Effects of Trapezius Muscle Self-Stretching on Muscle Stiffness and Choroidal Circulatory Dynamics: An Evaluation Using Ultrasound Strain Elastography and Laser Speckle Flowgraphy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Self-Stretching of the Trapezius Muscle
2.3. Intraocular Pressure and Systemic Hemodynamics
2.4. Autonomic Nervous System Assessment
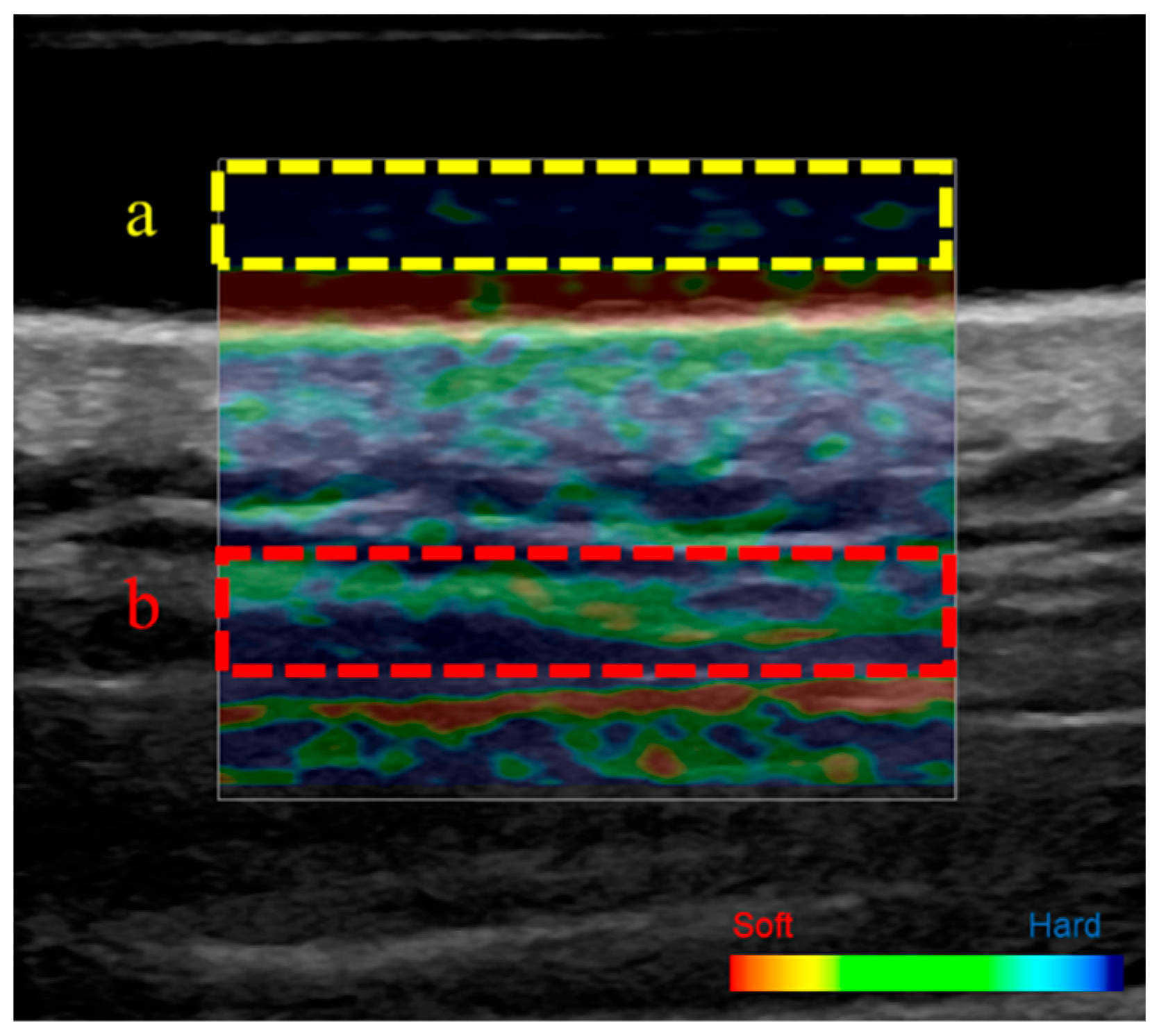
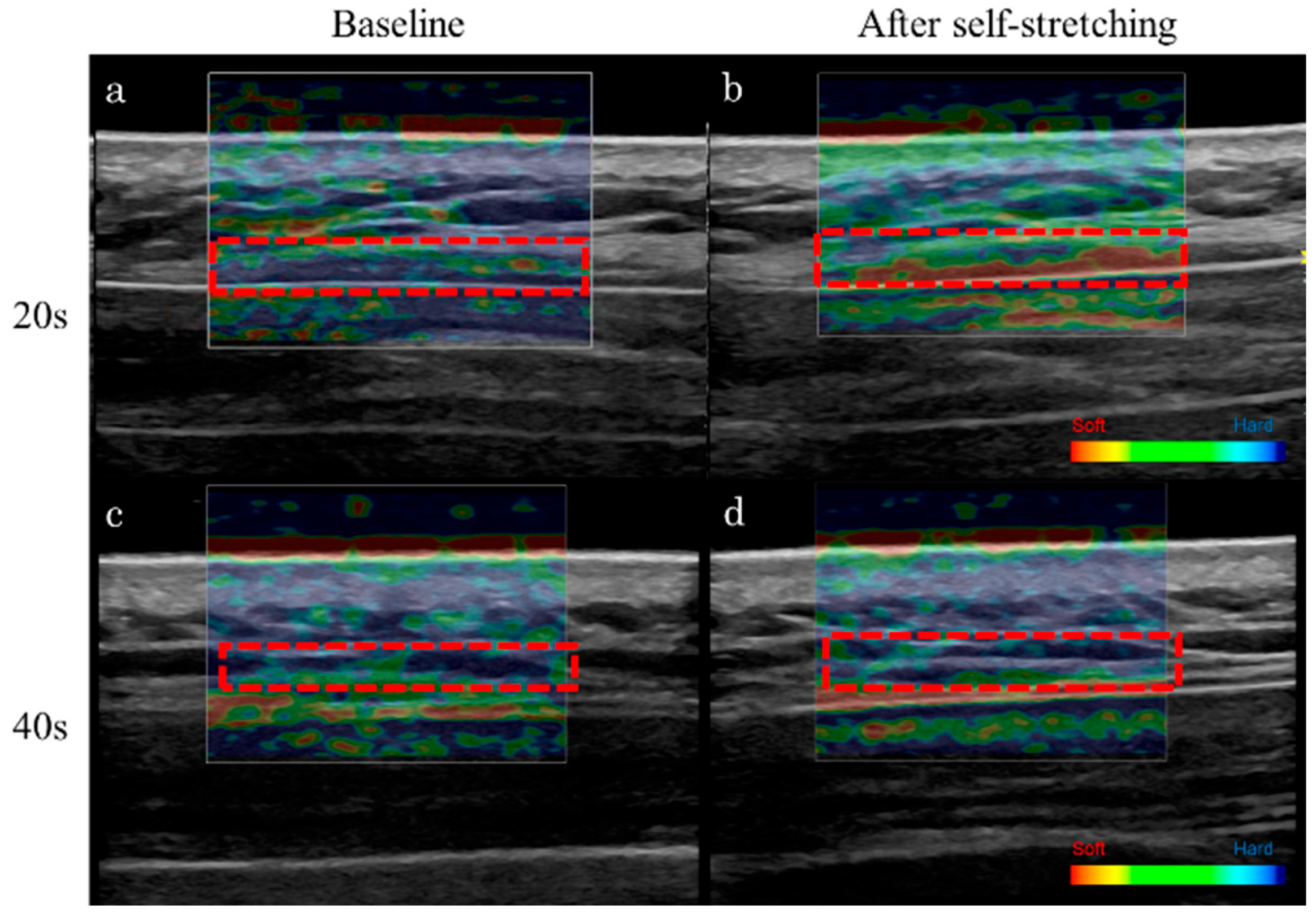
2.5. Ultrasound Strain Elastography
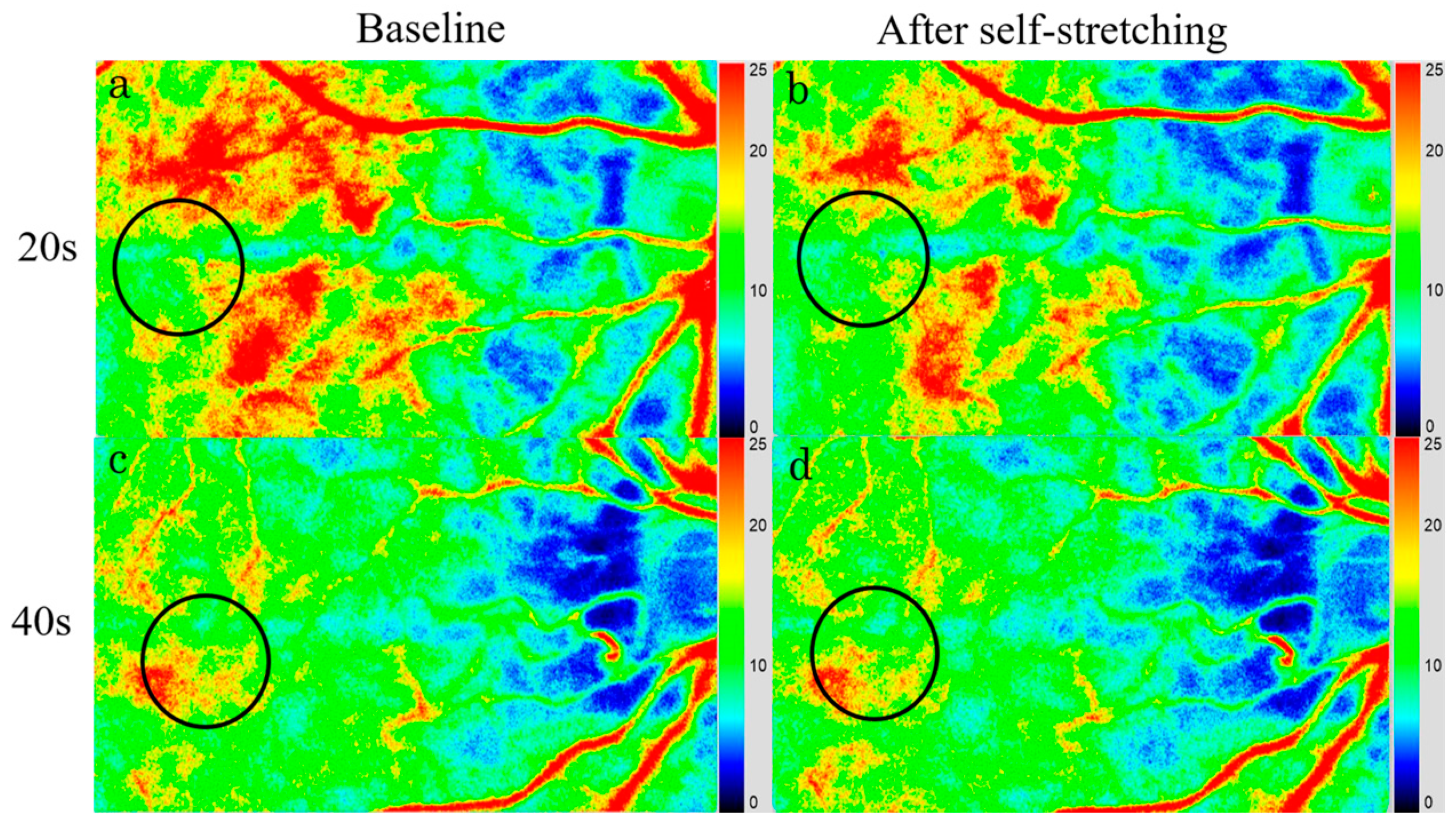
2.6. Laser Speckle Flowgraphy
2.7. Statistical Analyses
3. Results
3.1. IOP, Systemic Hemodynamics, and sAA Activity
3.2. Stiffness of the Upper Trapezius Muscle
3.3. Choroidal Blood Flow Velocity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IOP | intraocular pressure |
| BP | blood pressure |
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
| MBP | mean blood pressure |
| HR | heart rate |
| OPP | ocular perfusion pressure |
| sAA | salivary alpha-amylase |
| MBR | mean blur rate |
| RE | refractive error |
| D | diopter |
| ICC | intraclass correlation coefficient |
| LSFG | laser speckle flowgraphy |
References
- Hogg-Johnson, S.; van der Velde, G.; Carroll, L.J.; Holm, L.W.; Cassidy, J.D.; Guzman, J.; Côté, P.; Haldeman, S.; Ammendolia, C.; Carragee, E.; et al. The burden and determinants of neck pain in the general population: Results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. J. Manip. Physiol. Ther. 2009, 32, S46–S60. [Google Scholar] [CrossRef] [PubMed]
- Ming, Z.; Närhi, M.; Siivola, J. Neck and shoulder pain related to computer use. Pathophysiology 2004, 11, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Juul-Kristensen, B.; Kadefors, R.; Hansen, K.; Byström, P.; Sandsjö, L.; Sjøgaard, G. Clinical signs and physical function in neck and upper extremities among elderly female computer users: The NEW study. Eur. J. Appl. Physiol. 2006, 96, 136–145. [Google Scholar] [CrossRef]
- Kuo, W.H.; Jian, D.W.; Wang, T.G.; Wang, Y.C. Neck muscle stiffness quantified by sonoelastography is correlated with body mass index and chronic neck pain symptoms. Ultrasound Med. Biol. 2013, 39, 1356–1361. [Google Scholar] [CrossRef]
