Effect of Cardiac Phase on Cardiac Output Index Derived from Dynamic CT Myocardial Perfusion Imaging
Abstract
:1. Introduction
2. Methods
2.1. Study Subject
2.2. Dynamic CT Acquisition and Image Reconstruction
2.3. CT Derivation of CO Index
2.3.1. Implementation of Indicator Dilution Method
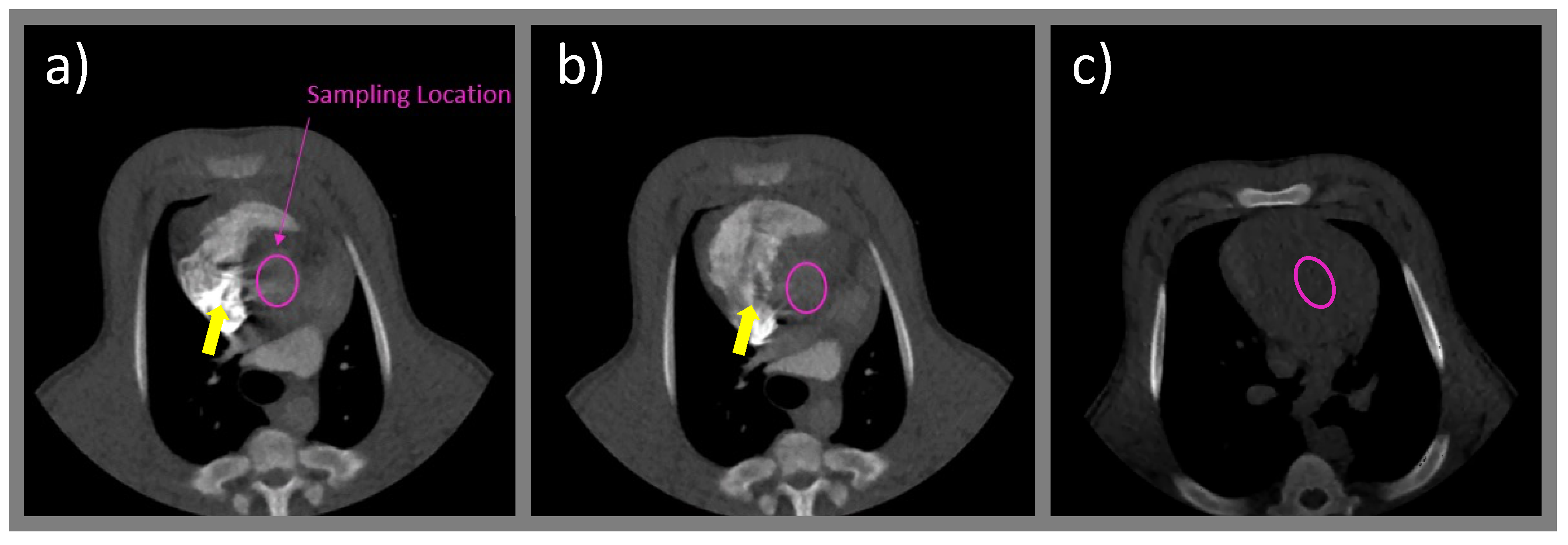
2.3.2. Sampling Location and Unit Conversion
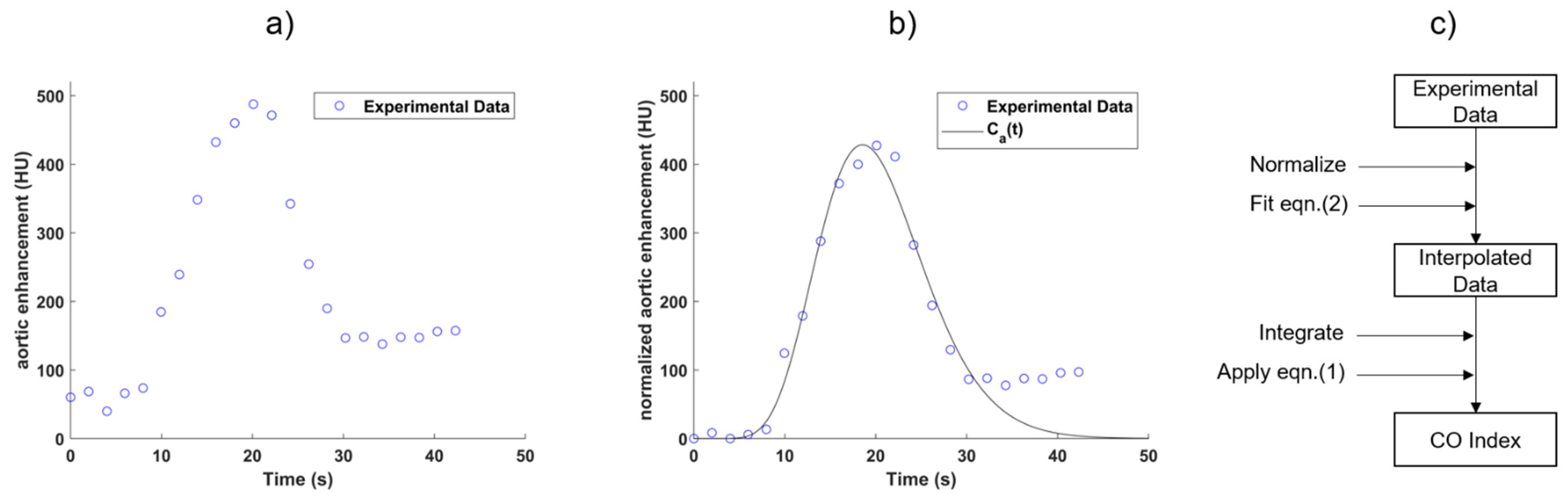
2.3.3. Smoothing and Normalization of Sampled Time-Enhancement Curve
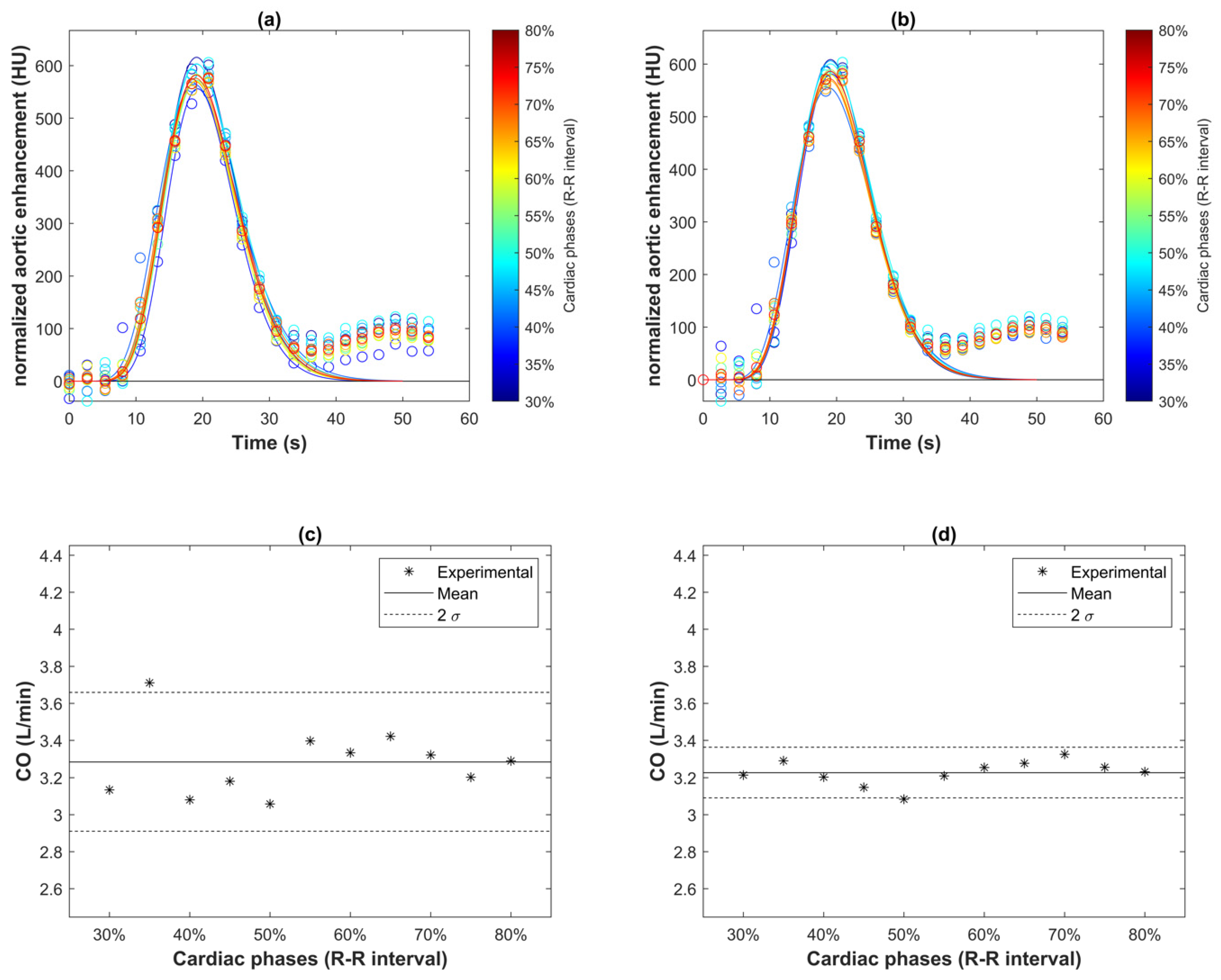
2.4. Derivation of CO Index at Different Cardiac Phases
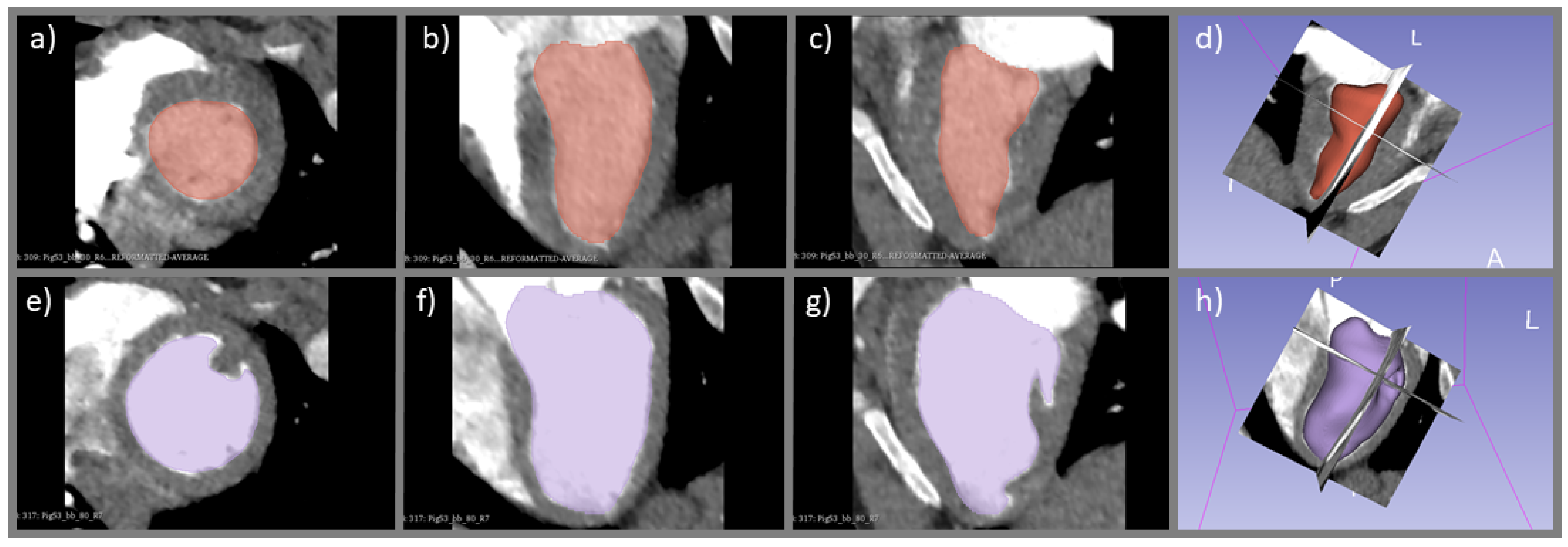
2.5. Derivation of CO Index with Ventricular Delineation Method
3. Results
3.1. Smoothing and Normalization of Aortic Time-Enhancement Curve Sampled from Dynamic CT Acquisition
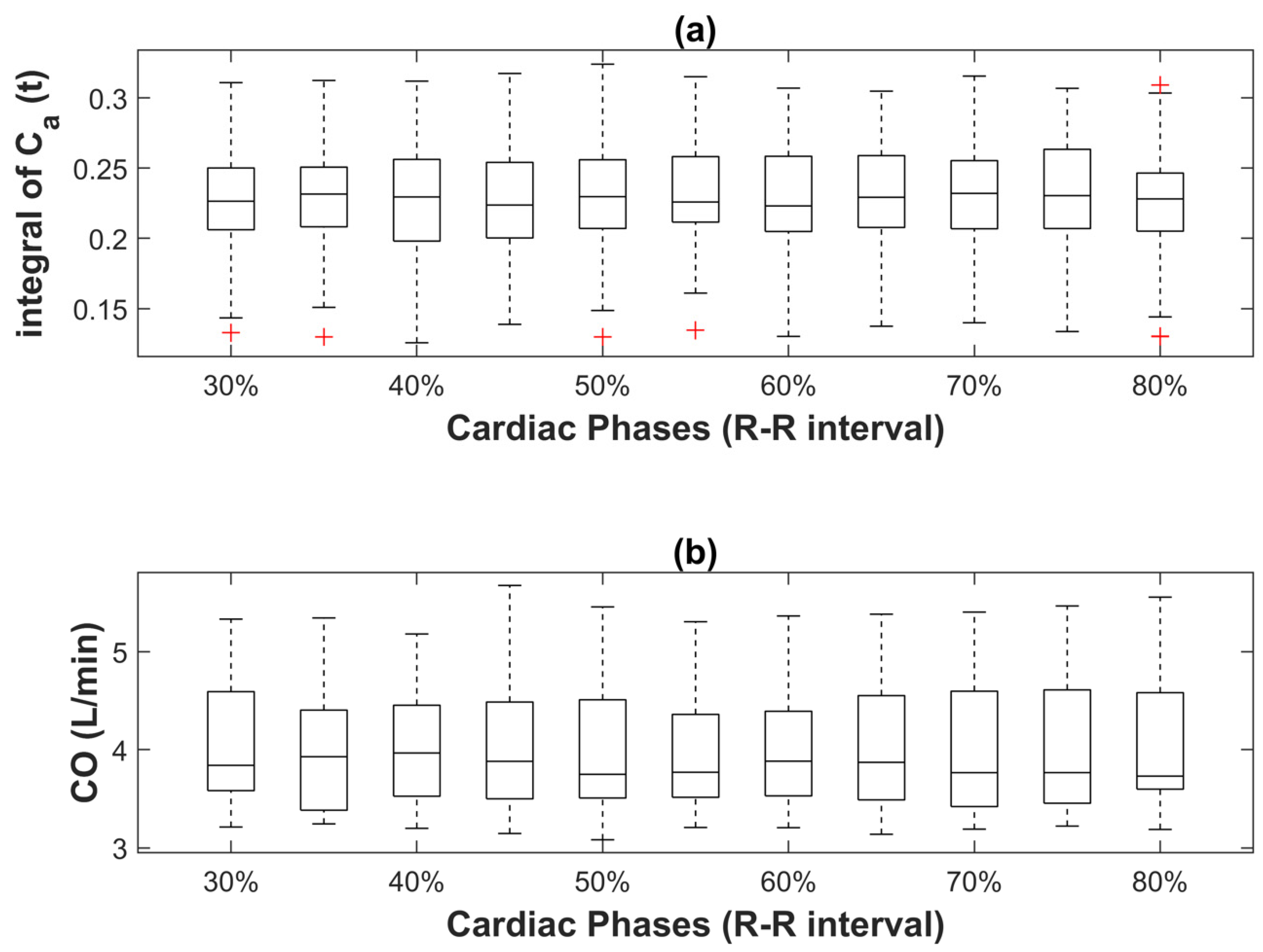
3.2. Effect of Cardiac Phase on CO Index Derived from Indicator Dilution Method
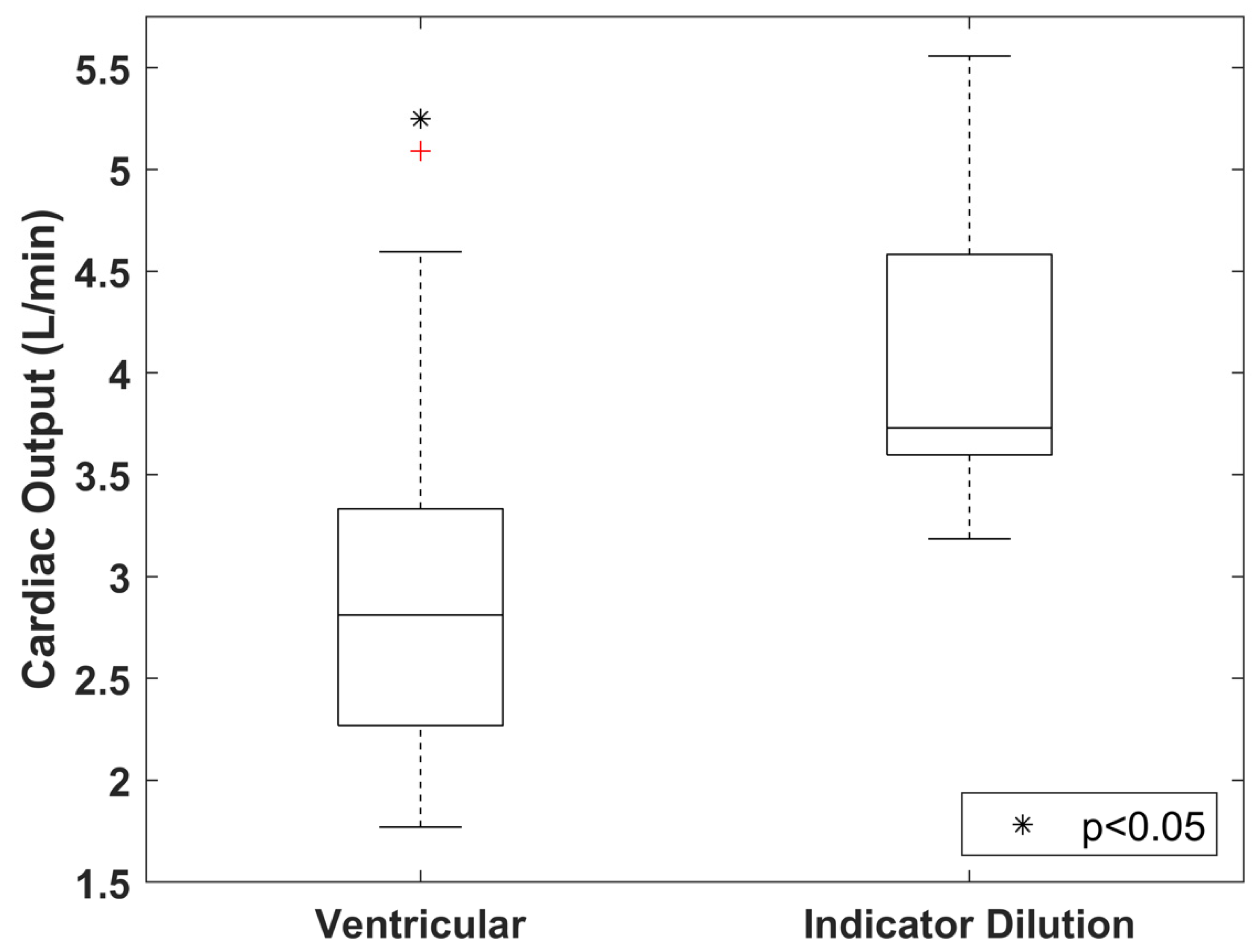
3.3. Difference in CO Index Derived from Indicator Dilution and Ventricular Delineation Methods
4. Discussion
4.1. Smoothing and Normalization of Sampled Aortic Time-Enhancement Curve
