Murine Functional Lung Imaging Using X-Ray Velocimetry for Longitudinal Noninvasive Quantitative Spatial Assessment of Pulmonary Airflow
Abstract
1. Introduction
2. Methods
2.1. Reversible Anesthesia and Tracheal Intubation Procedure
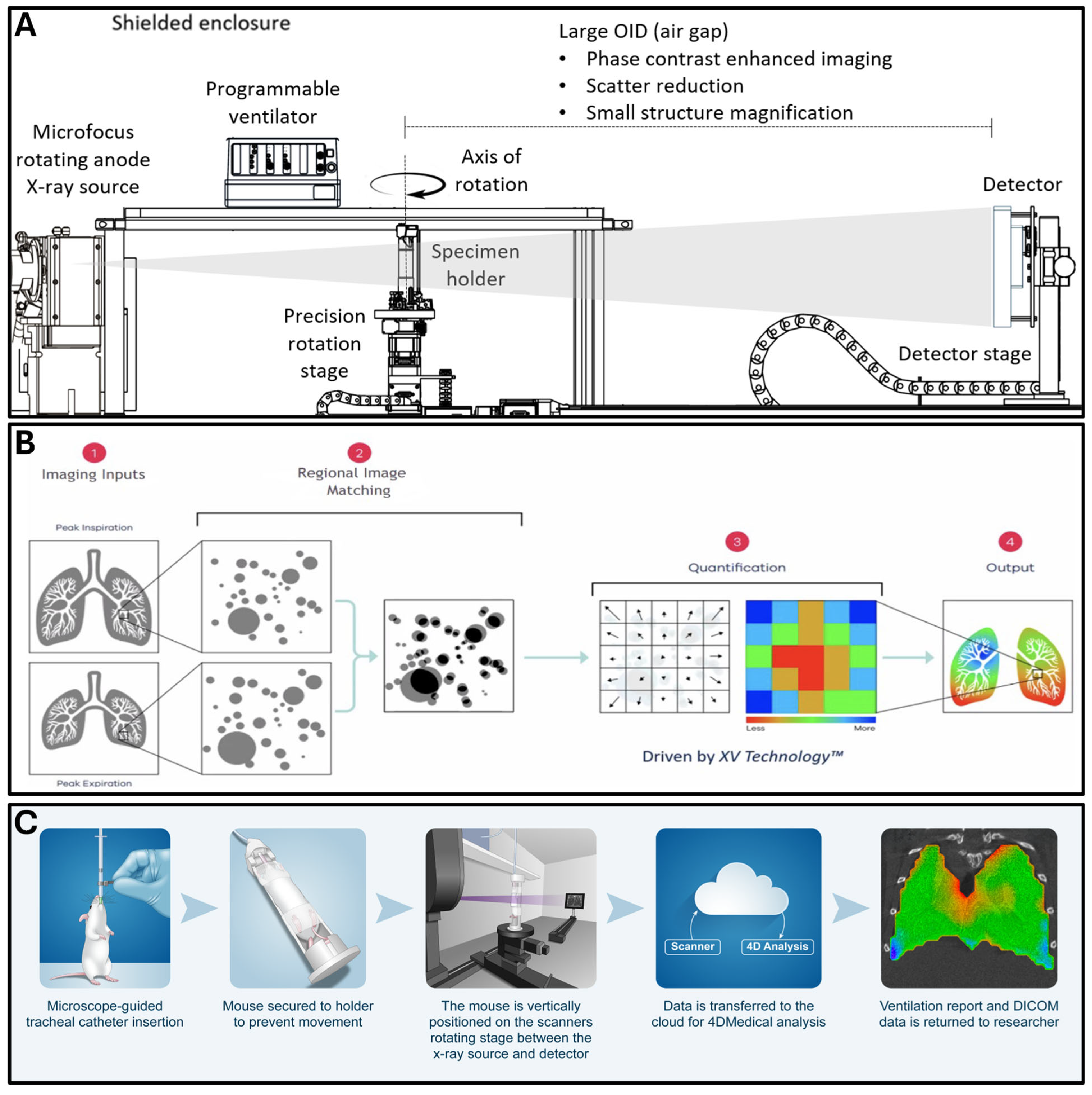
2.2. Dynamic Functional Lung Imaging
2.3. Radiation Dosimetry Measurement
2.4. Fibroproliferative Lung Mouse Model
2.5. Lung Inflation with Vascular Perfusion–Fixation
2.6. Histological Evaluation
2.7. Isolation of Lung Cells and Flow Cytometry
2.8. Scanning of Mouse Lungs
2.9. Digital Image Processing
2.10. Statistical Analysis
3. Results
3.1. Test–Retest Lung Scans
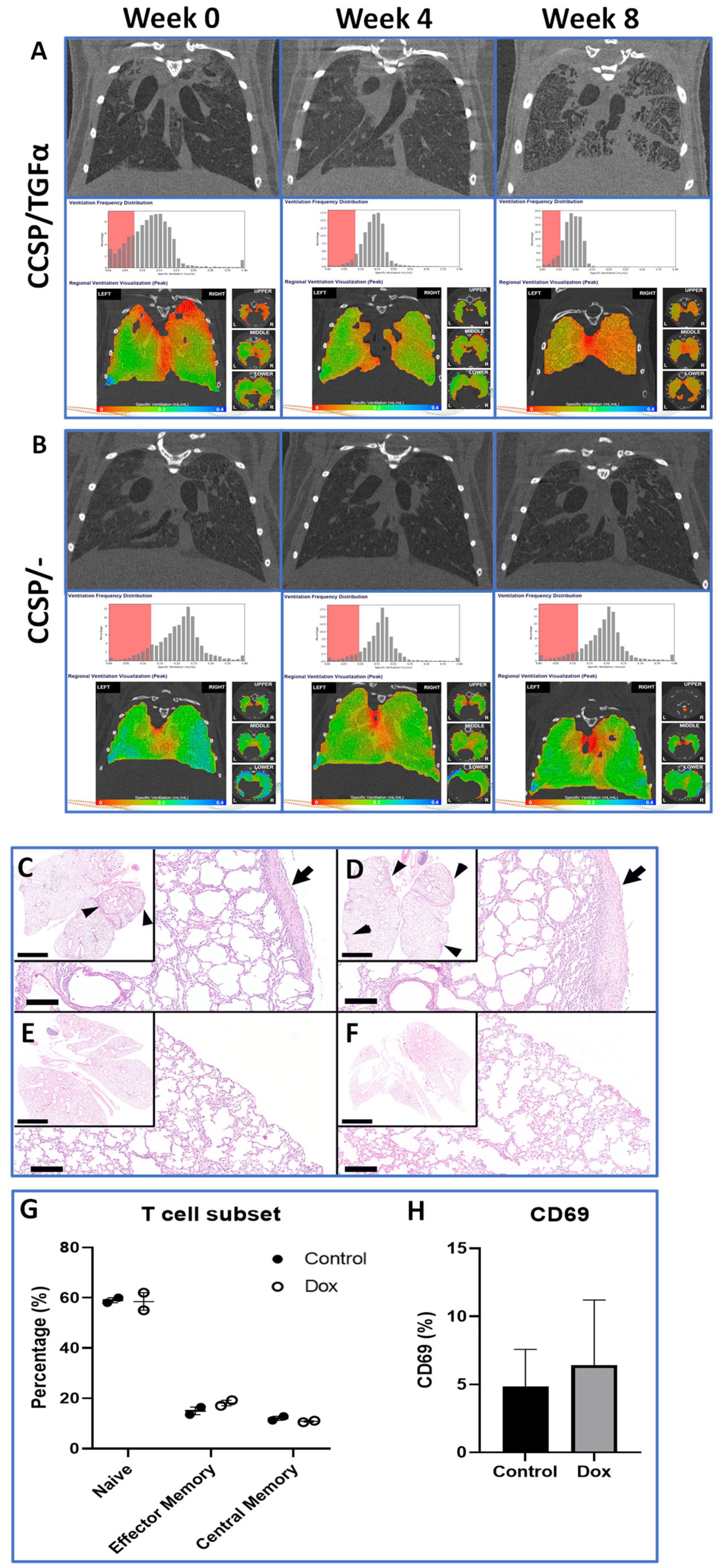
3.2. Longitudinal Assessment of Lung Fibroproliferative Disease Progression
3.3. Histology and Immune Infiltrate Analysis
3.4. Mouse Radiation Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
References
- McGovern, T.K.; Robichaud, A.; Fereydoonzad, L.; Schuessler, T.F.; Martin, J.G. Evaluation of respiratory system mechanics in mice using the forced oscillation technique. J. Vis. Exp. 2013, 75, e50172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Guia, R.M.; Zatecka, V.; Rozman, J.; Prochazka, J.; Sedlacek, R. Full Assessment of Lung Mechanics Using Computer-Controlled, Forced Oscillation Technique. Curr. Protoc. 2022, 2, e488. [Google Scholar] [CrossRef] [PubMed]
- Shofer, S.; Badea, C.; Auerbach, S.; Schwartz, D.A.; Johnson, G.A. A micro-computed tomography-based method for the measurement of pulmonary compliance in healthy and bleomycin-exposed mice. Exp. Lung Res. 2007, 33, 169–183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Redente, E.F.; Kopf, K.W.; Bahadur, A.N.; Robichaud, A.; Lundblad, L.K.; McDonald, L.T. Application-specific approaches to MicroCT for evaluation of mouse models of pulmonary disease. PLoS ONE 2023, 18, e0281452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parker, J.C. Acute lung injury and pulmonary vascular permeability: Use of transgenic models. Compr. Physiol. 2011, 1, 835–882. [Google Scholar] [CrossRef] [PubMed]