- Taş, S.; Korkusuz, F.; Erden, Z. Neck muscle stiffness in participants with and without chronic neck pain: A shear-wave elastography study. J. Manip. Physiol. Ther. 2018, 41, 580–588. [Google Scholar] [CrossRef]
- Leonard, J.H.; Kok, K.S.; Ayiesha, R.; Das, S.; Roslizawati, N.; Vikram, M.; Baharudin, O. Prolonged writing task: Comparison of electromyographic analysis of upper trapezius muscle in subjects with or without neck pain. Clin. Ter. 2010, 161, 29–33. [Google Scholar]
- Salavati, M.; Akhbari, B.; Ebrahimi Takamjani, I.; Ezzati, K.; Haghighatkhah, H. Reliability of the upper trapezius muscle and fascia thickness and strain ratio measures by ultrasonography and sonoelastography in participants with myofascial pain syndrome. J. Chiropr. Med. 2017, 16, 316–323. [Google Scholar] [CrossRef]
- Dieterich, A.V.; Andrade, R.J.; Le Sant, G.; Falla, D.; Petzke, F.; Hug, F.; Nordez, A. Shear wave elastography reveals different degrees of passive and active stiffness of the neck extensor muscles. Eur. J. Appl. Physiol. 2017, 117, 171–178. [Google Scholar] [CrossRef]
- Maïsetti, O.; Hug, F.; Bouillard, K.; Nordez, A. Characterization of passive elastic properties of the human medial gastrocnemius muscle belly using supersonic shear imaging. J. Biomech. 2012, 45, 978–984. [Google Scholar] [CrossRef]
- Creze, M.; Nordez, A.; Soubeyrand, M.; Rocher, L.; Maître, X.; Bellin, M.F. Shear wave sonoelastography of skeletal muscle: Basic principles, biomechanical concepts, clinical applications, and future perspectives. Skelet. Radiol. 2018, 47, 457–471. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, S.H.; Hahm, S.C.; Cho, H.Y. Thermotherapy plus neck stabilization exercise for chronic nonspecific neck pain in elderly: A single-blinded randomized controlled trial. Int. J. Environ. Res. Public Health 2020, 17, 5572. [Google Scholar] [CrossRef] [PubMed]
- Benjaboonyanupap, D.; Paungmali, A.; Pirunsan, U. Effect of therapeutic sequence of hot pack and ultrasound on physiological response over trigger point of upper trapezius. Asian J. Sports Med. 2015, 6, e23806. [Google Scholar] [CrossRef] [PubMed]
