Tips and Tricks in Thoracic Radiology for Beginners: A Findings-Based Approach
Abstract
1. Introduction
2. Differential Diagnosis of Mediastinal Masses
2.1. Prevascular Compartment
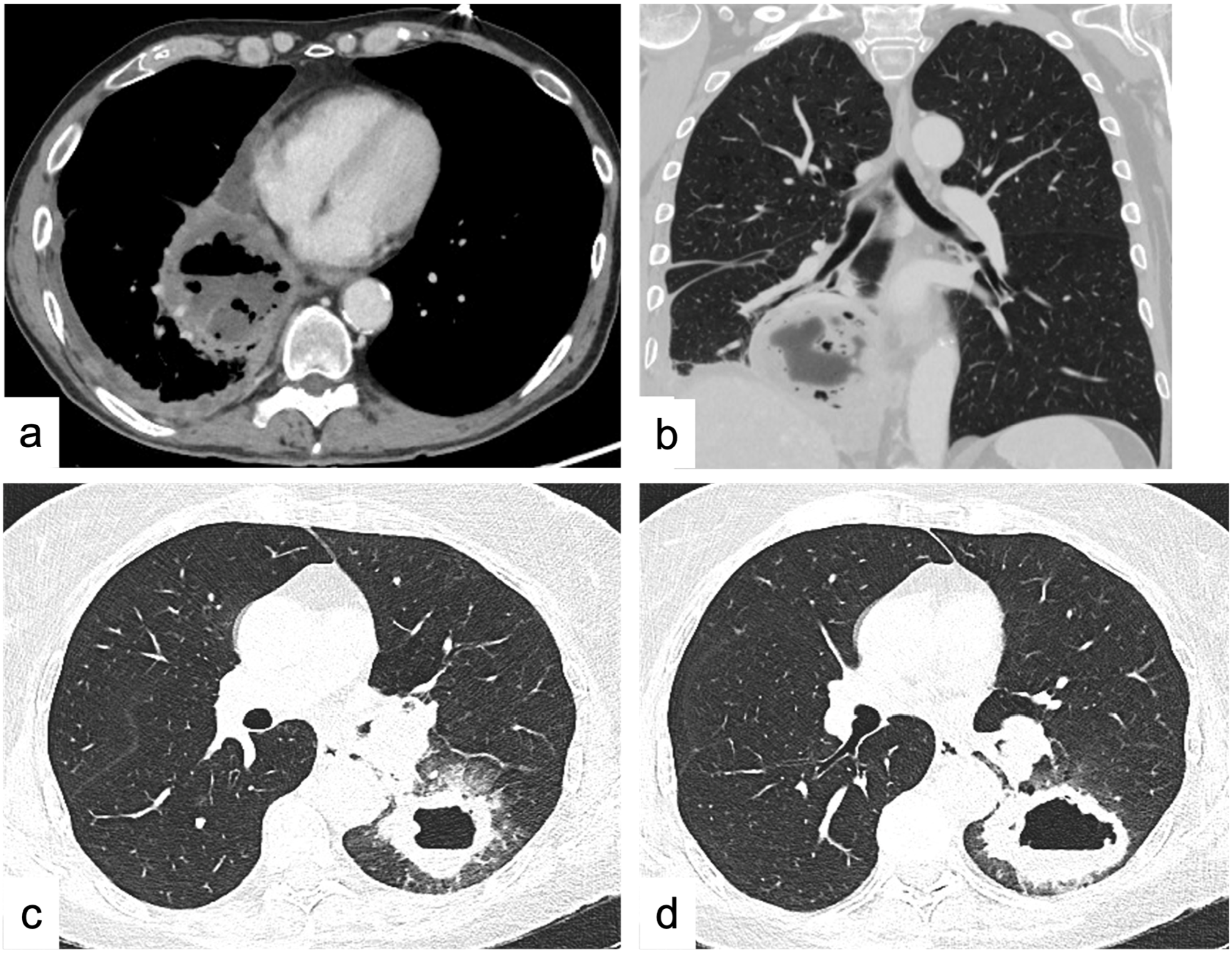
2.1.1. Fat-Containing Lesions
2.1.2. Cystic Lesions
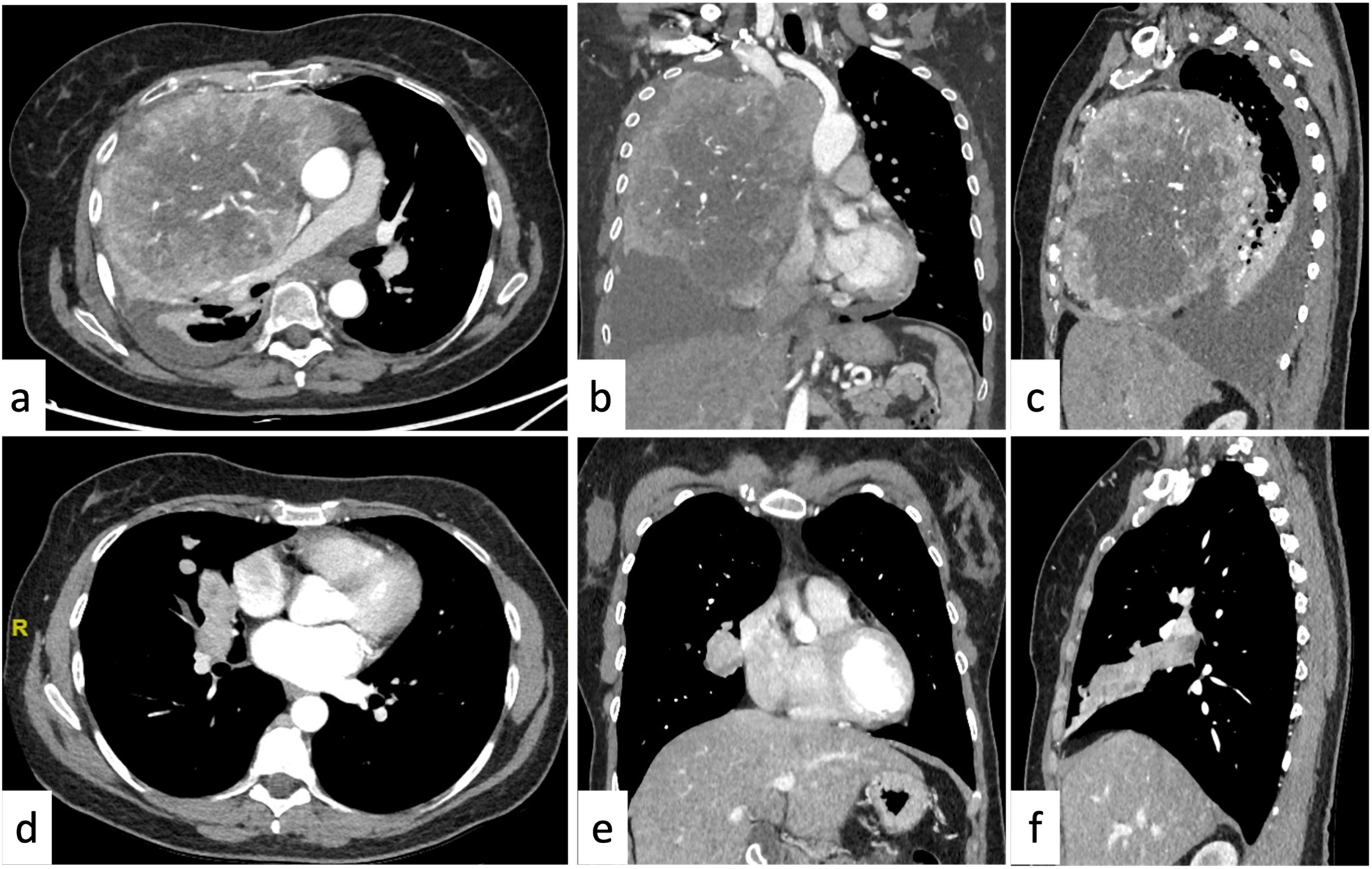
2.1.3. Soft-Tissue Enhancing Masses
2.2. Middle Compartment
2.2.1. Cystic Lesions
2.2.2. Soft-Tissue Enhancing Masses
2.3. Posterior Compartment
2.3.1. Cystic Lesions
2.3.2. Soft-Tissue Enhancing Masses
2.4. More than One Mediastinal Compartment
3. Differential Diagnosis of Pleural Lesions
3.1. Pleural Neoplasms
3.2. Tumorlike Pleural Lesions
4. Differential Diagnosis of the Parenchymal Disease
4.1. Focal Lung Involvement
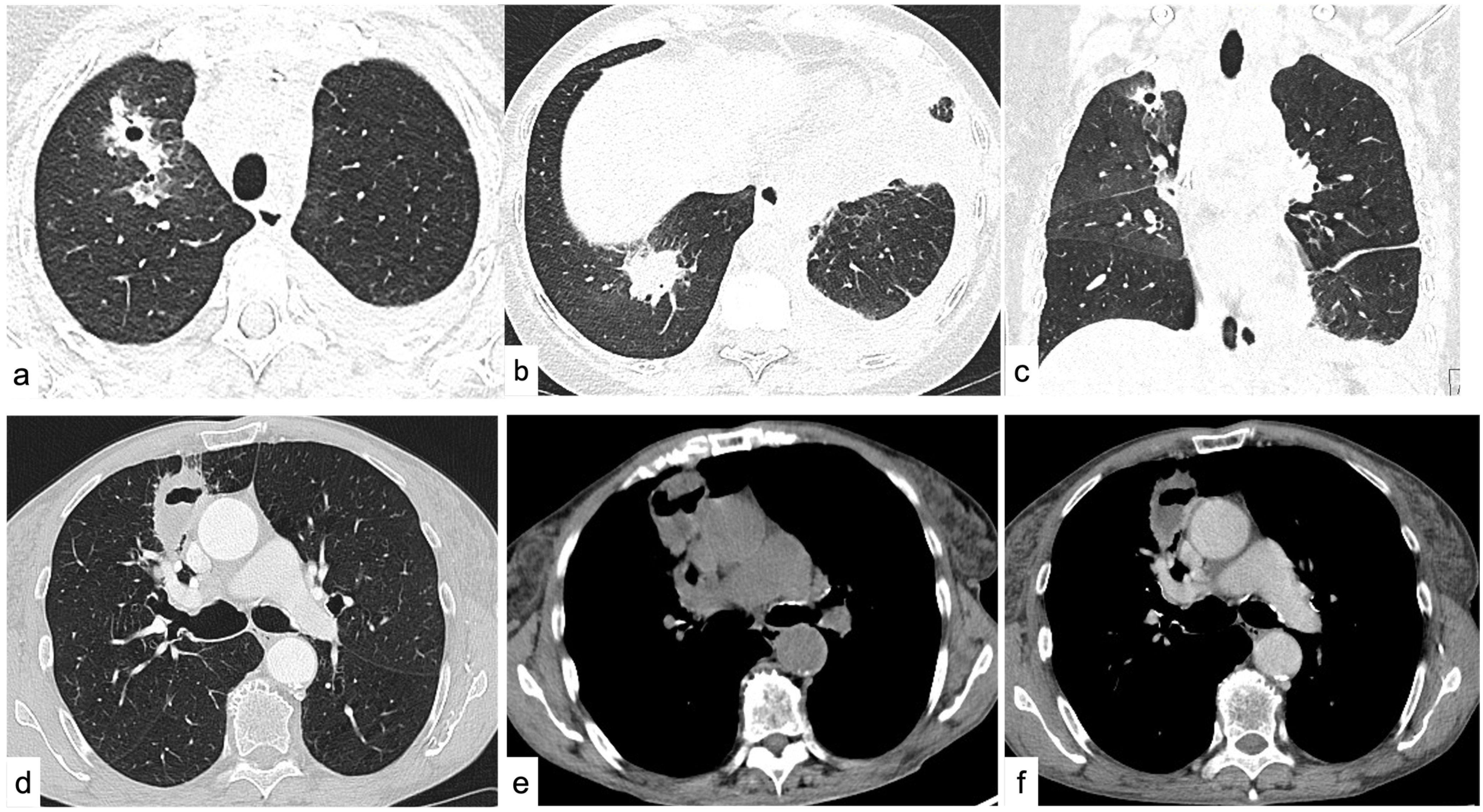
4.1.1. Density
4.1.2. Shape
4.1.3. Margins
4.1.4. Fat Attenuation
4.1.5. Calcifications
4.1.6. Cavitations and Cysts
4.2. Diffuse Lung Disease
4.2.1. Reticular Pattern
Interlobular Septal Thickening
Honeycombing
Irregular Reticulation
4.2.2. Nodular Pattern
Perilymphatic Nodules
Centrilobular Nodules
Random Nodules
4.2.3. Increased Lung Attenuation
Ground-Glass Opacities
Consolidations
4.2.4. Decreased Lung Attenuation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elicker, B.M.; Webb, W.R. Fundamental of High-Resolution Lung CT, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Hoop, B.D.; Schaefer-Prokop, C.; Gietema, H.A.; Jong, P.A.D.; Ginneken, B.V.; Klaveren, R.J.V.; Prokop, M. Screening for Lung Cancer with Digital Chest Radiography: Sensitivity and Number of Secondary Work-up CT Examinations. Radiology 2010, 255, 629–637. [Google Scholar] [CrossRef]
- Leung, S.C.; Churg, A.M.; Leipsic, J.A.; Levy, R.D.; Wilcox, P.G.; Ryerson, C.J. Unclassifiable Interstitial Lung Disease: An Unresolved Diagnostic Dilemma. Respirol. Case Rep. 2015, 3, 85–88. [Google Scholar] [CrossRef]
- Ghigna, M.R.; de Montpreville, V.T. Mediastinal Tumours and Pseudo-Tumours: A Comprehensive Review with Emphasis on Multidisciplinary Approach. Eur. Respir. Rev. 2021, 30, 200309. [Google Scholar] [CrossRef]
- Quint, L.E. Imaging of Anterior Mediastinal Masses. Cancer Imaging 2007, 7, S56–S62. [Google Scholar] [CrossRef]
- Juanpere, S.; Cañete, N.; Ortuño, P.; Martínez, S.; Sanchez, G.; Bernado, L. A Diagnostic Approach to the Mediastinal Masses. Insights Imaging 2013, 4, 29–52. [Google Scholar] [CrossRef]
- Carter, B.W.; Tomiyama, N.; Bhora, F.Y.; de Christenson, M.L.R.; Nakajima, J.; Boiselle, P.M.; Detterbeck, F.C.; Marom, E.M. A Modern Definition of Mediastinal Compartments. J. Thorac. Oncol. 2014, 9, S97–S101. [Google Scholar] [CrossRef]
- Carter, B.W.; Benveniste, M.F.; Madan, R.; Godoy, M.C.; Groot, P.M.D.; Truong, M.T.; Rosado-De-Christenson, M.L.; Marom, E.M. ITMIG Classification of Mediastinal Compartments and Multidisciplinary Approach to Mediastinal Masses. Radiographics 2017, 37, 413–436. [Google Scholar] [CrossRef]
- Whitten, C.R.; Khan, S.; Munneke, G.J.; Grubnic, S. A Diagnostic Approach to Mediastinal Abnormalities. Radiographics 2007, 27, 657–671. [Google Scholar] [CrossRef]
- Patnaik, S.; Malempati, A.; Uppin, M.; Susarla, R. Rare Mediastinal Masses—Imaging Review. J. Cancer Res. Ther. 2021, 17, 13–21. [Google Scholar] [CrossRef]
- Franquet, T.; Erasmus, J.J.; Giménez, A.; Rossi, S.; Prats, R. The Retrotracheal Space: Normal Anatomic and Pathologic Appearances. RadioGraphics 2002, 22, S231–S246. [Google Scholar] [CrossRef]
- Savoca, C.J.; Austin, J.H.M.; Goldberg, H.I. The Right Paratracheal Stripe. Radiology 1977, 122, 295–301. [Google Scholar] [CrossRef]
- Duwe, B.V.; Sterman, D.H.; Musani, A.I. Tumors of the Mediastinum. Chest 2005, 128, 2893–2909. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, K.S.; Han, J.; Yoon, H.-K.; Kim, T.S.; Han, B.K.; Kim, J.; Shim, Y.M. Spectrum of Neurogenic Tumors in the Thorax: CT and Pathologic Findings. J. Comput. Assist. Tomogr. 1999, 23, 399–406. [Google Scholar] [CrossRef]
- Takahashi, K.; Al-Janabi, N.J. Computed Tomography and Magnetic Resonance Imaging of Mediastinal Tumors. J. Magn. Reson. Imaging 2010, 32, 1325–1339. [Google Scholar] [CrossRef]
- McLoud, T.; Boiselle, P. Thoracic Radiology, the Requisites; Mosby: Maryland Heights, MO, USA, 2010. [Google Scholar]
- Carter, B.W.; Okumura, M.; Detterbeck, F.C.; Marom, E.M. Approaching the Patient with an Anterior Mediastinal Mass: A Guide for Radiologists. J. Thorac. Oncol. 2014, 9, S110–S118. [Google Scholar] [CrossRef]
- Park, J.W.; Jeong, W.G.; Lee, J.E.; Lee, H.J.; Ki, S.Y.; Lee, B.C.; Kim, H.O.; Kim, S.K.; Heo, S.H.; Lim, H.S.; et al. Pictorial Review of Mediastinal Masses with an Emphasis on Magnetic Resonance Imaging. Korean J. Radiol. 2021, 22, 139–154. [Google Scholar] [CrossRef]
- Roden, A.C.; Fang, W.; Shen, Y.; Carter, B.W.; White, D.B.; Jenkins, S.M.; Spears, G.M.; Molina, J.R.; Klang, E.; Segni, M.D.; et al. Distribution of Mediastinal Lesions Across Multi-Institutional, International, Radiology Databases. J. Thorac. Oncol. 2020, 15, 568–579. [Google Scholar] [CrossRef]
- Molinari, F.; Bankier, A.A.; Eisenberg, R.L. Fat-Containing Lesions in Adult Thoracic Imaging. Am. J. Roentgenol. 2011, 197, W795–W813. [Google Scholar] [CrossRef]
- Schneiter, S.M.; Warrier, R.; Lefkovits, L.; Laurie, C.; O’Brien, P.E.; Taylor, A.J. Effects of Weight Loss on Pericardial Fat and Left Ventricular Mass Assessed with Cardiac Magnetic Resonance Imaging in Morbid Obesity. Int. J. Clin. Med. 2011, 2, 360–366. [Google Scholar] [CrossRef]
- Tomiyama, N.; Honda, O.; Tsubamoto, M.; Inoue, A.; Sumikawa, H.; Kuriyama, K.; Kusumoto, M.; Johkoh, T.; Nakamura, H. Anterior Mediastinal Tumors: Diagnostic Accuracy of CT and MRI. Eur. J. Radiol. 2009, 69, 280–288. [Google Scholar] [CrossRef]
- Hartmann, T. Pearls and Pitfalls in Thoracic Imaging: Variants and Other Difficult Diagnoses, 1st ed.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Gaerte, S.C.; Meyer, C.A.; Winer-Muram, H.T.; Tarver, R.D.; Conces, D.J. Fat-Containing Lesions of the Chest. RadioGraphics 2002, 22, S61–S78. [Google Scholar] [CrossRef]
- Varma, V.; Alabousi, A.; Burute, N.; Haider, E. Thymic Masses and Mimics in Adults: Review of Common and Uncommon Pathologies. Clin. Imaging 2021, 77, 98–110. [Google Scholar] [CrossRef]
- Nishino, M.; Ashiku, S.K.; Kocher, O.N.; Thurer, R.L.; Boiselle, P.M.; Hatabu, H. The Thymus: A Comprehensive Review. RadioGraphics 2006, 26, 335–348. [Google Scholar] [CrossRef]
- Munden, R.F.; Nesbitt, J.C.; Kemp, B.L.; Chasen, M.H.; Whitman, G.J. Primary Liposarcoma of the Mediastinum. Am. J. Roentgenol. 2000, 175, 1340. [Google Scholar] [CrossRef]
- Hahn, H.P.; Fletcher, C.D.M. Primary Mediastinal Liposarcoma. Am. J. Surg. Pathol. 2007, 31, 1868–1874. [Google Scholar] [CrossRef]
- Sirmali, M.; Türüt, H.; Gezer, S.; Findik, G.; Kaya, S.; Tastepe, Ý.; Çetin, G. Clinical and Radiologic Evaluation of Foramen of Morgagni Hernias and the Transthoracic Approach. World J. Surg. 2005, 29, 1520–1524. [Google Scholar] [CrossRef]
- Choi, Y.W.; McAdams, H.P.; Jeon, S.C.; Hong, E.K.; Kim, Y.-H.; Im, J.-G.; Lee, S.R. Idiopathic Multilocular Thymic Cyst. Am. J. Roentgenol. 2001, 177, 881–885. [Google Scholar] [CrossRef]
- Nasseri, F.; Eftekhari, F. Clinical and Radiologic Review of the Normal and Abnormal Thymus: Pearls and Pitfalls. RadioGraphics 2010, 30, 413–428. [Google Scholar] [CrossRef]
- Feigin, D.S.; Fenoglio, J.J.; McAllister, H.A.; Madewell, J.E. Pericardial Cysts: A Radiologic-Pathologic Correlation and Review. Radiology 1977, 125, 15–20. [Google Scholar] [CrossRef]
- Jeung, M.-Y.; Gasser, B.; Gangi, A.; Bogorin, A.; Charneau, D.; Wihlm, J.M.; Dietemann, J.-L.; Roy, C. Imaging of Cystic Masses of the Mediastinum. RadioGraphics 2002, 22, S79–S93. [Google Scholar] [CrossRef]
- Nakazono, T.; Yamaguchi, K.; Egashira, R.; Mizuguchi, M.; Irie, H. Anterior Mediastinal Lesions: CT and MRI Features and Differential Diagnosis. Jpn. J. Radiol. 2021, 39, 101–117. [Google Scholar] [CrossRef]
- Faul, J.L.; Berry, G.J.; Colby, T.V.; Ruoss, S.J.; Walter, M.B.; Rosen, G.D.; Raffin, T.A. State of the Art Thoracic Lymphangiomas, Lymphangiectasis, Lymphangiomatosis, and Lymphatic Dysplasia Syndrome. Am. J. Respir. Crit. Care Med. 2000, 161, 1037–1046. [Google Scholar] [CrossRef]
- Giron, J.; Fajadet, P.; Sans, N.; Jarlaud, T.; Verhnet, H.; Galy-Fourcade, D.; Baunin, C.; Durand, G.; Sénac, J.P.; Railhac, J.J. Diagnostic Approach to Mediastinal Masses. Eur. J. Radiol. 1998, 27, 21–42. [Google Scholar] [CrossRef]