4.2. Effect of Cardiac Phase on CO Index Derived from Indicator Dilution Method
4.3. Difference in CO Index Derived from Indicator Dilution and Ventricular Delineation Methods
4.4. Study Limitations
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vincent, J.-L. Understanding cardiac output. Crit. Care 2008, 12, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, C.C.; Karlin, P.; Haythe, J.; Lim, T.K.; Mancini, D.M. Peak cardiac power output, measured noninvasively, is a powerful predictor of outcome in chronic heart failure. Circ. Heart Fail. 2009, 2, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignati, C.; Cattadori, G. Measuring Cardiac Output during Cardiopulmonary Exercise Testing. Ann. Am. Thorac Soc. 2017, 14 (Suppl. S1), S48–S52. [Google Scholar] [CrossRef] [PubMed]
- Klocke, F.J.; Greene, D.G.; Koberstein, R.C. Indicator-dilution measurement of cardiac output with dissolved hydrogen. Circ. Res. 1968, 22, 841–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herfkens, R.J.; Axel, L.; Lipton, M.J.; Napel, S.; Berninger, W.; Redington, R. Measurement of cardiac output by computed transmission tomography. Investig. Radiol. 1982, 17, 550–553. [Google Scholar] [CrossRef]
- Raman, S.V.; Tran, T.; Simonetti, O.P.; Sun, B. Dynamic computed tomography to determine cardiac output in patients with left ventricular assist devices. J. Thorac. Cardiovasc. Surg. 2009, 137, 1213–1217. [Google Scholar] [CrossRef] [Green Version]
- Pienn, M.; Kovacs, G.; Tscherner, M.; Johnson, T.R.; Kullnig, P.; Stollberger, R.; Olschewski, A.; Olschewski, H.; Bálint, Z. Determination of cardiac output with dynamic contrast-enhanced computed tomography. Int J. Cardiovasc. Imaging 2013, 29, 1871–1878. [Google Scholar] [CrossRef]
- Ludman, P.F.; Coats, A.J.S.; Poole-Wilson, P.A.; Rees, R.S.O. Measurement accuracy of cardiac output in humans: Indicator-dilution technique versus geometric analysis by ultrafast computed tomography. J. Am. Coll Cardiol. 1993, 21, 1482–1489. [Google Scholar] [CrossRef] [Green Version]
- Mahnken, A.H.; Klotz, E.; Hennemuth, A.; Jung, B.; Koos, R.; Wildberger, J.E.; Günther, R.W. Measurement of cardiac output from a test-bolus injection in multislice computed tomography. Eur. Radiol. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Garrett, J.S.; Lanzer, P.; Jaschke, W.; Botvinick, E.; Sievers, R.; Higgins, C.B.; Lipton, M.J. Measurement of cardiac output by cine computed tomography. Am. J. Cardiol. 1985, 56, 657–661. [Google Scholar] [CrossRef]
- Hsiao, E.M.; Rybicki, F.J.; Steigner, M. CT coronary angiography: 256-slice and 320-detector row scanners. Curr. Cardiol. Rep. 2010, 12, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Huang, I.-L.; Wu, M.-T.; Hu, C.; Mar, G.-Y.; Lee, T.-Y.; So, A. Quantitative low-dose rest and stress CT myocardial perfusion imaging with a whole-heart coverage scanner improves functional assessment of coronary artery disease. Int. J. Cardiol. Heart Vasc. 2019, 24, 100381. [Google Scholar] [CrossRef]
- Cademartiri, F.; Seitun, S.; Clemente, A.; La Grutta, L.; Toia, P.; Runza, G.; Midiri, M.; Maffei, E. Myocardial blood flow quantification for evaluation of coronary artery disease by computed tomography. Cardiovasc. Diagn Ther. 2017, 7, 129–150. [Google Scholar] [CrossRef] [Green Version]
- Abualsaud, A.O.; Lowe, B.S.; Guo, K.; Marelli, A.J.; Kaouache, M.; Guo, L.; Jutras, L.; Martucci, G.; Therrien, J. Cardiac output as a predictor in congenital heart disease: Are we stating the obvious? Int. J. Cardiol. 2016, 210, 143–148. [Google Scholar] [CrossRef]
- So, A.; Wisenberg, G.; Teefy, P.; Yadegari, A.; Bagur, R.; Hadway, J.; Morrison, L.; MacDonald, A.; Gaskin, D.; Butler, J.; et al. Functional CT assessment of extravascular contrast distribution volume and myocardial perfusion in acute myocardial infarction. Int. J. Cardiol. 2018, 266, 15–23. [Google Scholar] [CrossRef]
- Stewart, G.N. Researches on the Circulation Time and on the Influences which affect it. J. Physiol. 1987, 22, 159–183. [Google Scholar] [CrossRef]
- So, A.; Imai, Y.; Nett, B.; Jackson, J.; Hsieh, J.; Wisenberg, G.; Teefy, P.; Yadegari, A.; Islam, A.; Lee, T.-Y. Technical Note: Evaluation of a 160-mm/256-row CT scanner for whole-heart quantitative myocardial perfusion imaging. Med. Phys. 2016, 43, 4821–4832. [Google Scholar] [CrossRef]
