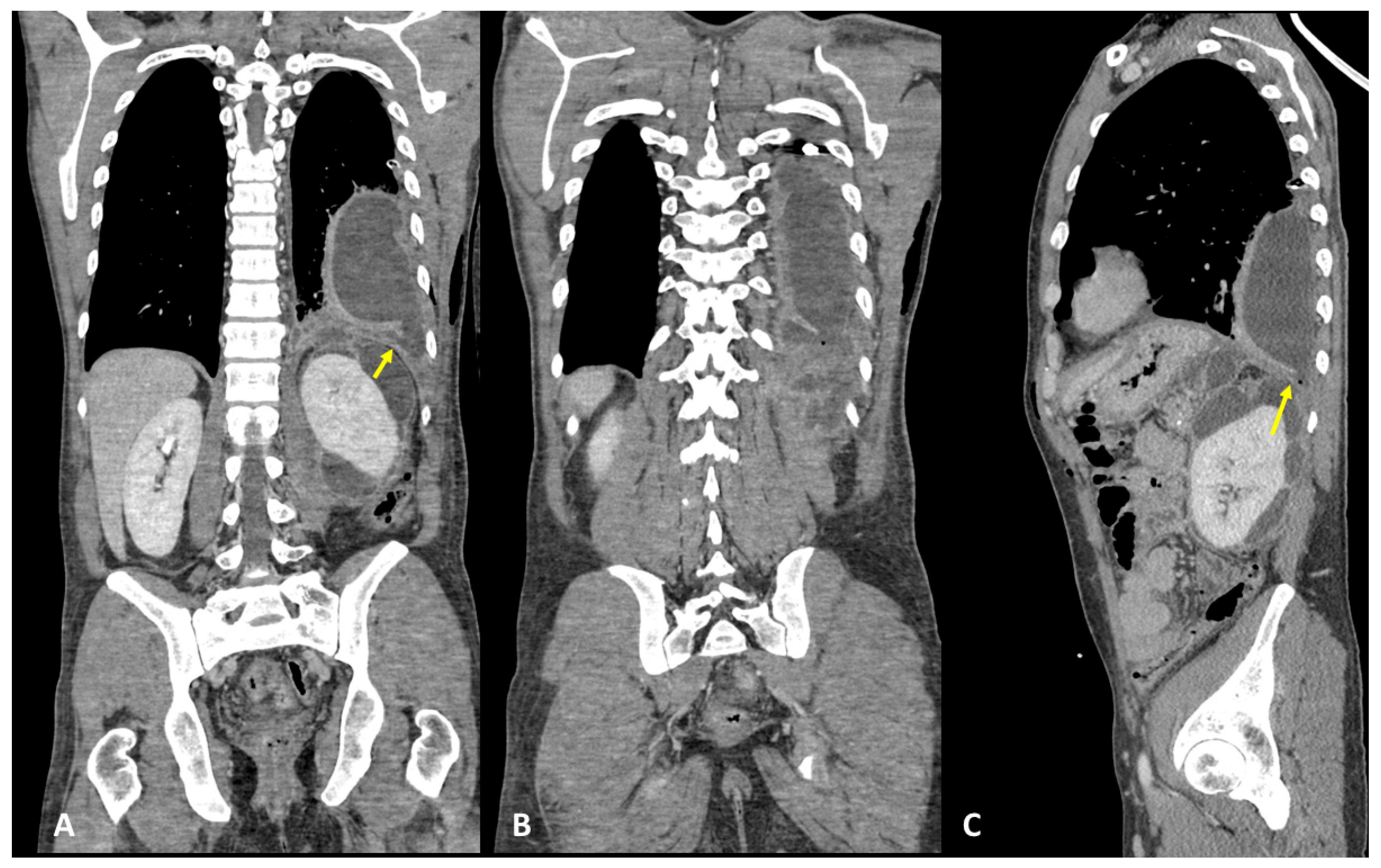
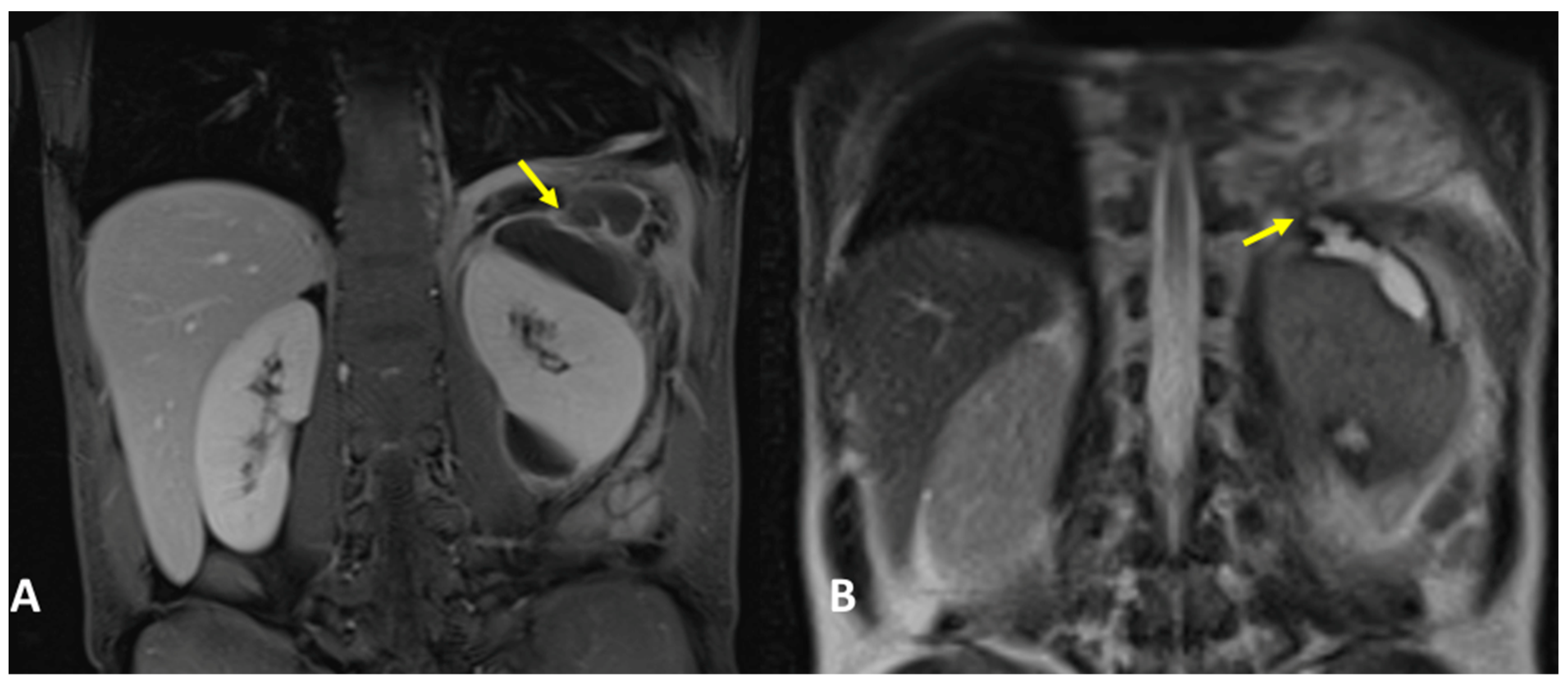
3.1. Case #1: Hepato-Thoracic Fistula
3.2. Case #2: Hepato-Thoracic Fistula
3.3. Case #3: Hepato-Thoracic Fistula
3.4. Case #4: Pancreaticopleural Fistula
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chaturvedi, A.; Rajiah, P.; Chaturvedi, A. Imaging of acquired transdiaphragmatic fistulae and communications. Clin. Imaging 2019, 53, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Bhalla, S.; Balfe, D.M. Acquired gastrointestinal fistulas: Classification, etiologies, and imaging evaluation. Radiology 2002, 224, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Tamburrini, S.; Fiorini, V.; Lugara, M.; Napodano, G.; Del Biondo, D.; Squame, F.; Sarti, G.; Quassone, P.; Coppola, M.G.; Iannuzzi, M.; et al. Nephrobronchial fistula a case report and review of the literature. Radiol. Case Rep. 2021, 16, 3470–3477. [Google Scholar] [CrossRef]
- Tamburrini, S.; Lugara, M.; Saturnino, P.P.; Ferrandino, G.; Quassone, P.; Leboffe, S.; Sarti, G.; Rocco, C.; Panico, C.; Raffaele, F.; et al. Pleural empyema secondary to nephropleural fistula in complicated pyonephrosis. Radiol. Case Rep. 2021, 16, 2714–2718. [Google Scholar] [CrossRef]
- Sharma, R.; Meyer, C.A.; Frazier, A.A.; Martin Rother, M.D.; Kusmirek, J.E.; Kanne, J.P. Routes of Transdiaphragmatic Migration from the Abdomen to the Chest. Radiographics 2020, 40, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Caporale, A.; Giuliani, A.; Teneriello, F.; Della Casa, U.; Aurello, P.; Iaricci, P.; Fegiz, G. Hydatid hepatothoracic fistulas. A report of 30 cases. Ital. J. Surg. Sci. 1987, 17, 327–333. [Google Scholar]
- Noora Sabah Jaber, M.A.N. Surgical Management of Thoraco-Biliary Fistula. Iraqui Med. J. 2014, 60, 21–26. [Google Scholar]
- Singh, B.; Moodley, J.; Sheik-Gafoor, M.H.; Dhooma, N.; Reddi, A. Conservative management of thoracobiliary fistula. Ann. Thorac. Surg. 2002, 73, 1088–1091. [Google Scholar] [CrossRef]