- Thongtipmak, S.; Buranruk, O.; Eungpinichpong, W.; Konharn, K. Immediate effects and acceptability of an application-based stretching exercise incorporating deep slow breathing for neck pain self-management. Healthc. Inform. Res. 2020, 26, 50–60. [Google Scholar] [CrossRef]
- Hanney, W.J.; Puentedura, E.J.; Kolber, M.J.; Liu, X.; Pabian, P.S.; Cheatham, S.W. The immediate effects of manual stretching and cervicothoracic junction manipulation on cervical range of motion and upper trapezius pressure pain thresholds. J. Back Musculoskelet. Rehabil. 2017, 30, 1005–1013. [Google Scholar] [CrossRef]
- Zetterberg, C.; Forsman, M.; Richter, H.O. Neck/shoulder discomfort due to visually demanding experimental near work is influenced by previous neck pain, task duration, astigmatism, internal eye discomfort and accommodation. PLoS ONE 2017, 12, e0182439. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Uchino, M.; Uchino, Y.; Dogru, M.; Kawashima, M.; Komuro, A.; Sonomura, Y.; Kato, H.; Tsubota, K.; Kinoshita, S. Importance of tear film instability in dry eye disease in office workers using visual display terminals: The Osaka study. Am. J. Ophthalmol. 2015, 159, 748–754. [Google Scholar] [CrossRef]
- Pucker, A.D.; Kerr, A.M.; Sanderson, J.; Lievens, C. Digital eye strain: Updated perspectives. Clin. Optom. 2024, 16, 233–246. [Google Scholar] [CrossRef]
- Saito, S.; Sotoyama, M.; Saito, S.; Taptagaporn, S. Physiological indices of visual fatigue due to VDT operation: Pupillary reflexes and accommodative responses. Ind. Health 1994, 32, 57–66. [Google Scholar] [CrossRef]
- Mohan, A.; Sen, P.; Shah, C.; Datt, K.; Jain, E. Binocular accommodation and vergence dysfunction in children attending online classes during the COVID-19 pandemic: Digital Eye Strain in Kids (DESK) Study-2. J. Pediatr. Ophthalmol. Strabismus 2021, 58, 224–231. [Google Scholar] [CrossRef]
- Flügel-Koch, C.; May, C.A.; Lütjen-Drecoll, E. Presence of a contractile cell network in the human choroid. Ophthalmologica 1996, 210, 296–302. [Google Scholar] [CrossRef]