- Wang, J.; Lu, H.; Liang, Z.; Eremina, D.; Zhang, G.; Wang, S.; Chen, J.; Manzione, J. An experimental study on the noise properties of x-ray CT sinogram data in Radon space. Phys. Med. Biol. 2008, 53, 3327–3341. [Google Scholar] [CrossRef]
- Zierler, K. Indicator dilution methods for measuring blood flow, volume, and other properties of biological systems: A brief history and memoir. Ann. Biomed. Eng. 2000, 28, 836–848. [Google Scholar] [CrossRef]
- Stephenson, J.L. Theory of Measurement of Blood Flow by Dye Dilution Technique. IRE Trans. Med. Electron. 1958, PGME-12, 82–88. [Google Scholar] [CrossRef]
- Thompson, H.K.; Starmer, C.F.; Whalen, R.E.; Mcintosh, H.D. Indicator Transit Time Considered as a Gamma Variate. Circ. Res. 1964, 14, 502–515. [Google Scholar] [CrossRef] [Green Version]
- Davenport, R. The Derivation of the Gamma-Variate Relationship for Tracer Dilution Curves. J. Nucl. Med. 1983, 24, 945–948. [Google Scholar]
- Band, D.M.; Linton, R.A.F.; O’Brien, T.K.; Jonas, M.M.; Linton, N.W.F. The shape of indicator dilution curves used for cardiac output measurement in man. J. Physiol. 1997, 498, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [Green Version]