- Shahrzad, M.; Le, T.S.M.; Silva, M.; Bankier, A.A.; Eisenberg, R.L. Anterior Mediastinal Masses. Am. J. Roentgenol. 2014, 203, W128–W138. [Google Scholar] [CrossRef]
- Nakazono, T.; Yamaguchi, K.; Egashira, R.; Takase, Y.; Nojiri, J.; Mizuguchi, M.; Irie, H. CT-Based Mediastinal Compartment Classifications and Differential Diagnosis of Mediastinal Tumors. Jpn. J. Radiol. 2019, 37, 117–134. [Google Scholar] [CrossRef]
- World Health Organization. Tumours of the Thymus. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th ed.; International Agency for Research on Cancer (IARC), Ed.; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Benveniste, M.F.K.; Rosado-de-Christenson, M.L.; Sabloff, B.S.; Moran, C.A.; Swisher, S.G.; Marom, E.M. Role of Imaging in the Diagnosis, Staging, and Treatment of Thymoma. RadioGraphics 2011, 31, 1847–1861. [Google Scholar] [CrossRef]
- Inoue, A.; Tomiyama, N.; Tatsumi, M.; Ikeda, N.; Okumura, M.; Shiono, H.; Inoue, M.; Higuchi, I.; Aozasa, K.; Johkoh, T.; et al. 18F-FDG PET for the Evaluation of Thymic Epithelial Tumors: Correlation with the World Health Organization Classification in Addition to Dual-Time-Point Imaging. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1219–1225. [Google Scholar] [CrossRef]
- Sadohara, J.; Fujimoto, K.; Müller, N.L.; Kato, S.; Takamori, S.; Ohkuma, K.; Terasaki, H.; Hayabuchi, N. Thymic Epithelial Tumors: Comparison of CT and MR Imaging Findings of Low-Risk Thymomas, High-Risk Thymomas, and Thymic Carcinomas. Eur. J. Radiol. 2006, 60, 70–79. [Google Scholar] [CrossRef]
- Rosado-de-Christenson, M.L.; Strollo, D.C.; Marom, E.M. Imaging of Thymic Epithelial Neoplasms. Hematol./Oncol. Clin. N. Am. 2008, 22, 409–431. [Google Scholar] [CrossRef]
- Endo, M.; Nakagawa, K.; Ohde, Y.; Okumura, T.; Kondo, H.; Igawa, S.; Nakamura, Y.; Tsuya, A.; Murakami, H.; Takahashi, T.; et al. Utility of 18FDG-PET for Differentiating the Grade of Malignancy in Thymic Epithelial Tumors. Lung Cancer 2008, 61, 350–355. [Google Scholar] [CrossRef]
- Strollo, D.C.; Rosado-de-Christenson, M.L. Primary Mediastinal Malignant Germ Cell Neoplasms: Imaging Features. Chest Surg. Clin. N. Am. 2002, 12, 645–658. [Google Scholar] [CrossRef]
- Lemarié, E.; Assouline, P.S.; Diot, P.; Regnard, J.F.; Levasseur, P.; Droz, J.P.; Ruffié, P. Primary Mediastinal Germ Cell Tumors. Chest 1992, 102, 1477–1483. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Nichols, C.R.; Droz, J.-P.; Schmoll, H.-J.; Horwich, A.; Gerl, A.; Fossa, S.D.; Beyer, J.; Pont, J.; Kanz, L.; et al. Extragonadal Germ Cell Tumors of the Mediastinum and Retroperitoneum: Results from an International Analysis. J. Clin. Oncol. 2002, 20, 1864–1873. [Google Scholar] [CrossRef]
- Bukowski, R.M.; Wolf, M.; Kulander, B.G.; Montie, J.; Crawford, E.D.; Blumenstein, B. Alternating Combination Chemotherapy in Patients with Extragonadal Germ Cell Tumors a Southwest Oncology Group Study. Cancer 1993, 71, 2631–2638. [Google Scholar] [CrossRef]
- Rosado-de-Christenson, M.L.; Templeton, P.A.; Moran, C.A. From the Archives of the AFIP. Mediastinal Germ Cell Tumors: Radiologic and Pathologic Correlation. RadioGraphics 1992, 12, 1013–1030. [Google Scholar] [CrossRef]
- Tian, L.; Liu, L.Z.; Cui, C.Y.; Zhang, W.D.; Kuang, Y.L. CT Findings of Primary Non-Teratomatous Germ Cell Tumors of the Mediastinum—A Report of 15 Cases. Eur. J. Radiol. 2012, 81, 1057–1061. [Google Scholar] [CrossRef]
- Fizazi, K.; Culine, S.; Droz, J.P.; Kramar, A.; Théodore, C.; Ruffié, P.; Chevalier, T.L. Primary Mediastinal Nonseminomatous Germ Cell Tumors: Results of Modern Therapy Including Cisplatin-Based Chemotherapy. J. Clin. Oncol. 1998, 16, 725–732. [Google Scholar] [CrossRef]
- Kesler, K.A.; Rieger, K.M.; Ganjoo, K.N.; Sharma, M.; Fineberg, N.S.; Einhorn, L.H.; Brown, J.W. Primary Mediastinal Nonseminomatous Germ Cell Tumors: The Influence of Postchemotherapy Pathology on Long-Term Survival after Surgery. J. Thorac. Cardiovasc. Surg. 1999, 118, 692–701. [Google Scholar] [CrossRef]
- Kissin, C.M.; Husband, J.E.; Nicholas, D.; Eversman, W. Benign Thymic Enlargement in Adults after Chemotherapy: CT Demonstration. Radiology 1987, 163, 67–70. [Google Scholar] [CrossRef]
- Shetty, A.S.; Sipe, A.L.; Zulfiqar, M.; Tsai, R.; Raptis, D.A.; Raptis, C.A.; Bhalla, S. In-Phase and Opposed-Phase Imaging: Applications of Chemical Shift and Magnetic Susceptibility in the Chest and Abdomen. RadioGraphics 2019, 39, 115–135. [Google Scholar] [CrossRef]
- Inaoka, T.; Takahashi, K.; Mineta, M.; Yamada, T.; Shuke, N.; Okizaki, A.; Nagasawa, K.; Sugimori, H.; Aburano, T. Thymic Hyperplasia and Thymus Gland Tumors: Differentiation with Chemical Shift MR Imaging. Radiology 2007, 243, 869–876. [Google Scholar] [CrossRef]
- McAdams, H.P.; Kirejczyk, W.M. Bronchogenic Cyst: Imaging Features with Clinical and Histopathologic Correlation. Radiology 2000, 217, 441–446. [Google Scholar] [CrossRef]
- Ferguson, C.C.; Young, L.N.; Sutherland, J.B.; Macpherson, R.I. Intrathoracic Gastrogenic Cyst-Preoperative Diagnosis by Technetium Pertechnetate Scan. J. Pediatr. Surg. 1973, 8, 827–828. [Google Scholar] [CrossRef]
- Balcombe, J.; Torigian, D.A.; Kim, W.; Miller, W.T. Cross-Sectional Imaging of Paragangliomas of the Aortic Body and Other Thoracic Branchiomeric Paraganglia. Am. J. Roentgenol. 2007, 188, 1054–1058. [Google Scholar] [CrossRef]
- Brown, M.L.; Zayas, G.E.; Abel, M.D.; Young, W.F.; Schaff, H.V. Mediastinal Paragangliomas: The Mayo Clinic Experience. Ann. Thorac. Surg. 2008, 86, 946–951. [Google Scholar] [CrossRef]
- Motwani, M.; Kidambi, A.; Herzog, B.A.; Uddin, A.; Greenwood, J.P.; Plein, S. MR Imaging of Cardiac Tumors and Masses: A Review of Methods and Clinical Applications. Radiology 2013, 268, 26–43. [Google Scholar] [CrossRef]
- Miles, J.; Pennybacker, J.; Sheldon, P. Intrathoracic Meningocele. Its Development and Association with Neurofibromatosis. J. Neurol. Neurosurg. Psychiatry 1969, 32, 99–110. [Google Scholar] [CrossRef]
- Glazer, H.; Siegel, M.; Sagel, S. Low-Attenuation Mediastinal Masses on CT. Am. J. Roentgenol. 1989, 152, 1173–1177. [Google Scholar] [CrossRef]
- Kirchner, S.G.; Heller, R.M.; Smith, C.W. Pancreatic Pseudocyst of the Mediastinum. Radiology 1977, 123, 37–42. [Google Scholar] [CrossRef]
- Kawashima, A.; Fishman, E.K.; Kuhlman, J.E.; Nixon, M.S. CT of Posterior Mediastinal Masses. Radiographics 1991, 11, 1045–1067. [Google Scholar] [CrossRef]
- Strollo, D.C.; Rosado-de-Christenson, L.M.L.; Jett, J.R. Primary Mediastinal Tumors. Chest 1997, 112, 1344–1357. [Google Scholar] [CrossRef]
- Bhargava, R.; Parham, D.M.; Lasater, O.E.; Chari, R.S.; Chen, G.; Fletcher, B.D. MR Imaging Differentiation of Benign and Malignant Peripheral Nerve Sheath Tumors: Use of the Target Sign. Pediatr. Radiol. 1997, 27, 124–129. [Google Scholar] [CrossRef]
- Murphey, M.D.; Smith, W.S.; Smith, S.E.; Kransdorf, M.J.; Temple, H.T. From the Archives of the AFIP. Imaging of Musculoskeletal Neurogenic Tumors: Radiologic-Pathologic Correlation. Radiographics 1999, 19, 1253–1280. [Google Scholar] [CrossRef]
- Bredella, M.A.; Torriani, M.; Hornicek, F.; Ouellette, H.A.; Plamer, W.E.; Williams, Z.; Fischman, A.J.; Plotkin, S.R. Value of PET in the Assessment of Patients with Neurofibromatosis Type 1. Am. J. Roentgenol. 2007, 189, 928–935. [Google Scholar] [CrossRef]
- Warbey, V.S.; Ferner, R.E.; Dunn, J.T.; Calonje, E.; O’Doherty, M.J. [18F]FDG PET/CT in the Diagnosis of Malignant Peripheral Nerve Sheath Tumours in Neurofibromatosis Type-1. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 751–757. [Google Scholar] [CrossRef]
- Berkmen, Y.M.; Zalta, B.A. Case 126: Extramedullary Hematopoiesis. Radiology 2007, 245, 905–908. [Google Scholar] [CrossRef]
- Georgiades, C.S.; Neyman, E.G.; Francis, I.R.; Sneider, M.B.; Fishman, E.K. Typical and Atypical Presentations of Extramedullary Hemopoiesis. Am. J. Roentgenol. 2002, 179, 1239–1243. [Google Scholar] [CrossRef]
- Georgakopoulou, V.E.; Damaskos, C.; Mantzouranis, K.; Melemeni, D.; Gkoufa, A.; Chlapoutakis, S.; Garmpis, N.; Sklapani, P.; Aravantinou, A.; Garmpi, A.; et al. Invasive Methods for the Diagnosis and Management of Intrathoracic Extramedullary Hematopoiesis: A Literature Review. Respir. Med. Res. 2021, 79, 100815. [Google Scholar] [CrossRef]
- Yang, M.; Covington, M.F.; Nguyen, B.D.; Johnson, G.B.; Mesa, R.A.; Roarke, M.C. 99mTc-Sulfur Colloid Bone Marrow Scintigraphy in Diagnosis of Diffuse Pulmonary Extramedullary Hematopoiesis Secondary to Myelofibrosis. J. Nucl. Med. Technol. 2018, 46, 368–372. [Google Scholar] [CrossRef]