- Tielemans, B.; Dekoster, K.; Verleden, S.E.; Sawall, S.; Leszczynski, B.; Laperre, K.; Vanstapel, A.; Verschakelen, J.; Kachelriess, M.; Verbeken, E.; et al. From Mouse to Man and Back: Closing the Correlation Gap between Imaging and Histopathology for Lung Diseases. Diagnostics 2020, 10, 636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schuster, D.P.; Kovacs, A.; Garbow, J.; Piwnica-Worms, D. Recent advances in imaging the lungs of intact small animals. Am. J. Respir. Cell Mol. Biol. 2004, 30, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Galban, C.J.; Han, M.K.; Boes, J.L.; Chughtai, K.A.; Meyer, C.R.; Johnson, T.D.; Galban, S.; Rehemtulla, A.; Kazerooni, E.A.; Martinez, F.J.; et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat. Med. 2012, 18, 1711–1715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kauczor, H.; Surkau, R.; Roberts, T. MRI using hyperpolarized noble gases. Eur. Radiol. 1998, 8, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Dugas, J.P.; Garbow, J.R.; Kobayashi, D.K.; Conradi, M.S. Hyperpolarized (3)He MRI of mouse lung. Magn. Reson. Med. 2004, 52, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Niedbalski, P.J.; Cochran, A.S.; Freeman, M.S.; Guo, J.; Fugate, E.M.; Davis, C.B.; Dahlke, J.; Quirk, J.D.; Varisco, B.M.; Woods, J.C.; et al. Validating in vivo hyperpolarized (129) Xe diffusion MRI and diffusion morphometry in the mouse lung. Magn. Reson. Med. 2021, 85, 2160–2173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wakayama, T.; Ueyama, T.; Imai, F.; Kimura, A.; Fujiwara, H. Quantitative assessment of regional lung ventilation in emphysematous mice using hyperpolarized (129)Xe MRI with a continuous flow hyperpolarizing system. Magn. Reson. Imaging 2022, 92, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Karmali, D.; Sowho, M.; Bose, S.; Pearce, J.; Tejwani, V.; Diamant, Z.; Yarlagadda, K.; Ponce, E.; Eikelis, N.; Otvos, T.; et al. Functional imaging for assessing regional lung ventilation in preclinical and clinical research. Front. Med. 2023, 10, 1160292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murrie, R.P.; Paganin, D.M.; Fouras, A.; Morgan, K.S. Phase contrast x-ray velocimetry of small animal lungs: Optimising imaging rates. Biomed. Opt. Express 2016, 7, 79–92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Werdiger, F.; Donnelley, M.; Dubsky, S.; Murrie, R.P.; Carnibella, R.P.; Samarage, C.R.; How, Y.Y.; Zosky, G.R.; Fouras, A.; Parsons, D.W.; et al. Quantification of muco-obstructive lung disease variability in mice via laboratory X-ray velocimetry. Sci. Rep. 2020, 10, 10859. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asosingh, K.; Frimel, M.; Zlojutro, V.; Grant, D.; Stephens, O.; Wenger, D.; Fouras, A.; DiFilippo, F.; Erzurum, S. Preclinical Four-Dimensional Functional Lung Imaging and Quantification of Regional Airflow: A New Standard in Lung Function Evaluation in Murine Models. Am. J. Respir. Cell Mol. Biol. 2022, 67, 423–429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reyne, N.; Smith, R.; Cmielewski, P.; Eikelis, N.; Nilsen, K.; Louise, J.; Duerr, J.; Mall, M.A.; Lawrence, M.; Parsons, D.; et al. Functional Lung Imaging Identifies Peripheral Ventilation Changes in β-ENaC Mice. Respirology 2025, 30, 335–345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reyne, N.; Smith, R.; Cmielewski, P.; Eikelis, N.; Lawrence, M.; Louise, J.; Pirakalathanan, P.; Parsons, D.; Donnelley, M. Assessment of respiratory mechanics and X-ray velocimetry functional imaging in two cystic fibrosis rat models. Sci. Rep. 2024, 14, 21646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Constantinou, C.; Attix, F.H.; Paliwal, B.R. A solid water phantom material for radiotherapy x-ray and gamma-ray beam calibrations. Med. Phys. 1982, 9, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.S.; Friedman, P.S.; Ferretti, C.; Ristow, N.; Tecchio, M.; Litzenberg, D.W.; Bashkirov, V.; Schulte, R. A Prototype Scintillator Real-Time Beam Monitor for Ultra-high Dose Rate Radiotherapy. Med. Phys. 2024, 51, 2905–2923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tichelaar, J.W.; Lu, W.; Whitsett, J.A. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J. Biol. Chem. 2000, 275, 11858–11864. [Google Scholar] [CrossRef] [PubMed]
- Hardie, W.D.; Le Cras, T.D.; Jiang, K.; Tichelaar, J.W.; Azhar, M.; Korfhagen, T.R. Conditional expression of transforming growth factor-alpha in adult mouse lung causes pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L741–L749. [Google Scholar] [CrossRef] [PubMed]
- Gage, G.J.; Kipke, D.R.; Shain, W. Whole animal perfusion fixation for rodents. J. Vis. Exp. 2012, e3564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas, S.M.; Bednarek, J.; Janssen, W.J.; Hume, P.S. Air-Inflation of Murine Lungs with Vascular Perfusion-Fixation. J. Vis. Exp. 2021, e62215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Renne, R.; Brix, A.; Harkema, J.; Herbert, R.; Kittel, B.; Lewis, D.; March, T.; Nagano, K.; Pino, M.; Rittinghausen, S.; et al. Proliferative and nonproliferative lesions of the rat and mouse respiratory tract. Toxicol. Pathol. 2009, 37, 5S–73S. [Google Scholar] [CrossRef] [PubMed]