- Delaey, C.; Van De Voorde, J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000, 32, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Nickla, D.L.; Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, M.; Tamaki, Y.; Tomidokoro, A.; Araie, M. In vivo measurement of blood velocity in human major retinal vessels using the laser speckle method. Investig. Ophthalmol. Vis. Sci. 2011, 52, 87–92. [Google Scholar] [CrossRef]
- Aizawa, N.; Yokoyama, Y.; Chiba, N.; Omodaka, K.; Yasuda, M.; Otomo, T.; Nakamura, M.; Fuse, N.; Nakazawa, T. Reproducibility of retinal circulation measurements obtained using laser speckle flowgraphy-NAVI in patients with glaucoma. Clin. Ophthalmol. 2011, 5, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Haneda, M.; Hashimoto, Y.; Mishima, A.; Saito, D.; Yoshitomi, T. Changes in choroidal circulation hemodynamics during the menstrual cycle in young, healthy women. PLoS ONE 2022, 17, e0270501. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Igawa, R.; Sakai, Y.; Yoshimura, M.; Yoshitomi, T. Seasonal variation of choroidal thickness and circulation in young, healthy participants. Acta Ophthalmol. 2023, 101, 708–709. [Google Scholar] [CrossRef]
- Imabayashi, S.; Hashimoto, Y.; Ishimaru, Y.; Umemoto, R.; Chiyozono, M.; Yamanokuchi, T.; Yoshitomi, T. Changes in choroidal circulation hemodynamics measured using laser speckle flowgraphy after a cold pressor test in young healthy participants. Tomography 2023, 9, 790–797. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ishimaru, Y.; Chiyozono, M.; Imabayashi, S.; Umemoto, R.; Yamanokuchi, T.; Yoshitomi, T. Changes in choroidal blood flow by diurnal variation in healthy young adults. Open Ophthalmol. J. 2023, 17, e187436412301300. [Google Scholar] [CrossRef]
- Kuwahara, F.; Hashimoto, Y.; Toh, N.; Imabayashi, S.; Sakamoto, A.; Shiraishi, K.; Igawa, R.; Yoshitomi, T. Parasympathetic dominance decreases the choroidal blood flow velocity measured using laser speckle flowgraphy. Cureus 2023, 15, e46996. [Google Scholar] [CrossRef]