- Yang, M.; Roarke, M. Diffuse Pulmonary Extramedullary Hematopoiesis in Myelofibrosis Diagnosed with Technetium-99m Sulfur Colloid Bone Marrow Scintigraphy and Single Photon Emission Computerized Tomography/CT. Am. J. Hematol. 2017, 92, 323–324. [Google Scholar] [CrossRef]
- Iyer, H.; Anand, A.; Sryma, P.; Gupta, K.; Naranje, P.; Damle, N.; Mittal, S.; Madan, N.K.; Mohan, A.; Hadda, V.; et al. Mediastinal Lymphadenopathy: A Practical Approach. Expert Rev. Respir. Med. 2021, 15, 1317–1334. [Google Scholar] [CrossRef]
- Rusch, V.W.; Asamura, H.; Watanabe, H.; Giroux, D.J.; Rami-Porta, R.; Goldstraw, P. The IASLC Lung Cancer Staging Project: A Proposal for a New International Lymph Node Map in the Forthcoming Seventh Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2009, 4, 568–577. [Google Scholar] [CrossRef]
- Suwatanapongched, T.; Gierada, D.S. CT of Thoracic Lymph Nodes. Part I: Anatomy and Drainage. Br. J. Radiol. 2006, 79, 922–928. [Google Scholar] [CrossRef]
- Suwatanapongched, T.; Gierada, D.S. CT of Thoracic Lymph Nodes. Part II: Diseases and Pitfalls. Br. J. Radiol. 2006, 79, 999–1000. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef]
- Martini, N.; Heelan, R.; Westcott, J.; Bains, M.S.; McCormack, P.; Caravelli, J.; Watson, R.; Zaman, M. Comparative Merits of Conventional, Computed Tomographic, and Magnetic Resonance Imaging in Assessing Mediastinal Involvement in Surgically Confirmed Lung Carcinoma. J. Thorac. Cardiovasc. Surg. 1985, 90, 639–648. [Google Scholar] [CrossRef]
- Glazer, G.; Gross, B.; Quint, L.; Francis, I.; Bookstein, F.; Orringer, M. Normal Mediastinal Lymph Nodes: Number and Size According to American Thoracic Society Mapping. Am. J. Roentgenol. 1985, 144, 261–265. [Google Scholar] [CrossRef]
- Remy-Jardin, M.; Duyck, P.; Remy, J.; Petyt, L.; Wurtz, A.; Mensier, E.; Copin, M.C.; Riquet, M. Hilar Lymph Nodes: Identification with Spiral CT and Histologic Correlation. Radiology 1995, 196, 387–394. [Google Scholar] [CrossRef]
- Tirumani, S.H.; Ramaiya, N.H.; Keraliya, A.; Bailey, N.D.; Ott, P.A.; Hodi, F.S.; Nishino, M. Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab. Cancer Immunol. 2015, 3, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Gkiozos, I.; Kopitopoulou, A.; Kalkanis, A.; Vamvakaris, I.N.; Judson, M.A.; Syrigos, K.N. Sarcoidosis-Like Reactions Induced by Checkpoint Inhibitors. J. Thorac. Oncol. 2018, 13, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Vega, F.; Quesada, A.E. Aggressive Mediastinal Lymphomas. Semin. Diagn. Pathol. 2021; in press. [Google Scholar] [CrossRef]
- Criado, E.; Sánchez, M.; Ramírez, J.; Arguis, P.; de Caralt, T.M.; Perea, R.J.; Xaubet, A. Pulmonary Sarcoidosis: Typical and Atypical Manifestations at High-Resolution CT with Pathologic Correlation1. Radiographics 2010, 30, 1567–1586. [Google Scholar] [CrossRef]
- Wynants, J.; Stroobants, S.; Dooms, C.; Vansteenkiste, J. Staging of Lung Cancer. Radiol. Clin. N. Am. 2007, 45, 609–625. [Google Scholar] [CrossRef]
- Mennini, M.L.; Catalano, C.; Monte, M.D.; Fraioli, F. Computed Tomography and Magnetic Resonance Imaging of the Thoracic Lymphatic System. Thorac. Surg. Clin. 2012, 22, 155–160. [Google Scholar] [CrossRef]
- Bhalla, A.; Goyal, A.; Guleria, R.; Gupta, A. Chest Tuberculosis: Radiological Review and Imaging Recommendations. Indian J. Radiol. Imaging 2015, 25, 213–225. [Google Scholar] [CrossRef]
- Harisinghani, M.G.; McLoud, T.C.; Shepard, J.-A.O.; Ko, J.P.; Shroff, M.M.; Mueller, P.R. Tuberculosis from Head to Toe1. Radiographics 2000, 20, 449–470. [Google Scholar] [CrossRef]
- Ganeshan, D.; Menias, C.O.; Lubner, M.G.; Pickhardt, P.J.; Sandrasegaran, K.; Bhalla, S. Sarcoidosis from Head to Toe: What the Radiologist Needs to Know. Radiographics 2018, 38, 1180–1200. [Google Scholar] [CrossRef]
- Begin, R.; Bergeron, D.; Samson, L.; Boctor, M.; Cantin, A. CT Assessment of Silicosis in Exposed Workers. Am. J. Roentgenol. 1987, 148, 509–514. [Google Scholar] [CrossRef]
- Homer, M.J.; Wechsler, R.J.; Carter, B.L. Mediastinal Lipomatosis. Radiology 1978, 128, 657–661. [Google Scholar] [CrossRef]
- Beaudoin, S.; Gonzalez, A.V. Evaluation of the Patient with Pleural effusion. CMAJ 2018, 190, E291–E295. [Google Scholar] [CrossRef]
- Helm, E.J.; Matin, T.N.; Gleeson, F.V. Imaging of the Pleura. J. Magn. Reson. Imaging 2010, 32, 1275–1286. [Google Scholar] [CrossRef]
- Tran, J.; Haussner, W.; Shah, K. Traumatic Pneumothorax: A Review of Current Diagnostic Practices and Evolving Management. J. Emerg. Med. 2021, 61, 517–528. [Google Scholar] [CrossRef]
- Sureka, B.; Thukral, B.B.; Mittal, M.K.; Mittal, A.; Sinha, M. Radiological Review of Pleural Tumors. Indian J. Radiol. Imaging 2013, 23, 313–320. [Google Scholar] [CrossRef]
- Walker, C.M.; Takasugi, J.E.; Chung, J.H.; Reddy, G.P.; Done, S.L.; Pipavath, S.N.; Schmidt, R.A.; Godwin, J.D. Tumorlike Conditions of the Pleura. Radiographics 2012, 32, 971–985. [Google Scholar] [CrossRef]
- Downer, N.J.; Ali, N.J.; Au-Yong, I.T.H. Investigating Pleural Thickening. BMJ 2013, 346, e8376. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, L.; Feragalli, B.; Sacco, R.; Merlino, B.; Storto, M.L. Malignant Pleural Disease. Eur. J. Radiol. 2000, 34, 98–118. [Google Scholar] [CrossRef] [PubMed]
- Bibby, A.C.; Tsim, S.; Kanellakis, N.; Ball, H.; Talbot, D.C.; Blyth, K.G.; Maskell, N.A.; Psallidas, I. Malignant Pleural Mesothelioma: An Update on Investigation, Diagnosis and Treatment. Eur. Respir. Rev. 2016, 25, 472–486. [Google Scholar] [CrossRef]
- Luerken, L.; Thurn, P.L.; Zeman, F.; Stroszczynski, C.; Hamer, O.W. Conspicuity of Malignant Pleural Mesothelioma in Contrast Enhanced MDCT—Arterial Phase or Late Phase? BMC Cancer 2021, 21, 1144. [Google Scholar] [CrossRef]
- Alfudhili, K.M.; Lynch, D.A.; Laurent, F.; Ferretti, G.R.; Dunet, V.; Beigelman-Aubry, C. Focal Pleural Thickening Mimicking Pleural Plaques on Chest Computed Tomography: Tips and Tricks. Br. J. Radiol. 2016, 89, 20150792. [Google Scholar] [CrossRef]
- Claude-Desroches, M.; Bierry, G.; Touitou-Gottenberg, D.; Golmard, J.-L.; Grenier, P.A.; Beigelman-Aubry, C. Focal Dependent Pleural Thickening at MDCT: Pleural Lesion or Functional Abnormality? Diagn. Interv. Imaging 2012, 93, 360–364. [Google Scholar] [CrossRef]
- Nickell, L.T.; Lichtenberger, J.P.; Khorashadi, L.; Abbott, G.F.; Carter, B.W. Multimodality Imaging for Characterization, Classification, and Staging of Malignant Pleural Mesothelioma. Radiographics 2014, 34, 1692–1706. [Google Scholar] [CrossRef]
- Metintas, M.; Ucgun, I.; Elbek, O.; Erginel, S.; Metintas, S.; Kolsuz, M.; Harmanci, E.; Alatas, F.; Hillerdal, G.; Ozkan, R.; et al. Computed Tomography Features in Malignant Pleural Mesothelioma and Other Commonly Seen Pleural Diseases. Eur. J. Radiol. 2002, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yamamuro, M.; Gerbaudo, V.H.; Gill, R.R.; Jacobson, F.L.; Sugarbaker, D.J.; Hatabu, H. Morphologic and Functional Imaging of Malignant Pleural Mesothelioma. Eur. J. Radiol. 2007, 64, 356–366. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, J.S.; Lee, K.W.; Yi, C.A.; Koo, J.M.; Jung, S.H. Multidetector CT Findings and Differential Diagnoses of Malignant Pleural Mesothelioma and Metastatic Pleural Diseases in Korea. Korean J. Radiol. 2016, 17, 545–553. [Google Scholar] [CrossRef]
- Robinson, B.W.S.; Lake, R.A. Advances in Malignant Mesothelioma. N. Engl. J. Med. 2005, 353, 1591–1603. [Google Scholar] [CrossRef]
- Qureshi, N.R.; Gleeson, F.V. Imaging of Pleural Disease. Clin. Chest Med. 2006, 27, 193–213. [Google Scholar] [CrossRef]
- Cardillo, G.; Facciolo, F.; Cavazzana, A.O.; Capece, G.; Gasparri, R.; Martelli, M. Localized (Solitary) Fibrous Tumors of the Pleura: An Analysis of 55 Patients. Ann. Thorac. Surg. 2000, 70, 1808–1812. [Google Scholar] [CrossRef]
- Rosado-de-Christenson, M.L.; Abbott, G.F.; McAdams, H.P.; Franks, T.J.; Galvin, J.R. From the Archives of the AFIP. Localized Fibrous Tumors of the Pleura1. RadioGraphics 2003, 23, 759–783. [Google Scholar] [CrossRef]
- Delgado, D.; Ramírez, O.; Sultan, N.; Miranda, P.; Delgado, A. Pleural Plaques by Inhalation of Asbestos Fibers. Rev. Bras. Med. Trab. 2020, 18, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Lennartz, S.; Blanc, M.L.; Zopfs, D.; Hokamp, N.G.; Abdullayev, N.; Laukamp, K.R.; Haneder, S.; Borggrefe, J.; Maintz, D.; Persigehl, T. Dual-Energy CT-Derived Iodine Maps: Use in Assessing Pleural Carcinomatosis. Radiology 2019, 290, 796–804. [Google Scholar] [CrossRef]