- Graham, J.E. Observations on Broncho-Biliary Fistula: (With the Reports of Two Cases). Br. Med. J. 1897, 1, 1397–1400. [Google Scholar] [CrossRef]
- Eryigit, H.; Oztas, S.; Urek, S.; Olgac, G.; Kurutepe, M.; Kutlu, C.A. Management of acquired bronchobiliary fistula: 3 case reports and a literature review. J. Cardiothorac. Surg. 2007, 2, 52. [Google Scholar] [CrossRef]
- Warren, K.W.; Christophi, C.; Armendariz, R.; Basu, S. Surgical treatment of bronchobiliary fistulas. Surg. Gynecol. Obstet. 1983, 157, 351–356. [Google Scholar] [PubMed]
- Gries, C.; Branding, G.; Ritz, J.P.; Golder, W. Bronchobiliary fistula as a complication of Bulau drainage. Rofo 1998, 169, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Gugenheim, J.; Ciardullo, M.; Traynor, O.; Bismuth, H. Bronchobiliary fistulas in adults. Ann. Surg. 1988, 207, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Crnjac, A.; Pivec, V.; Ivanecz, A. Thoracobiliary fistulas: Literature review and a case report of fistula closure with omentum majus. Radiol. Oncol. 2013, 47, 77–85. [Google Scholar] [CrossRef]
- Gandhi, N.; Kent, T.; Kaban, J.M.; Stone, M.; Teperman, S.; Simon, R. Bronchobiliary fistula after penetrating thoracoabdominal trauma: Case report and literature review. J. Trauma 2009, 67, E143–E145. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.D.; Johnson, N.A.; Metcalfe, M.S.; Neal, C.P.; Harrison, R.F.; Berry, D.P.; Dennison, A.R. Cholecystobronchial fistula secondary to adenomyomatosis of the gallbladder. Ann. R Coll. Surg. Engl. 2007, 89, W14–W16. [Google Scholar] [CrossRef]
- Karabulut, N.; Cakmak, V.; Kiter, G. Confident diagnosis of bronchobiliary fistula using contrast-enhanced magnetic resonance cholangiography. Korean J. Radiol. 2010, 11, 493–496. [Google Scholar] [CrossRef]