- Ujita, C.; Hashimto, Y.; Noguchi, K.; Nakamura, N.; Yoshimura, M.; Ichiki, S.; Uehara, M.; Nakazaki, A.; Taniguchi, T.; Yoshitomi, T. Investigation of choroidal circulation hemodynamics using laser speckle flowgraphy after periocular skin warming. Cureus 2024, 16, e75118. [Google Scholar] [CrossRef]
- Imagawa, N.; Mizuno, Y.; Nakata, I.; Komoto, N.; Sakebayashi, H.; Shigetoh, H.; Kodama, T.; Miyazaki, J. The impact of stretching intensities on neural and autonomic responses: Implications for relaxation. Sensors 2023, 23, 6890. [Google Scholar] [CrossRef] [PubMed]
- Eda, N.; Ito, H.; Akama, T. Beneficial effects of yoga stretching on salivary stress hormones and parasympathetic nerve activity. J. Sports Sci. Med. 2020, 19, 695–702. [Google Scholar] [PubMed]
- Jelen, A.; Javornik, E.; Meh, S.G.; Kozinc, Ž. The effect of a 5-week therapeutic massage on erector spinae and upper trapezius muscle stiffness as determined by shear-wave elastography: A randomized controlled trial. Front. Sports Act. Living 2024, 6, 1428301. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Okawara, H.; Nakashima, D.; Iwabuchi, S.; Matsumoto, M.; Nakamura, M.; Nagura, T. Reliability of trapezius muscle hardness measurement: A comparison between portable muscle hardness meter and ultrasound strain elastography. Sensors 2020, 20, 7200. [Google Scholar] [CrossRef]
- Inami, T.; Tsujimura, T.; Shimizu, T.; Watanabe, T.; Lau, W.Y.; Nosaka, K. Relationship between isometric contraction intensity and muscle hardness assessed by ultrasound strain elastography. Eur. J. Appl. Physiol. 2017, 117, 843–852. [Google Scholar] [CrossRef]
- Mizuno, T.; Matsumoto, M.; Umemura, Y. Viscoelasticity of the muscle-tendon unit is returned more rapidly than range of motion after stretching. Scand. J. Med. Sci. Sports 2013, 23, 23–30. [Google Scholar] [CrossRef]
- Kruse, N.T.; Silette, C.R.; Scheuermann, B.W. Influence of passive stretch on muscle blood flow, oxygenation and central cardiovascular responses in healthy young males. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1210–H1221. [Google Scholar] [CrossRef]
- Zullo, A.; Fleckenstein, J.; Schleip, R.; Hoppe, K.; Wearing, S.; Klingler, W. Structural and functional changes in the coupling of fascial tissue, skeletal muscle, and nerves during aging. Front. Physiol. 2020, 11, 592. [Google Scholar] [CrossRef]