- Jaramillo, F.A.; Gutierrez, F.; Bhalla, S. Pleural Tumours and Tumour-like Lesions. Clin. Radiol. 2018, 73, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Rousset, P.; Rousset-Jablonski, C.; Alifano, M.; Mansuet-Lupo, A.; Buy, J.N.; Revel, M.P. Thoracic Endometriosis Syndrome: CT and MRI Features. Clin. Radiol. 2014, 69, 323–330. [Google Scholar] [CrossRef]
- Arnaud, L.; Pierre, I.; Beigelman-Aubry, C.; Capron, F.; Brun, A.L.; Rigolet, A.; Girerd, X.; Weber, N.; Piette, J.C.; Grenier, P.A.; et al. Pulmonary Involvement in Erdheim-Chester Disease: A Single-Center Study of Thirty-Four Patients and a Review of the Literature. Arthritis Rheum. 2010, 62, 3504–3512. [Google Scholar] [CrossRef]
- Martínez-de-Alegría, A.; Baleato-González, S.; García-Figueiras, R.; Bermúdez-Naveira, A.; Abdulkader-Nallib, I.; Díaz-Peromingo, J.A.; Villalba-Martín, C. IgG4-Related Disease from Head to Toe. Radiographics 2015, 35, 2007–2025. [Google Scholar] [CrossRef]
- Hirano, K.; Kawabe, T.; Komatsu, Y.; Matsubara, S.; Togawa, O.; Arizumi, T.; Yamamoto, N.; Nakai, Y.; Sasahira, N.; Tsujino, T.; et al. High-rate Pulmonary Involvement in Autoimmune Pancreatitis. Intern. Med. J. 2006, 36, 58–61. [Google Scholar] [CrossRef]
- Zen, Y.; Inoue, D.; Kitao, A.; Onodera, M.; Abo, H.; Miyayama, S.; Gabata, T.; Matsui, O.; Nakanuma, Y. IgG4-Related Lung and Pleural Disease: A Clinicopathologic Study of 21 Cases. Am. J. Surg. Pathol. 2009, 33, 1886–1893. [Google Scholar] [CrossRef]
- Inoue, D.; Zen, Y.; Abo, H.; Gabata, T.; Demachi, H.; Kobayashi, T.; Yoshikawa, J.; Miyayama, S.; Yasui, M.; Nakanuma, Y.; et al. Immunoglobulin G4–Related Lung Disease: CT Findings with Pathologic Correlations. Radiology 2009, 251, 260–270. [Google Scholar] [CrossRef]
- Murray, J.F.; Schraufnagel, D.E.; Hopewell, P.C. Treatment of Tuberculosis. A Historical Perspective. Ann. Am. Thorac. Soc. 2015, 12, 1749–1759. [Google Scholar] [CrossRef]
- Eagle, K.; Mond, D.J.; Khan, A. Lucite-Ball Plombage. N. Engl. J. Med. 1994, 330, 1723. [Google Scholar] [CrossRef] [PubMed]
- Koratala, A.; Bhatti, V. Incidental Finding of Oleothorax. N. Engl. J. Med. 2017, 376, e21. [Google Scholar] [CrossRef] [PubMed]
- Ledda, R.E.; Silva, M.; McMichael, N.; Sartorio, C.; Branchi, C.; Milanese, G.; Nayak, S.M.; Sverzellati, N. The Diagnostic Value of Grey-Scale Inversion Technique in Chest Radiography. Radiol. Med. 2022, 127, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.G.; Levin, D.L.; Sykes, A.-M.G.; Lindell, R.M.; White, D.B.; Kuzo, R.S.; Suresh, V.; Yu, L.; Leng, S.; Holmes, D.R.; et al. Observer Performance for Detection of Pulmonary Nodules at Chest CT over a Large Range of Radiation Dose Levels. Radiology 2020, 297, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; Borgheresi, A.; Carotti, M.; Ottaviani, L.; Badaloni, M.; Floridi, C.; Giovagnoni, A. Third-Generation Iterative Reconstruction on a Dual-Source, High-Pitch, Low-Dose Chest CT Protocol with Tin Filter for Spectral Shaping at 100 KV: A Study on a Small Series of COVID-19 Patients. Radiol. Med. 2021, 126, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Rawashdeh, M.A.; Saade, C. Radiation Dose Reduction Considerations and Imaging Patterns of Ground Glass Opacities in Coronavirus: Risk of over Exposure in Computed Tomography. Radiol. Med. 2021, 126, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Tagliati, C.; Lanza, C.; Pieroni, G.; Amici, L.; Carotti, M.; Giuseppetti, G.M.; Giovagnoni, A. Ultra-Low-Dose Chest CT in Adult Patients with Cystic Fibrosis Using a Third-Generation Dual-Source CT Scanner. Radiol. Med. 2021, 126, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.; Daskareh, M.; Gholamrezanezhad, A. The Lingering Manifestations of COVID-19 during and after Convalescence: Update on Long-Term Pulmonary Consequences of Coronavirus Disease 2019 (COVID-19). Radiol. Med. 2021, 126, 40–46. [Google Scholar] [CrossRef]
- Silva, M.; Picozzi, G.; Sverzellati, N.; Anglesio, S.; Bartolucci, M.; Cavigli, E.; Deliperi, A.; Falchini, M.; Falaschi, F.; Ghio, D.; et al. Low-Dose CT for Lung Cancer Screening: Position Paper from the Italian College of Thoracic Radiology. Radiol. Med. 2022, 127, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Peerlings, J.; Troost, E.G.C.; Nelemans, P.J.; Cobben, D.C.P.; Boer, J.C.J.D.; Hoffmann, A.L.; Beets-Tan, R.G.H. The Diagnostic Value of MR Imaging in Determining the Lymph Node Status of Patients with Non-Small Cell Lung Cancer: A Meta-Analysis. Radiology 2016, 281, 86–98. [Google Scholar] [CrossRef]
- Scialpi, M.; Moschini, T.O.; Filippis, G.D. PET/Contrast-Enhanced CT in Oncology: “To Do, or Not to Do, That Is the Question”. Radiol. Med. 2022, 127, 925–927. [Google Scholar] [CrossRef]
- Sakai, H.; Takeda, M. Percutaneous Transthoracic Needle Biopsy of the Lung in the Era of Precision Medicine. J. Thorac. Dis. 2019, 11, S1213–S1215. [Google Scholar] [CrossRef]
- Sabatino, V.; Russo, U.; D’Amuri, F.; Bevilacqua, A.; Pagnini, F.; Milanese, G.; Gentili, F.; Nizzoli, R.; Tiseo, M.; Pedrazzi, G.; et al. Pneumothorax and Pulmonary Hemorrhage after CT-Guided Lung Biopsy: Incidence, Clinical Significance and Correlation. Radiol. Med. 2021, 126, 170–177. [Google Scholar] [CrossRef]
- Loverdos, K.; Fotiadis, A.; Kontogianni, C.; Iliopoulou, M.; Gaga, M. Lung Nodules: A Comprehensive Review on Current Approach and Management. Ann. Thorac. Med. 2019, 14, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Gohagan, J.; Marcus, P.; Fagerstrom, R.; Pinsky, P.; Kramer, B.; Prorok, P. Baseline Findings of a Randomized Feasibility Trial of Lung Cancer Screening With Spiral CT Scan vs Chest Radiograph. Chest 2004, 126, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Swensen, S.J.; Jett, J.R.; Hartman, T.E.; Midthun, D.E.; Sloan, J.A.; Sykes, A.-M.; Aughenbaugh, G.L.; Clemens, M.A. Lung Cancer Screening with CT: Mayo Clinic Experience. Radiology 2003, 226, 756–761. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef]
- de Hoop, B.; van Ginneken, B.; Gietema, H.; Prokop, M. Pulmonary Perifissural Nodules on CT Scans: Rapid Growth Is Not a Predictor of Malignancy. Radiology 2012, 265, 611–616. [Google Scholar] [CrossRef]
- Chung, K.; Jacobs, C.; Scholten, E.T.; Goo, J.M.; Prosch, H.; Sverzellati, N.; Ciompi, F.; Mets, O.M.; Gerke, P.K.; Prokop, M.; et al. Lung-RADS Category 4X: Does It Improve Prediction of Malignancy in Subsolid Nodules? Radiology 2017, 284, 264–271. [Google Scholar] [CrossRef]
- Erasmus, J.J.; Connolly, J.E.; McAdams, H.P.; Roggli, V.L. Solitary Pulmonary Nodules: Part I. Morphologic Evaluation for Differentiation of Benign and Malignant Lesions. RadioGraphics 2000, 20, 43–58. [Google Scholar] [CrossRef]
- Li, J.; Xia, T.; Yang, X.; Dong, X.; Liang, J.; Zhong, N.; Guan, Y. Malignant Solitary Pulmonary Nodules: Assessment of Mass Growth Rate and Doubling Time at Follow-up CT. J. Thorac. Dis. 2018, 10, S797–S806. [Google Scholar] [CrossRef]
- Khan, A.N.; Al-Jahdali, H.H.; Allen, C.M.; Irion, K.L.; Ghanem, S.A.; Koteyar, S.S. The Calcified Lung Nodule: What Does It Mean. Ann. Thorac. Med. 2010, 5, 67–79. [Google Scholar] [CrossRef]
- Devaraj, A.; van Ginneken, B.; Nair, A.; Baldwin, D. Use of Volumetry for Lung Nodule Management: Theory and Practice. Radiology 2017, 284, 630–644. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sone, S.; Takashima, S.; Li, F.; Yang, Z.G.; Maruyama, Y.; Watanabe, T. Growth Rate of Small Lung Cancers Detected on Mass CT Screening. Br. J. Radiol. 2000, 73, 1252–1259. [Google Scholar] [CrossRef]
- Yankelevitz, D.F.; Yip, R.; Smith, J.P.; Liang, M.; Liu, Y.; Xu, D.M.; Salvatore, M.M.; Wolf, A.S.; Flores, R.M.; Henschke, C.I.; et al. CT Screening for Lung Cancer: Nonsolid Nodules in Baseline and Annual Repeat Rounds. Radiology 2015, 277, 555–564. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Mitsudomi, T. Management of Ground-Glass Opacities: Should All Pulmonary Lesions with Ground-Glass Opacity Be Surgically Resected? Transl. Lung Cancer Res. 2013, 2, 354–363. [Google Scholar] [CrossRef]
- Seo, J.B.; Im, J.-G.; Goo, J.M.; Chung, M.J.; Kim, M.-Y. Atypical Pulmonary Metastases: Spectrum of Radiologic Findings1. Radiographics 2001, 21, 403–417. [Google Scholar] [CrossRef]
- American College of Radiology Lung CT Screening Reporting & Data System (Lung-RADS®). Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads (accessed on 2 June 2023).