- Kantarci, M.; Pirimoglu, B.; Karabulut, N.; Bayraktutan, U.; Ogul, H.; Ozturk, G.; Aydinli, B.; Kizrak, Y.; Eren, S.; Yilmaz, S. Non-invasive detection of biliary leaks using Gd-EOB-DTPA-enhanced MR cholangiography: Comparison with T2-weighted MR cholangiography. Eur. Radiol. 2013, 23, 2713–2722. [Google Scholar] [CrossRef]
- Argiro, R.; Sensi, B.; Siragusa, L.; Bellini, L.; Conte, L.E.; Riccetti, C.; Del Vecchio Blanco, G.; Troncone, E.; Floris, R.; Salavracos, M.; et al. Liver-Specific Contrast-Enhanced Magnetic Resonance Cholangio-Pancreatography (Ce-MRCP) in Non-Invasive Diagnosis of Iatrogenic Biliary Leakage. Diagnostics 2023, 13, 1681. [Google Scholar] [CrossRef]
- Navsaria, P.H.; Adams, S.; Nicol, A.J. Traumatic thoracobiliary fistulae: A case report with a review of the current management options. Injury 2002, 33, 639–643. [Google Scholar] [CrossRef]
- Sut, M.; Gray, R.; Ramachandran, M.; Diamond, T. Pancreaticopleural fistula: A rare complication of ERCP-induced pancreatitis. Ulster Med. J. 2009, 78, 185–186. [Google Scholar] [PubMed]
- Fulcher, A.S.; Capps, G.W.; Turner, M.A. Thoracopancreatic fistula: Clinical and imaging findings. J. Comput. Assist. Tomogr. 1999, 23, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Aswani, Y.; Hira, P. Pancreaticopleural fistula: A review. JOP 2015, 16, 90–94. [Google Scholar]
- Cameron, J.L.; Kieffer, R.S.; Anderson, W.J.; Zuidema, G.D. Internal pancreatic fistulas: Pancreatic ascites and pleural effusions. Ann. Surg. 1976, 184, 587–593. [Google Scholar] [CrossRef]
- Wypych, K.; Serafin, Z.; Galazka, P.; Strzesniewski, P.; Matuszczak, W.; Nierzwicka, K.; Lasek, W.; Prokurat, A.I.; Bak, M. Pancreaticopleural fistulas of different origin: Report of two cases and a review of literature. Pol. J. Radiol. 2011, 76, 56–60. [Google Scholar]