- Brook, R.A.; Chiro, G.D. Principles of computer assisted tomography (CAT) in radiographic and radioisotopic imaging. Phys. Med. Biol. 1976, 21, 689–732. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dempsey, S.C.H.; Lee, T.-Y.; Samani, A.; So, A. Effect of Cardiac Phase on Cardiac Output Index Derived from Dynamic CT Myocardial Perfusion Imaging. Tomography 2022, 8, 1129-1140. https://doi.org/10.3390/tomography8020092
Dempsey SCH, Lee T-Y, Samani A, So A. Effect of Cardiac Phase on Cardiac Output Index Derived from Dynamic CT Myocardial Perfusion Imaging. Tomography. 2022; 8(2):1129-1140. https://doi.org/10.3390/tomography8020092
Chicago/Turabian StyleDempsey, Sergio C. H., Ting-Yim Lee, Abbas Samani, and Aaron So. 2022. "Effect of Cardiac Phase on Cardiac Output Index Derived from Dynamic CT Myocardial Perfusion Imaging" Tomography 8, no. 2: 1129-1140. https://doi.org/10.3390/tomography8020092
APA StyleDempsey, S. C. H., Lee, T.-Y., Samani, A., & So, A. (2022). Effect of Cardiac Phase on Cardiac Output Index Derived from Dynamic CT Myocardial Perfusion Imaging. Tomography, 8(2), 1129-1140. https://doi.org/10.3390/tomography8020092