- Donato, A.J.; Uberoi, A.; Wray, D.W.; Nishiyama, S.; Lawrenson, L.; Richardson, R.S. Differential effects of aging on limb blood flow in humans. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H272–H278. [Google Scholar] [CrossRef]
- Henry, M.; Baudry, S. Age-related changes in leg proprioception: Implications for postural control. J. Neurophysiol. 2019, 122, 525–538. [Google Scholar] [CrossRef]
- Heizelmann, A.; Tasdemir, S.; Schmidberger, J.; Gräter, T.; Kratzer, W.; Grüner, B. Measurements of the trapezius and erector spinae muscles using virtual touch imaging quantification ultrasound-elastography: A cross section study. BMC Musculoskelet. Disord. 2017, 18, 370. [Google Scholar] [CrossRef] [PubMed]
- Sahu, G.K.; Upadhyay, S.; Panna, S.M. Salivary alpha amylase activity in human beings of different age groups subjected to psychological stress. Indian. J. Clin. Biochem. 2014, 29, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Pulopulos, M.M.; Hidalgo, V.; Puig-Pérez, S.; Salvador, A. Psychophysiological response to social stressors: Relevance of sex and age. Psicothema 2018, 30, 171–176. [Google Scholar] [CrossRef]
- Hamidovic, A.; Van Hedger, K.; Choi, S.H.; Flowers, S.; Wardle, M.; Childs, E. Quantitative meta-analysis of heart rate variability finds reduced parasympathetic cardiac tone in women compared to men during laboratory-based social stress. Neurosci. Biobehav. Rev. 2020, 114, 194–200. [Google Scholar] [CrossRef]

| 20 s Group | 40 s Group | p Value | |
|---|---|---|---|
| Sex (women/men) | 13/5 | 1/7 | 0.009 |
| Age | 21.0 ± 4.9 | 43.0 ± 15.2 | 0.593 |
| RE (D) | −2.63 ± 0.64 | −2.50 ± 0.77 | 0.696 |
| Baseline | After Self-Stretching | p Value (Wilcoxon Signed-Rank Test) | |
|---|---|---|---|
| IOP (mmHg) | 15.0 ± 3.4 | 14.0 ± 3.3 | 0.170 |
| SBP (mmHg) | 109.5 ± 26.2 | 106.0 ± 25.3 | 0.002 ** |
| DBP (mmHg) | 72.0 ± 16.8 | 70.3 ± 16.3 | 0.014 * |
| MBP (mmHg) | 84.7 ± 19.9 | 82.6 ± 19.3 | <0.001 *** |
| HR (bpm) | 76.0 ± 18.0 | 74.0 ± 17.6 | 0.239 |
| OPP (mmHg) | 46.0 ± 11.0 | 45.3 ± 10.7 | 0.004 ** |