- American College of Radiology Lung-RADS V2022 Assessment Categories. Available online: https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/Lung-RADS-2022.pdf (accessed on 2 June 2023).
- Yip, R.; Yankelevitz, D.F.; Hu, M.; Li, K.; Xu, D.M.; Jirapatnakul, A.; Henschke, C.I. Lung Cancer Deaths in the National Lung Screening Trial Attributed to Nonsolid Nodules. Radiology 2016, 281, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Henschke, C.I.; Lee, I.J.; Wu, N.; Farooqi, A.; Khan, A.; Yankelevitz, D.; Altorki, N.K. CT Screening for Lung Cancer: Prevalence and Incidence of Mediastinal Masses. Radiology 2006, 239, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.S. The CT Halo Sign. Radiology 2004, 230, 109–110. [Google Scholar] [CrossRef]
- Lee, H.J.; Goo, J.M.; Lee, C.H.; Park, C.M.; Kim, K.G.; Park, E.A.; Lee, H.Y. Predictive CT Findings of Malignancy in Ground-Glass Nodules on Thin-Section Chest CT: The Effects on Radiologist Performance. Eur. Radiol. 2009, 19, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Kwon, S.Y.; Yoon, H.I.; Lee, S.M.; Yim, J.J.; Lee, J.H.; Yoo, C.G.; Kim, Y.W.; Han, S.K.; Shim, Y.S.; et al. Clinical Significance of a Solitary Ground-Glass Opacity (GGO) Lesion of the Lung Detected by Chest CT. Lung Cancer 2007, 55, 67–73. [Google Scholar] [CrossRef]
- Kim, H.; Park, C.M.; Koh, J.M.; Lee, S.M.; Goo, J.M. Pulmonary Subsolid Nodules: What Radiologists Need to Know about the Imaging Features and Management Strategy. Diagn. Interv. Radiol. 2014, 20, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Awai, K.; Murao, K.; Ozawa, A.; Utsunomiya, D.; Yanaga, Y.; Kawanaka, K.; Yamashita, Y. Volume-Doubling Time of Pulmonary Nodules with Ground Glass Opacity at Multidetector CT. Assessment with Computer-Aided Three-Dimensional Volumetry. Acad. Radiol. 2011, 18, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hoop, B.D.; Gietema, H.; Vorst, S.V.D.; Murphy, K.; Klaveren, R.J.V.; Prokop, M. Pulmonary Ground-Glass Nodules: Increase in Mass as an Early Indicator of Growth. Radiology 2010, 255, 199–206. [Google Scholar] [CrossRef]
- Godoy, M.C.B.; Naidich, D.P. Subsolid Pulmonary Nodules and the Spectrum of Peripheral Adenocarcinomas of the Lung: Recommended Interim Guidelines for Assessment and Management. Radiology 2009, 253, 606–622. [Google Scholar] [CrossRef] [PubMed]
- Marchiori, E.; Zanetti, G.; Meirelles, G.S.P.; Escuissato, D.L.; Souza, A.S.; Hochhegger, B. The Reversed Halo Sign on High-Resolution CT in Infectious and Noninfectious Pulmonary Diseases. Am. J. Roentgenol. 2011, 197, W69–W75. [Google Scholar] [CrossRef]
- Li, F.; Sone, S.; Abe, H.; MacMahon, H.; Doi, K. Malignant versus Benign Nodules at CT Screening for Lung Cancer: Comparison of Thin-Section CT Findings. Radiology 2004, 233, 793–798. [Google Scholar] [CrossRef]
- Edey, A.J.; Hansell, D.M. Incidentally Detected Small Pulmonary Nodules on CT. Clin. Radiol. 2009, 64, 872–884. [Google Scholar] [CrossRef]
- Ahn, M.I.; Gleeson, T.G.; Chan, I.H.; McWilliams, A.M.; MacDonald, S.L.; Lam, S.; Atkar-Khattra, S.; Mayo, J.R. Perifissural Nodules Seen at CT Screening for Lung Cancer. Radiology 2010, 254, 949–956. [Google Scholar] [CrossRef]
- Revel, M.-P. Avoiding Overdiagnosis in Lung Cancer Screening: The Volume Doubling Time Strategy. Eur. Respir. J. 2013, 42, 1459–1463. [Google Scholar] [CrossRef]
- Takashima, S.; Sone, S.; Li, F.; Maruyama, Y.; Hasegawa, M.; Matsushita, T.; Takayama, F.; Kadoya, M. Small Solitary Pulmonary Nodules (≤1 cm) Detected at Population-Based CT Screening for Lung Cancer: Reliable High-Resolution CT Features of Benign Lesions. Am. J. Roentgenol. 2003, 180, 955–964. [Google Scholar] [CrossRef]
- Serafin, Z. Rounded Atelectasis of the Lung: A Pictorial Review. Pol. J. Radiol. 2014, 79, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.; Lawler, G. CT Features of Rounded Atelectasis of the Lung. Am. J. Roentgenol. 1984, 143, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Yudin, A. Rounded Atelectasis, Comet-Tail Sign, and Crow’s Foot Sign. In Metaphorical Signs in Computed Tomography of Chest and Abdomen; Springer International Publishing: Cham, Switzerland, 2014; p. 45. [Google Scholar]
- Partap, V.A. The Comet Tail Sign. Radiology 1999, 213, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Siegelman, S.S.; Khouri, N.F.; Scott, W.W.; Leo, F.P.; Hamper, U.M.; Fishman, E.K.; Zerhouni, E.A. Pulmonary Hamartoma: CT Findings. Radiology 1986, 160, 313–317. [Google Scholar] [CrossRef]
- Winer-Muram, H.T. The Solitary Pulmonary Nodule. Radiology 2006, 239, 34–49. [Google Scholar] [CrossRef]
- Seemann, M.D.; Staebler, A.; Beinert, T.; Dienemann, H.; Obst, B.; Matzko, M.; Pistitsch, C.; Reiser, M.F. Usefulness of Morphological Characteristics for the Differentiation of Benign from Malignant Solitary Pulmonary Lesions Using HRCT. Eur. Radiol. 1999, 9, 409–417. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, C.M.; Goo, J.M.; Lee, H.-J.; Wi, J.Y.; Kang, C.H. Invasive Pulmonary Adenocarcinomas versus Preinvasive Lesions Appearing as Ground-Glass Nodules: Differentiation by Using CT Features. Radiology 2013, 268, 265–273. [Google Scholar] [CrossRef]
- Hsu, J.-S.; Han, I.-T.; Tsai, T.-H.; Lin, S.-F.; Jaw, T.-S.; Liu, G.-C.; Chou, S.-H.; Chong, I.-W.; Chen, C.-Y. Pleural Tags on CT Scans to Predict Visceral Pleural Invasion of Non–Small Cell Lung Cancer That Does Not Abut the Pleura. Radiology 2016, 279, 590–596. [Google Scholar] [CrossRef]
- Harders, S.W.; Madsen, H.H.; Rasmussen, T.R.; Hager, H.; Rasmussen, F. High Resolution Spiral CT for Determining the Malignant Potential of Solitary Pulmonary Nodules: Refining and Testing the Test. Acta Radiol. 2011, 52, 401–409. [Google Scholar] [CrossRef]
- Hochhegger, B.; Nin, C.S.; Alves, G.R.T.; Hochhegger, D.R.; Souza, V.V.S.D.; Watte, G.; Irion, K.L.; Guimarães, M.D.; Marchiori, E. Multidetector Computed Tomography Findings in Pulmonary Hamartomas a New Fat Detection Threshold. J. Thorac. Imaging 2016, 31, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Hochhegger, B.; Marchiori, E.; Reis, D.Q.D.; Souza, A.S.; Souza, L.S.; Brum, T.; Irion, K.L. Chemical-Shift MRI of Pulmonary Hamartomas: Initial Experience Using a Modified Technique to Assess Nodule Fat. Am. J. Roentgenol. 2012, 199, W331–W334. [Google Scholar] [CrossRef] [PubMed]
- Grewal, R.G.; Austin, J.H.M. CT Demonstration of Calcification in Carcinoma of the Lung. J. Comput. Assist. Tomogr. 1994, 18, 867–871. [Google Scholar] [CrossRef]
- Diederich, S.; Wormanns, D.; Semik, M.; Thomas, M.; Lenzen, H.; Roos, N.; Heindel, W. Screening for Early Lung Cancer with Low-Dose Spiral CT: Prevalence in 817 Asymptomatic Smokers. Radiology 2002, 222, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, M.C.; Shipley, R.T.; Corcoran, H.L.; Dickson, B.A. CT Demonstration of Calcification in Carcinoma of the Lung. Am. J. Roentgenol. 1990, 154, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Gurney, J.W. Determining the Likelihood of Malignancy in Solitary Pulmonary Nodules with Bayesian Analysis. Part I. Theory. Radiology 1993, 186, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Thalinger, A.R.; Rosenthal, S.N.; Borg, S.; Arseneau, J.C. Cavitation of Pulmonary Metastases as a Response to Chemotherapy. Cancer 1980, 46, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Truong, M.T.; Ko, J.P.; Rossi, S.E.; Rossi, I.; Viswanathan, C.; Bruzzi, J.F.; Marom, E.M.; Erasmus, J.J. Update in the Evaluation of the Solitary Pulmonary Nodule. RadioGraphics 2014, 34, 1658–1679. [Google Scholar] [CrossRef]
- Stark, D.; Federle, M.; Goodman, P.; Podrasky, A.; Webb, W. Differentiating Lung Abscess and Empyema: Radiography and Computed Tomography. Am. J. Roentgenol. 1983, 141, 163–167. [Google Scholar] [CrossRef]
- Inchaustegui, C.A.; Wang, K.Y.; Teniola, O.; de Rosen, V.L. Large Septic Pulmonary Embolus Complicating Streptococcus Mutans Pulmonary Valve Endocarditis. J. Radiol. Case Rep. 2018, 12, 18–27. [Google Scholar] [CrossRef]
- Watanabe, T.; Yokoe, M.; Noguchi, Y. Septic Pulmonary Embolism Associated with Periodontal Disease: A Case Report and Literature Review. BMC Infect. Dis. 2019, 19, 74. [Google Scholar] [CrossRef]
- Baidya, A.; Ganakumar, V.; Jadon, R.S.; Ranjan, P.; Manchanda, S.; Sood, R. Septic Pulmonary Emboli as a Complication of Peripheral Venous Cannula Insertion. Drug Discov. Ther. 2018, 12, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Huang, Y.; Song, W.; Zheng, F.; Wang, X.; Xu, X.; Wang, Z.; Jiang, J.; Jin, Z. Clinical Features of Pulmonary Cryptococcosis in Thin-Section CT in Immunocompetent and Non-AIDS Immunocompromised Patients. Radiol. Med. 2020, 125, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.; Li, J. Thoracic Manifestation of Wegener’s Granulomatosis: Computed Tomography Findings and Analysis of Misdiagnosis. Exp. Ther. Med. 2018, 16, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.; Chung, J.H.; Digumarthy, S.R.; Kanne, J.P.; Abbott, G.F.; Shepard, J.A.O.; Mark, E.J.; Sharma, A. Common and Uncommon Manifestations of Wegener Granulomatosis at Chest CT: Radiologic-Pathologic Correlation. Radiographics 2012, 32, 51–69. [Google Scholar] [CrossRef]
- Ananthakrishnan, L.; Sharma, N.; Kanne, J.P. Wegener’s Granulomatosis in the Chest: High-Resolution CT Findings. Am. J. Roentgenol. 2009, 192, 676–682. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Nagata, K.; Nakanishi, M.; Natuhara, A.; Harada, H.; Kubota, Y.; Yokomura, I.; Hashimoto, S.; Nakagawa, M. Spiral CT Findings in Septic Pulmonary Emboli. Eur. J. Radiol. 2001, 37, 190–194. [Google Scholar] [CrossRef]
- Kui, N.A.; Templeton, P.A.; White, C.S.; Zu-Long, C.; You-Xian, B.; You-Quan, C. Evaluation of the Air Bronchogram Sign on CT in Solitary Pulmonary Lesions. J. Comput. Assist. Tomogr. 1996, 20, 983–986. [Google Scholar] [CrossRef]
- Qiang, J.W.; Zhou, K.R.; Lu, G.; Wang, Q.; Ye, X.G.; Xu, S.T.; Tan, L.J. The Relationship between Solitary Pulmonary Nodules and Bronchi: Multi-Slice CT–Pathological Correlation. Clin. Radiol. 2004, 59, 1121–1127. [Google Scholar] [CrossRef]
- Kim, T.J.; Goo, J.M.; Lee, K.W.; Park, C.M.; Lee, H.J. Clinical, Pathological and Thin-Section CT Features of Persistent Multiple Ground-Glass Opacity Nodules: Comparison with Solitary Ground-Glass Opacity Nodule. Lung Cancer 2009, 64, 171–178. [Google Scholar] [CrossRef]
- Farooqi, A.O.; Cham, M.; Zhang, L.; Beasley, M.B.; Austin, J.H.M.; Miller, A.; Zulueta, J.J.; Roberts, H.; Enser, C.; Kao, S.-J.; et al. Lung Cancer Associated With Cystic Airspaces. Am. J. Roentgenol. 2012, 199, 781–786. [Google Scholar] [CrossRef]
- Scholten, E.T.; Horeweg, N.; de Koning, H.J.; Vliegenthart, R.; Oudkerk, M.; Mali, W.P.T.M.; de Jong, P.A. Computed Tomographic Characteristics of Interval and Post Screen Carcinomas in Lung Cancer Screening. Eur. Radiol. 2015, 25, 81–88. [Google Scholar] [CrossRef]
- Mascalchi, M.; Attinà, D.; Bertelli, E.; Falchini, M.; Vella, A.; Pegna, A.L.; Ambrosini, V.; Zompatori, M. Lung Cancer Associated With Cystic Airspaces. J. Comput. Assist. Tomogr. 2015, 39, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Sheard, S.; Moser, J.; Sayer, C.; Stefanidis, K.; Devaraj, A.; Vlahos, I. Lung Cancers Associated with Cystic Airspaces: Underrecognized Features of Early Disease. Radiographics 2018, 38, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Gruden, J.F.; Naidich, D.P.; Machnicki, S.C.; Cohen, S.L.; Girvin, F.; Raoof, S. An Algorithmic Approach to the Interpretation of Diffuse Lung Disease on Chest CT Imaging: A Theory of Almost Everything. Chest 2020, 157, 612–635. [Google Scholar] [CrossRef] [PubMed]
- Ajith Kumar, A.K.; Mantri, S.N. Lymphangitic Carcinomatosis; [Updated 2022 Sep 19]; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560921/ (accessed on 2 June 2023).