- Sonoda, S.; Taniguchi, M.; Sato, T.; Yamasaki, M.; Enjoji, M.; Mae, S.; Irie, T.; Ina, H.; Sumi, Y.; Inase, N.; et al. Bilateral pleural fluid caused by a pancreaticopleural fistula requiring surgical treatment. Intern. Med. 2012, 51, 2655–2661. [Google Scholar] [CrossRef]
- King, J.C.; Reber, H.A.; Shiraga, S.; Hines, O.J. Pancreatic-pleural fistula is best managed by early operative intervention. Surgery 2010, 147, 154–159. [Google Scholar] [CrossRef]
- Ali, T.; Srinivasan, N.; Le, V.; Chimpiri, A.R.; Tierney, W.M. Pancreaticopleural fistula. Pancreas 2009, 38, e26–e31. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Gogoi, D.; Sinha, S.K.; Singh, P.; Yadav, T.D.; Khandelwal, N. Pancreaticopleural fistula: An unusual complication of pancreatitis diagnosed with magnetic resonance cholangiopancreatography. JOP 2009, 10, 671–673. [Google Scholar] [PubMed]
- Cocieru, A.; Saldinger, P.F. Frey procedure for pancreaticopleural fistula. J. Gastrointest. Surg. 2010, 14, 929–930. [Google Scholar] [CrossRef]
- Materne, R.; Vranckx, P.; Pauls, C.; Coche, E.E.; Deprez, P.; Van Beers, B.E. Pancreaticopleural fistula: Diagnosis with magnetic resonance pancreatography. Chest 2000, 117, 912–914. [Google Scholar] [CrossRef] [PubMed]
- Wronski, M.; Slodkowski, M.; Cebulski, W.; Moronczyk, D.; Krasnodebski, I.W. Optimizing management of pancreaticopleural fistulas. World J. Gastroenterol. 2011, 17, 4696–4703. [Google Scholar] [CrossRef] [PubMed]
- Akahane, T.; Kuriyama, S.; Matsumoto, M.; Kikuchi, E.; Kikukawa, M.; Yoshiji, H.; Masui, K.; Fukui, H. Pancreatic pleural effusion with a pancreaticopleural fistula diagnosed by magnetic resonance cholangiopancreatography and cured by somatostatin analogue treatment. Abdom. Imaging 2003, 28, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.Q.; Wang, H.; Zhu, G.Y.; Zhu, K.B.; Lv, F.X.; Tai, S. Management of acquired bronchobiliary fistula: A systematic literature review of 68 cases published in 30 years. World J. Gastroenterol. 2011, 17, 3842–3849. [Google Scholar] [CrossRef] [PubMed]