| sAA (KU/L) | 3.0 ± 1.5 | 3.0 ± 1.0 | 0.038 * |
| MBR | 13.9 ± 3.5 | 12.4 ± 3.1 | <0.001 *** |
| MBR (%) | 100.0 | 90.7 ± 21.3 | <0.001 *** |
| Strain ratio | 0.5 ± 0.1 | 0.4 ± 0.1 | <0.001 *** |
| Baseline | After Self-Stretching | p Value (Wilcoxon Signed-Rank Test) | |
|---|---|---|---|
| IOP (mmHg) | 15.5 ± 5.3 | 14.4 ± 5.2 | 0.262 |
| SBP (mmHg) | 130.0 ± 45.8 | 122.0 ± 44.0 | 0.030 * |
| DBP (mmHg) | 86.5 ± 30.1 | 85.5 ± 29.6 | 0.575 |
| MBP (mmHg) | 102.8 ± 35.3 | 99.6 ± 34.4 | 0.017 * |
| HR (bpm) | 77.8 ± 28.8 | 77.3 ± 27.6 | 0.068 |
| OPP (mmHg) | 57.6 ± 20.0 | 56.6 ± 19.5 | 0.035 * |
| sAA (KU/L) | 9.5 ± 4.2 | 4.0 ± 1.7 | 0.027 * |
| MBR | 10.7 ± 3.9 | 9.8 ± 3.6 | 0.011 * |
| MBR (%) | 100.0 | 91.4 ± 32.3 | 0.011 * |
| Strain ratio | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.179 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimura, M.; Taniguchi, T.; Yoshitomi, T.; Hashimoto, Y. Effects of Trapezius Muscle Self-Stretching on Muscle Stiffness and Choroidal Circulatory Dynamics: An Evaluation Using Ultrasound Strain Elastography and Laser Speckle Flowgraphy. Tomography 2025, 11, 73. https://doi.org/10.3390/tomography11070073
Yoshimura M, Taniguchi T, Yoshitomi T, Hashimoto Y. Effects of Trapezius Muscle Self-Stretching on Muscle Stiffness and Choroidal Circulatory Dynamics: An Evaluation Using Ultrasound Strain Elastography and Laser Speckle Flowgraphy. Tomography. 2025; 11(7):73. https://doi.org/10.3390/tomography11070073
Chicago/Turabian StyleYoshimura, Miki, Takanori Taniguchi, Takeshi Yoshitomi, and Yuki Hashimoto. 2025. "Effects of Trapezius Muscle Self-Stretching on Muscle Stiffness and Choroidal Circulatory Dynamics: An Evaluation Using Ultrasound Strain Elastography and Laser Speckle Flowgraphy" Tomography 11, no. 7: 73. https://doi.org/10.3390/tomography11070073
APA StyleYoshimura, M., Taniguchi, T., Yoshitomi, T., & Hashimoto, Y. (2025). Effects of Trapezius Muscle Self-Stretching on Muscle Stiffness and Choroidal Circulatory Dynamics: An Evaluation Using Ultrasound Strain Elastography and Laser Speckle Flowgraphy. Tomography, 11(7), 73. https://doi.org/10.3390/tomography11070073