- Cellina, M.; Gibelli, D.; Martinenghi, C.; Giardini, D.; Soresina, M.; Menozzi, A.; Oliva, G.; Carrafiello, G. Non-Contrast Magnetic Resonance Lymphography (NCMRL) in Cancer-Related Secondary Lymphedema: Acquisition Technique and Imaging Findings. Radiol. Med. 2021, 126, 1477–1486. [Google Scholar] [CrossRef]
- Klimek, M. Pulmonary Lymphangitis Carcinomatosis: Systematic Review and Meta-Analysis of Case Reports, 1970–2018. Postgrad. Med. 2019, 131, 309–318. [Google Scholar] [CrossRef]
- Bordonaro, V.; Ciancarella, P.; Ciliberti, P.; Curione, D.; Napolitano, C.; Santangelo, T.P.; Natali, G.L.; Rollo, M.; Guccione, P.; Pasquini, L.; et al. Dynamic Contrast-Enhanced Magnetic Resonance Lymphangiography in Pediatric Patients with Central Lymphatic System Disorders. Radiol. Med. 2021, 126, 737–743. [Google Scholar] [CrossRef]
- Andreu, J.; Hidalgo, A.; Pallisa, E.; Majó, J.; Martinez-Rodriguez, M.; Cáceres, J. Septal Thickening: HRCT Findings and Differential Diagnosis. Curr. Probl. Diagn. Radiol. 2004, 33, 226–237. [Google Scholar] [CrossRef]
- Fusco, R.; Simonetti, I.; Ianniello, S.; Villanacci, A.; Grassi, F.; Dell’Aversana, F.; Grassi, R.; Cozzi, D.; Bicci, E.; Palumbo, P.; et al. Pulmonary Lymphangitis Poses a Major Challenge for Radiologists in an Oncological Setting during the COVID-19 Pandemic. J. Pers. Med. 2022, 12, 624. [Google Scholar] [CrossRef]
- Silva, C.I.S.; Flint, J.D.; Levy, R.D.; Müller, N.L. Diffuse Lung Cysts in Lymphoid Interstitial Pneumonia. J. Thorac. Imag. 2006, 21, 241–244. [Google Scholar] [CrossRef]
- Zaveri, J.; La, Q.; Yarmish, G.; Neuman, J. More than Just Langerhans Cell Histiocytosis: A Radiologic Review of Histiocytic Disorders. Radiographics 2014, 34, 2008–2024. [Google Scholar] [CrossRef]
- Mendelson, D.S.; Wasserstein, M.P.; Desnick, R.J.; Glass, R.; Simpson, W.; Skloot, G.; Vanier, M.; Bembi, B.; Giugliani, R.; Mengel, E.; et al. Type B Niemann-Pick Disease: Findings at Chest Radiography, Thin-Section CT, and Pulmonary Function Testing. Radiology 2006, 238, 339–345. [Google Scholar] [CrossRef]
- Miller, B.H.; Rosado-de-Christenson, M.L.; McAdams, H.P.; Fishback, N.F. Thoracic Sarcoidosis: Radiologic-Pathologic Correlation. Radiographics 1995, 15, 421–437. [Google Scholar] [CrossRef]
- Polverosi, R.; Russo, R.; Coran, A.; Battista, A.; Agostini, C.; Pomerri, F.; Giraudo, C. Typical and Atypical Pattern of Pulmonary Sarcoidosis at High-Resolution CT: Relation to Clinical Evolution and Therapeutic Procedures. Radiol. Med. 2014, 119, 384–392. [Google Scholar] [CrossRef]
- Schaefer-Prokop, C.; Prokop, M.; Fleischmann, D.; Herold, C. High-Resolution CT of Diffuse Interstitial Lung Disease: Key Findings in Common Disorders. Eur. Radiol. 2001, 11, 373–392. [Google Scholar] [CrossRef]
- Chong, S.; Lee, K.S.; Chung, M.J.; Han, J.; Kwon, O.J.; Kim, T.S. Pneumoconiosis: Comparison of Imaging and Pathologic Findings. RadioGraphics 2006, 26, 59–77. [Google Scholar] [CrossRef]
- Georgiades, C.S.; Neyman, E.G.; Barish, M.A.; Fishman, E.K. Amyloidosis: Review and CT Manifestations. RadioGraphics 2004, 24, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Mang, C.; Grosse, C.; Schmid, K.; Stiebellehner, L.; Bankier, A.A. What Every Radiologist Should Know about Idiopathic Interstitial Pneumonias. Radiographics 2007, 27, 595–615. [Google Scholar] [CrossRef]
- Attili, A.K.; Kazerooni, E.A.; Gross, B.H.; Flaherty, K.R.; Myers, J.L.; Martinez, F.J. Smoking-Related Interstitial Lung Disease: Radiologic-Clinical-Pathologic Correlation. Radiographics 2008, 28, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Yagihashi, K.; Huckleberry, J.; Colby, T.V.; Tazelaar, H.D.; Zach, J.; Sundaram, B.; Pipavath, S.; Schwarz, M.I.; Lynch, D.A.; (IPFnet), I.P.F.C.R.N. Radiologic–Pathologic Discordance in Biopsy-Proven Usual Interstitial Pneumonia. Eur. Respir. J. 2016, 47, 1189–1197. [Google Scholar] [CrossRef]
- Rea, G.; Martino, M.D.; Capaccio, A.; Dolce, P.; Valente, T.; Castaldo, S.; Canora, A.; Lassandro, F.; Bocchino, M. Comparative Analysis of Density Histograms and Visual Scores in Incremental and Volumetric High-Resolution Computed Tomography of the Chest in Idiopathic Pulmonary Fibrosis Patients. Radiol. Med. 2021, 126, 599–607. [Google Scholar] [CrossRef]
- Lynch, D.A.; Austin, J.H.M.; Hogg, J.C.; Grenier, P.A.; Kauczor, H.U.; Bankier, A.A.; Barr, R.G.; Colby, T.V.; Galvin, J.R.; Gevenois, P.A.; et al. CT-Definable Subtypes of Chronic Obstructive Pulmonary Disease: A Statement of the Fleischner Society1. Radiology 2015, 277, 192–205. [Google Scholar] [CrossRef]
- Bellardita, L.; Colciago, R.R.; Frasca, S.; Santis, M.C.D.; Gay, S.; Palorini, F.; Rocca, E.L.; Valdagni, R.; Rancati, T.; Lozza, L. Breast Cancer Patient Perspective on Opportunities and Challenges of a Genetic Test Aimed to Predict Radio-Induced Side Effects before Treatment: Analysis of the Italian Branch of the REQUITE Project. Radiol. Med. 2021, 126, 1366–1373. [Google Scholar] [CrossRef]
- Borghetti, P.; Branz, J.; Volpi, G.; Pancera, S.; Buraschi, R.; Bianchi, L.N.C.; Bonù, M.L.; Greco, D.; Facheris, G.; Tomasi, C.; et al. Home-Based Pulmonary Rehabilitation in Patients Undergoing (Chemo)Radiation Therapy for Unresectable Lung Cancer: A Prospective Explorative Study. Radiol. Med. 2022, 127, 1322–1332. [Google Scholar] [CrossRef]
- Mega, S.; Fiore, M.; Carpenito, M.; Novembre, M.L.; Miele, M.; Trodella, L.E.; Grigioni, F.; Ippolito, E.; Ramella, S. Early GLS Changes Detection after Chemoradiation in Locally Advanced Non-Small Cell Lung Cancer (NSCLC). Radiol. Med. 2022, 127, 1355–1363. [Google Scholar] [CrossRef]
- Arslan, A.; Aktas, E.; Sengul, B.; Tekin, B. Dosimetric Evaluation of Left Ventricle and Left Anterior Descending Artery in Left Breast Radiotherapy. Radiol. Med. 2021, 126, 14–21. [Google Scholar] [CrossRef]
- Choi, Y.W.; Munden, R.F.; Erasmus, J.J.; Park, K.J.; Chung, W.K.; Jeon, S.C.; Park, C.K. Effects of Radiation Therapy on the Lung: Radiologic Appearances and Differential Diagnosis. Radiographics 2004, 24, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Akira, M.; Ishikawa, H.; Yamamoto, S. Drug-Induced Pneumonitis: Thin-Section CT Findings in 60 Patients. Radiology 2002, 224, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.E.; Erasmus, J.J.; McAdams, H.P.; Sporn, T.A.; Goodman, P.C. Pulmonary Drug Toxicity: Radiologic and Pathologic Manifestations. Radiographics 2000, 20, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Hino, T.; Han, J.; Franks, T.J.; Im, Y.; Hatabu, H.; Chung, M.P.; Lee, K.S. Connective Tissue Disease-Related Interstitial Lung Disease (CTD-ILD) and Interstitial Lung Abnormality (ILA): Evolving Concept of CT Findings, Pathology and Management. Eur. J. Radiol. Open 2021, 8, 100311. [Google Scholar] [CrossRef]
- Sverzellati, N.; Odone, A.; Silva, M.; Polverosi, R.; Florio, C.; Cardinale, L.; Cortese, G.; Addonisio, G.; Zompatori, M.; Dalpiaz, G.; et al. Structured Reporting for Fibrosing Lung Disease: A Model Shared by Radiologist and Pulmonologist. Radiol. Med. 2018, 123, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Watadani, T.; Sakai, F.; Johkoh, T.; Noma, S.; Akira, M.; Fujimoto, K.; Bankier, A.A.; Lee, K.S.; Müller, N.L.; Song, J.W.; et al. Interobserver Variability in the CT Assessment of Honeycombing in the Lungs. Radiology 2013, 266, 936–944. [Google Scholar] [CrossRef]
- Boitsios, G.; Bankier, A.A.; Eisenberg, R.L. Diffuse Pulmonary Nodules. Am. J. Roentgenol. 2010, 194, W354–W366. [Google Scholar] [CrossRef]
- Nakatsu, M.; Hatabu, H.; Morikawa, K.; Uematsu, H.; Ohno, Y.; Nishimura, K.; Nagai, S.; Izumi, T.; Konishi, J.; Itoh, H. Large Coalescent Parenchymal Nodules in Pulmonary Sarcoidosis: “Sarcoid Galaxy” Sign. Am. J. Roentgenol. 2002, 178, 1389–1393. [Google Scholar] [CrossRef]
- Gruden, J.F.; Webb, W.R.; Warnock, M. Centrilobular Opacities in the Lung on High-Resolution CT: Diagnostic Considerations and Pathologic Correlation. Am. J. Roentgenol. 1994, 162, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.M.; Schmidt, R.A.; Godwin, J.D.; Raghu, G. High Resolution CT in Respiratory Bronchiolitis-Associated Interstitial Lung Disease. J. Comput. Assist. Tomogr. 1993, 17, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.I.S.; Churg, A.; Müller, N.L. Hypersensitivity Pneumonitis: Spectrum of High-Resolution CT and Pathologic Findings. Am. J. Roentgenol. 2007, 188, 334–344. [Google Scholar] [CrossRef]
- Hirschmann, J.V.; Pipavath, S.N.J.; Godwin, J.D. Hypersensitivity Pneumonitis: A Historical, Clinical, and Radiologic Review. Radiographics 2009, 29, 1921–1938. [Google Scholar] [CrossRef]
- Rossi, S.E.; Franquet, T.; Volpacchio, M.; Giménez, A.; Aguilar, G. Tree-in-Bud Pattern at Thin-Section CT of the Lungs: Radiologic-Pathologic Overview. RadioGraphics 2005, 25, 789–801. [Google Scholar] [CrossRef]
- Palmucci, S.; Inì, C.; Cosentino, S.; Fanzone, L.; Pietro, S.D.; Mari, A.D.; Galioto, F.; Tiralongo, F.; Vignigni, G.; Toscano, S.; et al. Pulmonary Vasculitides: A Radiological Review Emphasizing Parenchymal HRCT Features. Diagnostics 2021, 11, 2318. [Google Scholar] [CrossRef]
- Specks, U. Pulmonary Manifestations of Vasculitis. In Pulmonary Manifestations of Rheumatic Disease; Dellaripa, P., Fischer, A., Flaherty, K., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Nachiappan, A.C.; Rahbar, K.; Shi, X.; Guy, E.S.; Barbosa, E.J.M.; Shroff, G.S.; Ocazionez, D.; Schlesinger, A.E.; Katz, S.I.; Hammer, M.M. Pulmonary Tuberculosis: Role of Radiology in Diagnosis and Management. Radiographics 2017, 37, 52–72. [Google Scholar] [CrossRef]
- Miller, W.T.; Shah, R.M. Isolated Diffuse Ground-Glass Opacity in Thoracic CT: Causes and Clinical Presentations. Am. J. Roentgenol. 2005, 184, 613–622. [Google Scholar] [CrossRef]