- Stahr, C.S.; Samarage, C.R.; Donnelley, M.; Farrow, N.; Morgan, K.S.; Zosky, G.; Boucher, R.C.; Siu, K.K.; Mall, M.A.; Parsons, D.W.; et al. Quantification of heterogeneity in lung disease with image-based pulmonary function testing. Sci. Rep. 2016, 6, 29438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diamond, V.M.; Bell, L.C.; Bone, J.N.; Driehuys, B.; Menchaca, M.; Santyr, G.; Svenningsen, S.; Thomen, R.P.; Marshall, H.; Smith, L.J.; et al. A Systematic Review of the Variability of Ventilation Defect Percent Generated From Hyperpolarized Noble Gas Pulmonary Magnetic Resonance Imaging. J. Magn. Reason. Imaging 2025, 62, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Raunig, D.L.; McShane, L.M.; Pennello, G.; Gatsonis, C.; Carson, P.L.; Voyvodic, J.T.; Wahl, R.L.; Kurland, B.F.; Schwarz, A.J.; Gonen, M.; et al. Quantitative imaging biomarkers: A review of statistical methods for technical performance assessment. Stat. Methods Med. Res. 2015, 24, 27–67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Primers 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Muri, J.; Durcova, B.; Slivka, R.; Vrbenska, A.; Makovicka, M.; Makovicky, P.; Skarda, J.; Delongova, P.; Kamarad, V.; Vecanova, J. Idiopathic Pulmonary Fibrosis: Review of Current Knowledge. Physiol. Res. 2024, 73, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Wijsenbeek, M.; Cottin, V. Spectrum of Fibrotic Lung Diseases. N. Engl. J. Med. 2020, 383, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kim, G.H.; Salisbury, M.L.; Barber, D.; Bartholmai, B.J.; Brown, K.K.; Conoscenti, C.S.; De Backer, J.; Flaherty, K.R.; Gruden, J.F.; et al. Computed Tomographic Biomarkers in Idiopathic Pulmonary Fibrosis. The Future of Quantitative Analysis. Am. J. Respir. Crit. Care Med. 2019, 199, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hardie, W.D.; Cleveland, Z.I.; Davidson, C.; Xu, X.; Madala, S.K.; Woods, J.C. Longitudinal free-breathing MRI measurement of murine lung physiology in a progressive model of lung fibrosis. J. Appl. Physiol. 2019, 126, 1138–1149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karmali, D.; Afanador-Castiblanco, S.; Otvos, T.; Aguilar, G.; Hossen, S.; Eikelis, N.; Nilsen, K.; Punjabi, N.M.; Siddharthan, T.; Kirkness, J.P. Regional lung volume changes with noninvasive positive pressure ventilation in healthy adults. J. Appl. Physiol. 