- Chua, H.K.; Allen, M.S.; Deschamps, C.; Miller, D.L.; Pairolero, P.C. Bronchobiliary fistula: Principles of management. Ann. Thorac. Surg. 2000, 70, 1392–1394. [Google Scholar] [CrossRef]


| Patient No. | Transdiaphragmatic Fistula Type | Age | Sex |
|---|---|---|---|
| 1 | Pancreaticopleural | 31 | F |
| 2 | Thoracobiliary | 61 | F |
| 3 | Thoracobiliary | 63 | F |
| 4 | Thoracobiliary | 91 | M |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picchi, S.G.; Lassandro, G.; Comune, R.; Pezzullo, F.; Fiorini, V.; Lassandro, F.; Tonerini, M.; Masala, S.; Tamburro, F.; Scaglione, M.; et al. Case Series of MRI and CT Assessment of Acquired Hepato-Biliary and Pancreatic Transdiaphragmatic Fistulae. Tomography 2023, 9, 1356-1368. https://doi.org/10.3390/tomography9040108
Picchi SG, Lassandro G, Comune R, Pezzullo F, Fiorini V, Lassandro F, Tonerini M, Masala S, Tamburro F, Scaglione M, et al. Case Series of MRI and CT Assessment of Acquired Hepato-Biliary and Pancreatic Transdiaphragmatic Fistulae. Tomography. 2023; 9(4):1356-1368. https://doi.org/10.3390/tomography9040108
Chicago/Turabian StylePicchi, Stefano Giusto, Giulia Lassandro, Rosita Comune, Filomena Pezzullo, Valeria Fiorini, Francesco Lassandro, Michele Tonerini, Salvatore Masala, Fabio Tamburro, Mariano Scaglione, and et al. 2023. "Case Series of MRI and CT Assessment of Acquired Hepato-Biliary and Pancreatic Transdiaphragmatic Fistulae" Tomography 9, no. 4: 1356-1368. https://doi.org/10.3390/tomography9040108
APA StylePicchi, S. G., Lassandro, G., Comune, R., Pezzullo, F., Fiorini, V., Lassandro, F., Tonerini, M., Masala, S., Tamburro, F., Scaglione, M., & Tamburrini, S. (2023). Case Series of MRI and CT Assessment of Acquired Hepato-Biliary and Pancreatic Transdiaphragmatic Fistulae. Tomography, 9(4), 1356-1368. https://doi.org/10.3390/tomography9040108