- Park, C.M.; Goo, J.M.; Lee, H.J.; Lee, C.H.; Chun, E.J.; Im, J.-G. Nodular Ground-Glass Opacity at Thin-Section CT: Histologic Correlation and Evaluation of Change at Follow-Up. RadioGraphics 2007, 27, 391–408. [Google Scholar] [CrossRef]
- Özel, M.; Aslan, A.; Araç, S. Use of the COVID-19 Reporting and Data System (CO-RADS) Classification and Chest Computed Tomography Involvement Score (CT-IS) in COVID-19 Pneumonia. Radiol. Med. 2021, 126, 679–687. [Google Scholar] [CrossRef]
- Rizzo, S.; Catanese, C.; Puligheddu, C.; Epistolio, S.; Ramelli, G.; Frattini, M.; Mestre, R.P.; Nadarajah, N.; Rezzonico, E.; Magoga, F.; et al. CT Evaluation of Lung Infiltrates in the Two Months Preceding the Coronavirus Disease 19 Pandemic in Canton Ticino (Switzerland): Were There Suspicious Cases before the Official First Case? Radiol. Med. 2022, 127, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Cereser, L.; Girometti, R.; Re, J.D.; Marchesini, F.; Como, G.; Zuiani, C. Inter-Reader Agreement of High-Resolution Computed Tomography Findings in Patients with COVID-19 Pneumonia: A Multi-Reader Study. Radiol. Med. 2021, 126, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Gerasia, R.; Mamone, G.; Amato, S.; Cucchiara, A.; Gallo, G.S.; Tafaro, C.; Fiorello, G.; Caruso, C.; Miraglia, R. COVID-19 Safety Measures at the Radiology Unit of a Transplant Institute: The Non-COVID-19 Patient’s Confidence with Safety Procedures. Radiol. Med. 2022, 127, 426–432. [Google Scholar] [CrossRef]
- Moroni, C.; Cozzi, D.; Albanesi, M.; Cavigli, E.; Bindi, A.; Luvarà, S.; Busoni, S.; Mazzoni, L.N.; Grifoni, S.; Nazerian, P.; et al. Chest X-Ray in the Emergency Department during COVID-19 Pandemic Descending Phase in Italy: Correlation with Patients’ Outcome. Radiol. Med. 2021, 126, 661–668. [Google Scholar] [CrossRef]
- Novelli, F.; Pinelli, V.; Chiaffi, L.; Carletti, A.M.; Sivori, M.; Giannoni, U.; Chiesa, F.; Celi, A. Prognostic Significance of Peripheral Consolidations at Chest X-Ray in Severe COVID-19 Pneumonia. Radiol. Med. 2022, 127, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Gabelloni, M.; Faggioni, L.; Cioni, D.; Mendola, V.; Falaschi, Z.; Coppola, S.; Corradi, F.; Isirdi, A.; Brandi, N.; Coppola, F.; et al. Extracorporeal Membrane Oxygenation (ECMO) in COVID-19 Patients: A Pocket Guide for Radiologists. Radiol. Med. 2022, 127, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, A.; Golemi, S.; Scrimieri, A.; Nicosia, C.M.C.; Zigliani, A.; Farina, D.; Maroldi, R. Chest X-Ray versus Chest Computed Tomography for Outcome Prediction in Hospitalized Patients with COVID-19. Radiol. Med. 2022, 127, 305–308. [Google Scholar] [CrossRef]
- Pecoraro, M.; Cipollari, S.; Marchitelli, L.; Messina, E.; Monte, M.D.; Galea, N.; Ciardi, M.R.; Francone, M.; Catalano, C.; Panebianco, V. Cross-Sectional Analysis of Follow-up Chest MRI and Chest CT Scans in Patients Previously Affected by COVID-19. Radiol. Med. 2021, 126, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Dalpiaz, G.; Gamberini, L.; Carnevale, A.; Spadaro, S.; Mazzoli, C.A.; Piciucchi, S.; Allegri, D.; Capozzi, C.; Neziri, E.; Bartolucci, M.; et al. Clinical Implications of Microvascular CT Scan Signs in COVID-19 Patients Requiring Invasive Mechanical Ventilation. Radiol. Med. 2022, 127, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Palmisano, A.; Cao, R.; Rancoita, P.; Landoni, G.; Grippaldi, D.; Boccia, E.; Cosenza, M.; Messina, A.; Marca, S.L.; et al. Quantitative Assessment of Lung Involvement on Chest CT at Admission: Impact on Hypoxia and Outcome in COVID-19 Patients. Clin. Imaging 2021, 77, 194–201. [Google Scholar] [CrossRef]
- Kao, Y.S.; Lin, K.T. A Meta-Analysis of the Diagnostic Test Accuracy of CT-Based Radiomics for the Prediction of COVID-19 Severity. Radiol. Med. 2022, 127, 754–762. [Google Scholar] [CrossRef]
- Cardobi, N.; Benetti, G.; Cardano, G.; Arena, C.; Micheletto, C.; Cavedon, C.; Montemezzi, S. CT Radiomic Models to Distinguish COVID-19 Pneumonia from Other Interstitial Pneumonias. Radiol. Med. 2021, 126, 1037–1043. [Google Scholar] [CrossRef]
- Grassi, R.; Belfiore, M.P.; Montanelli, A.; Patelli, G.; Urraro, F.; Giacobbe, G.; Fusco, R.; Granata, V.; Petrillo, A.; Sacco, P.; et al. COVID-19 Pneumonia: Computer-Aided Quantification of Healthy Lung Parenchyma, Emphysema, Ground Glass and Consolidation on Chest Computed Tomography (CT). Radiol. Med. 2021, 126, 553–560. [Google Scholar] [CrossRef]
- Salvatore, C.; Roberta, F.; Angela, D.L.; Cesare, P.; Alfredo, C.; Giuliano, G.; Giulio, L.; Giuliana, G.; Maria, R.G.; Paola, B.M.; et al. Clinical and Laboratory Data, Radiological Structured Report Findings and Quantitative Evaluation of Lung Involvement on Baseline Chest CT in COVID-19 Patients to Predict Prognosis. Radiol. Med. 2021, 126, 29–39. [Google Scholar] [CrossRef]
- Neri, E.; Miele, V.; Coppola, F.; Grassi, R. Use of CT and Artificial Intelligence in Suspected or COVID-19 Positive Patients: Statement of the Italian Society of Medical and Interventional Radiology. Radiol. Med. 2020, 125, 505–508. [Google Scholar] [CrossRef]
- Caruso, D.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Guido, G.; Polidori, T.; Rucci, C.; Bracci, B.; Tremamunno, G.; Laghi, A. Diagnostic Performance of CT Lung Severity Score and Quantitative Chest CT for Stratification of COVID-19 Patients. Radiol. Med. 2022, 127, 309–317. [Google Scholar] [CrossRef]
- Caruso, D.; Polici, M.; Zerunian, M.; Pucciarelli, F.; Polidori, T.; Guido, G.; Rucci, C.; Bracci, B.; Muscogiuri, E.; Dominicis, C.D.; et al. Quantitative Chest CT Analysis in Discriminating COVID-19 from Non-COVID-19 Patients. Radiol. Med. 2021, 126, 243–249. [Google Scholar] [CrossRef]
- Masci, G.M.; Iafrate, F.; Ciccarelli, F.; Pambianchi, G.; Panebianco, V.; Pasculli, P.; Ciardi, M.R.; Mastroianni, C.M.; Ricci, P.; Catalano, C.; et al. Tocilizumab Effects in COVID-19 Pneumonia: Role of CT Texture Analysis in Quantitative Assessment of Response to Therapy. Radiol. Med. 2021, 126, 1170–1180. [Google Scholar] [CrossRef]
- Agarwal, M.; van der Pol, C.B.; Patlas, M.N.; Udare, A.; Chung, A.D.; Rubino, J. Optimizing the Radiologist Work Environment: Actionable Tips to Improve Workplace Satisfaction, Efficiency, and Minimize Burnout. Radiol. Med. 2021, 126, 1255–1257. [Google Scholar] [CrossRef]
- Palmisano, A.; Vignale, D.; Boccia, E.; Nonis, A.; Gnasso, C.; Leone, R.; Montagna, M.; Nicoletti, V.; Bianchi, A.G.; Brusamolino, S.; et al. AI-SCoRE (Artificial Intelligence-SARS CoV2 Risk Evaluation): A Fast, Objective and Fully Automated Platform to Predict the Outcome in COVID-19 Patients. Radiol. Med. 2022, 127, 960–972. [Google Scholar] [CrossRef]
- Coppola, F.; Faggioni, L.; Regge, D.; Giovagnoni, A.; Golfieri, R.; Bibbolino, C.; Miele, V.; Neri, E.; Grassi, R. Artificial Intelligence: Radiologists’ Expectations and Opinions Gleaned from a Nationwide Online Survey. Radiol. Med. 2021, 126, 63–71. [Google Scholar] [CrossRef]
- Caliandro, M.; Fabiana, G.; Surgo, A.; Carbonara, R.; Ciliberti, M.P.; Bonaparte, I.; Caputo, S.; Fiorentino, A. Impact on Mental Health of the COVID-19 Pandemic in a Radiation Oncology Department. Radiol. Med. 2022, 127, 220–224. [Google Scholar] [CrossRef]
- Francolini, G.; Desideri, I.; Stocchi, G.; Ciccone, L.P.; Salvestrini, V.; Garlatti, P.; Aquilano, M.; Greto, D.; Bonomo, P.; Meattini, I.; et al. Impact of COVID-19 on Workload Burden of a Complex Radiotherapy Facility. Radiol. Med. 2021, 126, 717–721. [Google Scholar] [CrossRef]
- Cellini, F.; Franco, R.D.; Manfrida, S.; Borzillo, V.; Maranzano, E.; Pergolizzi, S.; Morganti, A.G.; Fusco, V.; Deodato, F.; Santarelli, M.; et al. Palliative Radiotherapy Indications during the COVID-19 Pandemic and in Future Complex Logistic Settings: The NORMALITY Model. Radiol. Med. 2021, 126, 1619–1656. [Google Scholar] [CrossRef]
- Felice, F.D.; D’Angelo, E.; Ingargiola, R.; Iacovelli, N.A.; Alterio, D.; Franco, P.; Bonomo, P.; Merlotti, A.; Bacigalupo, A.; Maddalo, M.; et al. A Snapshot on Radiotherapy for Head and Neck Cancer Patients during the COVID-19 Pandemic: A Survey of the Italian Association of Radiotherapy and Clinical Oncology (AIRO) Head and Neck Working Group. Radiol. Med. 2021, 126, 343–347. [Google Scholar] [CrossRef]
- Lorut, C.; Ghossains, M.; Horellou, M.-H.; Achkar, A.; Fretault, J.; Laaban, J.-P. A Noninvasive Diagnostic Strategy Including Spiral Computed Tomography in Patients with Suspected Pulmonary Embolism. Am. J. Respir. Crit. Care 2000, 162, 1413–1418. [Google Scholar] [CrossRef]
- Mirabile, A.; Lucarelli, N.M.; Sollazzo, E.P.; Ianora, A.A.S.; Sardaro, A.; Mirabile, G.; Lorusso, F.; Racanelli, V.; Maggialetti, N.; Scardapane, A. CT Pulmonary Angiography Appropriateness in a Single Emergency Department: Does the Use of Revised Geneva Score Matter? Radiol. Med. 2021, 126, 1544–1552. [Google Scholar] [CrossRef]
- Cozzi, D.; Moroni, C.; Cavigli, E.; Bindi, A.; Caviglioli, C.; Nazerian, P.; Vanni, S.; Miele, V.; Bartolucci, M. Prognostic Value of CT Pulmonary Angiography Parameters in Acute Pulmonary Embolism. Radiol. Med. 2021, 126, 1030–1036. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Gaibazzi, N.; Tuttolomondo, D.; Fusco, S.; Mura, V.L.; Peyvandi, F.; Aliberti, S.; Blasi, F.; Cozzi, D.; Carrafiello, G.; et al. Deep Vein Thrombosis in COVID-19 Patients in General Wards: Prevalence and Association with Clinical and Laboratory Variables. Radiol. Med. 2021, 126, 722–728. [Google Scholar] [CrossRef]
- Ippolito, D.; Giandola, T.; Maino, C.; Pecorelli, A.; Capodaglio, C.; Ragusi, M.; Porta, M.; Gandola, D.; Masetto, A.; Drago, S.; et al. Acute Pulmonary Embolism in Hospitalized Patients with SARS-CoV-2-Related Pneumonia: Multicentric Experience from Italian Endemic Area. Radiol. Med. 2021, 126, 669–678. [Google Scholar] [CrossRef]