2025, 138, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Kallman, R.F.; Kohn, H.I. The influence of strain on acute x-ray lethality in the mouse. I. LD50 and death rate studies. Radiat. Res. 1956, 5, 309–317. [Google Scholar] [PubMed]
- Grahn, D.; Hamilton, K.F. Genetic Variation in the Acute Lethal Response of Four Inbred Mouse Strains to Whole Body X-Irradiation. Genetics 1957, 42, 189–198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grahn, D. Acute Radiation Response of Mice from a Cross between Radiosensitive and Radioresistant Strains. Genetics 1958, 43, 835–843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mori, N.; Okumoto, M.; Morimoto, J.; Imai, S.; Matsuyama, T.; Takamori, Y.; Yagasaki, O. Genetic analysis of susceptibility to radiation-induced apoptosis of thymocytes in mice. Int. J. Radiat. Biol. 1992, 62, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Iwakawa, M.; Noda, S.; Ohta, T.; Ohira, C.; Lee, R.; Goto, M.; Wakabayashi, M.; Matsui, Y.; Harada, Y.; Imai, T. Different radiation susceptibility among five strains of mice detected by a skin reaction. J. Radiat. Res. 2003, 44, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Iwakawa, M.; Oohira, C.; Noda, S.; Minfu, Y.; Goto, M.; Tanaka, H.; Harada, Y.; Imai, T. Fractionated irradiation augments inter-strain variation of skin reactions among three strains of mice. J. Radiat. Res. 2004, 45, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, K.A.; Sun, J.R.; Giaccia, A.J.; Tosto, L.M.; Brown, J.M. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc. Natl. Acad. Sci. USA 1991, 88, 1394–1397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jackson, I.L.; Vujaskovic, Z.; Down, J.D. Revisiting strain-related differences in radiation sensitivity of the mouse lung: Recognizing and avoiding the confounding effects of pleural effusions. Radiat. Res. 2010, 173, 10–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boria, A.J.; Perez-Torres, C.J. Impact of mouse strain and sex when modeling radiation necrosis. Radiat. Oncol. 