- Masselli, G.; Almberger, M.; Tortora, A.; Capoccia, L.; Dolciami, M.; D’Aprile, M.R.; Valentini, C.; Avventurieri, G.; Bracci, S.; Ricci, P. Role of CT Angiography in Detecting Acute Pulmonary Embolism Associated with COVID-19 Pneumonia. Radiol. Med. 2021, 126, 1553–1560. [Google Scholar] [CrossRef]
- Gul, M.H.; Htun, Z.M.; Perez, V.d.J.; Suleman, M.; Arshad, S.; Imran, M.; Vyasabattu, M.; Wood, J.P.; Anstead, M.; Morris, P.E. Predictors and Outcomes of Acute Pulmonary Embolism in COVID-19; Insights from US National COVID Cohort Collaborative. Respir. Res. 2023, 24, 59. [Google Scholar] [CrossRef]
- Lombardi, A.F.; Afsahi, A.M.; Gupta, A.; Gholamrezanezhad, A. Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), Influenza, and COVID-19, beyond the Lungs: A Review Article. Radiol. Med. 2021, 126, 561–569. [Google Scholar] [CrossRef]
- Otrakji, A.; Digumarthy, S.R.; Gullo, R.L.; Flores, E.J.; Shepard, J.A.O.; Kalra, M.K. Dual-Energy CT: Spectrum of Thoracic Abnormalities. Radiographics 2016, 36, 38–52. [Google Scholar] [CrossRef]
- Yang, L.; Sun, J.; Li, J.; Peng, Y. Dual-Energy Spectral CT Imaging of Pulmonary Embolism with Mycoplasma Pneumoniae Pneumonia in Children. Radiol. Med. 2022, 127, 154–161. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Gao, J.; Li, J.; Li, M.; Zhou, Z.; Peng, Y. Performance Evaluation of a Deep Learning Image Reconstruction (DLIR) Algorithm in “Double Low” Chest CTA in Children: A Feasibility Study. Radiol. Med. 2021, 126, 1181–1188. [Google Scholar] [CrossRef]
- Gluecker, T.; Capasso, P.; Schnyder, P.; Gudinchet, F.; Schaller, M.-D.; Revelly, J.-P.; Chiolero, R.; Vock, P.; Wicky, S. Clinical and Radiologic Features of Pulmonary Edema. RadioGraphics 1999, 19, 1507–1531. [Google Scholar] [CrossRef]
- Martínez-Martínez, M.U.; van Oostdam, D.A.H.; Abud-Mendoza, C. Diffuse Alveolar Hemorrhage in Autoimmune Diseases. Curr. Rheumatol. Rep. 2017, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Lara, A.R.; Schwarz, M.I. Diffuse Alveolar Hemorrhage. Chest 2010, 137, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Hardak, E.; Brook, O.; Yigla, M. Radiological Features of Pneumocystis Jirovecii Pneumonia in Immunocompromised Patients with and without AIDS. Lung 2010, 188, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Parekh, M.; Donuru, A.; Balasubramanya, R.; Kapur, S. Review of the Chest CT Differential Diagnosis of Ground-Glass Opacities in the COVID Era. Radiology 2020, 297, E289–E302. [Google Scholar] [CrossRef] [PubMed]
- King, M.A.; Pope-Harman, A.L.; Allen, J.N.; Christoforidis, G.A.; Christoforidis, A.J. Acute Eosinophilic Pneumonia: Radiologic and Clinical Features. Radiology 1997, 203, 715–719. [Google Scholar] [CrossRef]
- Yeon, J.J.; Kim, K.I.; Im, J.S.; Chang, H.L.; Ki, N.L.; Ki, N.K.; Jeung, S.K.; Woon, J.K. Eosinophilic Lung Diseases: A Clinical, Radiologic, and Pathologic Overview. Radiographics 2007, 27, 617–637. [Google Scholar] [CrossRef]
- Mochimaru, H.; Kawamoto, M.; Fukuda, Y.; Kudoh, S. Clinicopathological Differences between Acute and Chronic Eosinophilic Pneumonia. Respirology 2005, 10, 76–85. [Google Scholar] [CrossRef]
- Heyneman, L.E.; Ward, S.; Lynch, D.A.; Remy-Jardin, M.; Johkoh, T.; Müller, N.L. Respiratory Bronchiolitis, Respiratory Bronchiolitis-Associated Interstitial Lung Disease, and Desquamative Interstitial Pneumonia: Different Entities or Part of the Spectrum of the Same Disease Process? Am. J. Roentgenol. 1999, 173, 1617–1622. [Google Scholar] [CrossRef]
- Frazier, A.A.; Franks, T.J.; Cooke, E.O.; Mohammed, T.L.H.; Pugatch, R.D.; Galvin, J.R. Pulmonary Alveolar Proteinosis. Radiographics 2008, 28, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Ioachimescu, O.C.; Kavuru, M.S. Pulmonary Alveolar Proteinosis. Chronic Respir. Dis. 2006, 3, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, D.; Bindi, A.; Cavigli, E.; Grosso, A.M.; Luvarà, S.; Morelli, N.; Moroni, C.; Piperio, R.; Miele, V.; Bartolucci, M. Exogenous Lipoid Pneumonia: When Radiologist Makes the Difference. Radiol. Med. 2021, 126, 22–28. [Google Scholar] [CrossRef]
- Betancourt, S.L.; Martinez-Jimenez, S.; Rossi, S.E.; Truong, M.T.; Carrillo, J.; Erasmus, J.J. Lipoid Pneumonia: Spectrum of Clinical and Radiologic Manifestations. Am. J. Roentgenol. 2010, 194, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, J.; Kanne, J.P.; Meyer, C.A.; Pipavath, S.N.J.; Schmidt, R.A.; Swanson, J.O.; Godwin, J.D. Histiocytic Disorders of the Chest: Imaging Findings. Radiographics 2015, 35, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Abbott, G.F.; Rosado-De-Christenson, M.L.; Frazier, A.A.; Franks, T.J.; Pugatch, R.D.; Galvin, J.R. Lymphangioleiomyomatosis: Radiologic-Pathologic Correlation. Radiographics 2005, 25, 803–828. [Google Scholar] [CrossRef]
| Compartment | Boundaries | Content | PA Chest X-ray Sign |
|---|---|---|---|
| Prevascular (anterior) | Anterior: posterior cortex of the sternum (mammary vessels excluded). Posterior: anterior aspect of the pericardium. | Thymus, mediastinal fat, lymph nodes, the left brachiocephalic vein. | Hilum overlay sign. Anterior junction line not visible. Preservation of the posterior mediastinal lines. If located over the level of the clavicles: not sharp margins. |
| Visceral (middle) | Anterior: posterior boundaries of the prevascular compartment. Posterior: a plane 1 cm beyond the anterior aspect of the vertebral bodies. | Nonvascular: pericardium, trachea, esophagus, lymph nodes. Vascular: heart, superior vena cava, ascending and descending thoracic aorta, thoracic duct, and intra-pericardial pulmonary arteries. | Widening or obliteration of the right paratracheal stripe. Widening of the aortopulmonary window. |
| Paravertebral (posterior) | Anterior: posterior boundaries of the visceral compartment. Posterolateral: a plane along the posterior margin of the chest wall at the lateral margin of the transverse processes. | Thoracic spine. Paravertebral soft tissues. | Hilum overlay sign. Deviation or disruption of the azygoesophageal line or paraspinal lines. If located superior to the level of the aortic arch: the obliteration of the posterior junction line. If located above the level of the clavicles: sharp margins. |
| Compartment | Fat Attenuation | Cystic Component | Soft-Tissue-Enhancing Masses |
|---|---|---|---|
| Anterior (prevascular) (69.8%) | (8.4%): Thymolipoma Lipoma Liposarcoma Epicardial fat pad Morgagni hernia | (24%): Pericardial cyst Cystic thymoma Lymphangioma Cystic teratoma | Thyroid goiter * Thymoma (30.8%) * Thymic carcinoma (7.5%) * Lymphoma (14.4%) Mature teratoma * Thymic hyperplasia Parathyroid adenomas * |
| Middle (visceral) (13.5%) | Bronchogenic cyst (16.8%) * | Thyroid goiter (13%) * Lymphadenopathy/metastasis (22.4%) Esophageal cancer | |
| Posterior (paravertebral) (5.4%) | Extramedullary hematopoiesis | (13.9%): Lateral meningocele Pseudocyst | Neurogenic neoplasms (53.9%) |
| >1 compartment (11.2%) | Liposarcoma Lipomatosis | Lymphangioma | Lymphadenopathy Lung cancer |
| Benignity | Malignancy | |
|---|---|---|
| Size | <6 mm | >3 cm |
| Volume doubling time (VDT) | VDT of <30 days or >400 days | VDT between 30 and 400 days |
| Margin | Smooth, rounded | Irregular, lobulated, or spiculated |
| Density | Fat | |
| Calcification (Central laminated, popcorn, and diffuse patterns of calcification) | Calcification (Punctate, eccentric patterns of calcification) | |
| Air (thin and regular walls, <5 mm) | Air (thick, irregular walls, <15 mm) | |
| Shape | Round lesions with smooth margin | Irregular shape with spiculated or lobulated margin, pleural tags |
| Triangular lesions in subpleural and perifissural locations | ||
| Polygonal, elongated, elliptical, linear, or plaque-like shape | ||
| Location | Perifissural nodules, predominately represent perifissural lymph nodes | Upper lobe distribution is associated with an increased risk of malignancy with an odds ratio of 1.9 |
| Paraseptal Emphysema | Honeycombing | |
|---|---|---|
| Layers | Always one layer | One or more layers |
| Wall Thickness | Very thin | Thick |
| Associated findings | Centrilobular emphysema | Traction bronchiectasis |
| Distribution | Upper lobes | Lower lobes |
| Size | Large | Small |
| Overall lung volume | Increased | Decreased |
| Associated reticulation | Absent | Present |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgheresi, A.; Agostini, A.; Pierpaoli, L.; Bruno, A.; Valeri, T.; Danti, G.; Bicci, E.; Gabelloni, M.; De Muzio, F.; Brunese, M.C.; et al. Tips and Tricks in Thoracic Radiology for Beginners: A Findings-Based Approach. Tomography 2023, 9, 1153-1186. https://doi.org/10.3390/tomography9030095
Borgheresi A, Agostini A, Pierpaoli L, Bruno A, Valeri T, Danti G, Bicci E, Gabelloni M, De Muzio F, Brunese MC, et al. Tips and Tricks in Thoracic Radiology for Beginners: A Findings-Based Approach. Tomography. 2023; 9(3):1153-1186. https://doi.org/10.3390/tomography9030095
Chicago/Turabian StyleBorgheresi, Alessandra, Andrea Agostini, Luca Pierpaoli, Alessandra Bruno, Tommaso Valeri, Ginevra Danti, Eleonora Bicci, Michela Gabelloni, Federica De Muzio, Maria Chiara Brunese, and et al. 2023. "Tips and Tricks in Thoracic Radiology for Beginners: A Findings-Based Approach" Tomography 9, no. 3: 1153-1186. https://doi.org/10.3390/tomography9030095
APA StyleBorgheresi, A., Agostini, A., Pierpaoli, L., Bruno, A., Valeri, T., Danti, G., Bicci, E., Gabelloni, M., De Muzio, F., Brunese, M. C., Bruno, F., Palumbo, P., Fusco, R., Granata, V., Gandolfo, N., Miele, V., Barile, A., & Giovagnoni, A. (2023). Tips and Tricks in Thoracic Radiology for Beginners: A Findings-Based Approach. Tomography, 9(3), 1153-1186. https://doi.org/10.3390/tomography9030095