2020, 15, 141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spalding, J.F.; Trujillo, T.T. Radiosensitivity of mice as a function of age. Radiat. Res. 1962, 16, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Ninomiya, Y.; Unno, K.; Morioka, T.; Nishimura, M.; Kakinuma, S. Impacts of psychological stress on high dose-rate radiation acute effects in a mouse experimental model. J. Radiat. Res. 2022, 63, 602–608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pizzarello, D.J.; Witcofski, R.L. A possible link between diurnal variations in radiation sensitivity and cell division in bone marrow of male mice. Radiology 1970, 97, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.Z.; Hisha, H.; Yang, G.X.; Fan, T.X.; Jin, T.; Li, Q.; Lian, Z.; Ikehara, S. Optimal protocol for total body irradiation for allogeneic bone marrow transplantation in mice. Bone Marrow Transplant. 2002, 30, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Lee, S.; Kang, H.; Kim, J.; Kim, K.; Youn, H.; Jin, Y.W.; Seo, S.; Youn, B. Organ-Specific Effects of Low Dose Radiation Exposure: A Comprehensive Review. Front. Genet. 2020, 11, 566244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruorton, M.; Donnelley, M.; Goddard, T.; O’Connor, A.; Parsons, D.; Phillips, J.; Carson-Chahhoud, K.; Tai, A. Pilot study of paediatric regional lung function assessment via X-ray velocimetry (XV) imaging in children with normal lungs and in children with cystic fibrosis. BMJ Open 2024, 14, e080034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kirkness, J.P.; Dusting, J.; Eikelis, N.; Pirakalathanan, P.; DeMarco, J.; Shiao, S.L.; Fouras, A. Association of x-ray velocimetry (XV) ventilation analysis compared to spirometry. Front. Med. Technol. 2023, 5, 1148310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siddharthan, T.; Grealis, K.; Kirkness, J.P.; Ötvös, T.; Stefanovski, D.; Tombleson, A.; Dalzell, M.; Gonzalez, E.; Nakrani, K.B.; Wenger, D.; et al. Quantifying ventilation by X-ray velocimetry in healthy adults. Respir. Res. 2023, 24, 215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richmond, B.W.; Lester, M.G.; Lui, V.; Dusting, J.; Raju, S.; Snell, G.I.; Blackburn, J.B.; Douglas, K.; Miller, R.F.; Siddharthan, T.; et al. X-ray velocimetry provides temporally and spatially-resolved biomarkers of lung ventilation in small airways disease. Respir. Res. 2025, 26, 226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

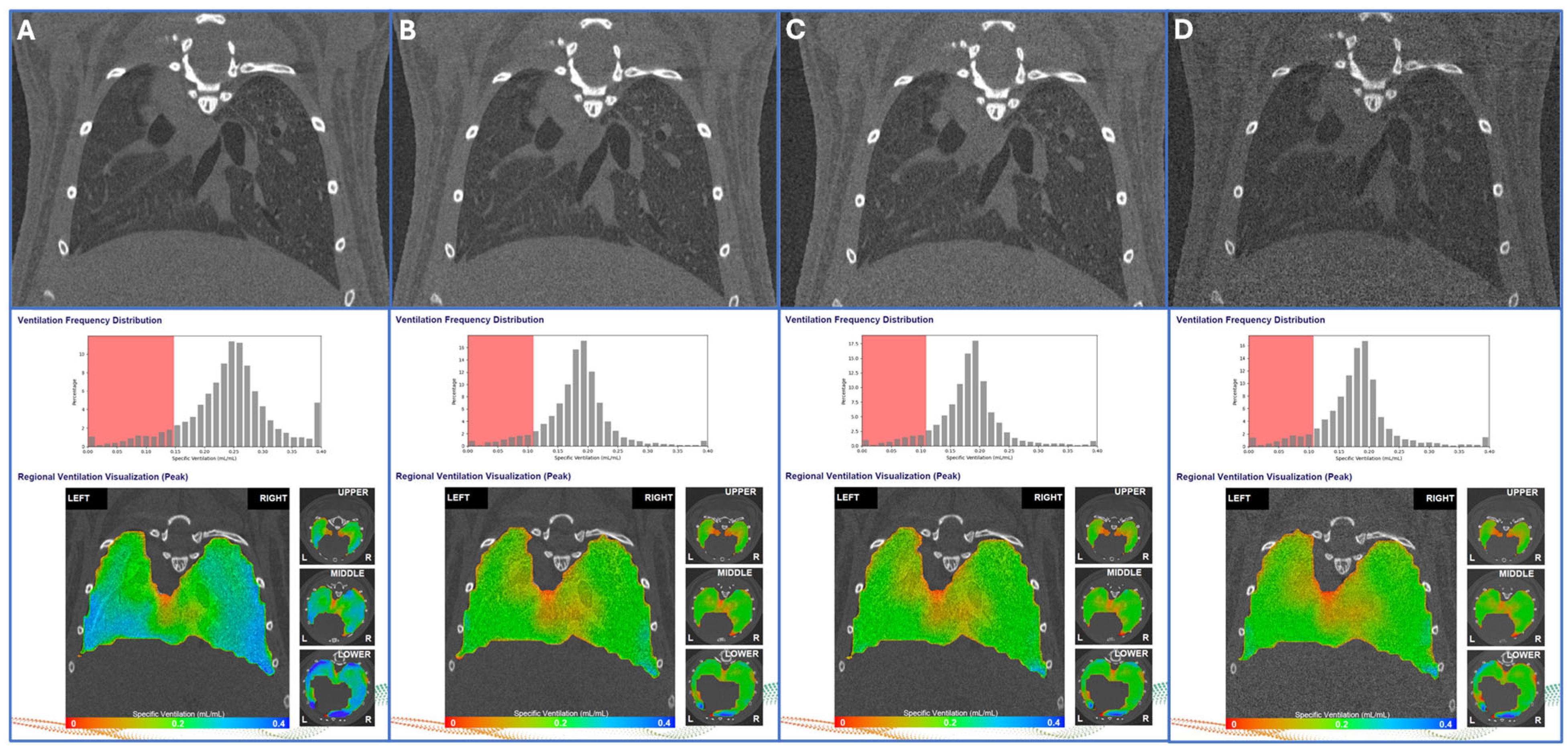
| Metric | Week 0 | Week 2 | Week 4 | Week 6 | Week 8 | wCV (%) |
|---|---|---|---|---|---|---|
| Body Weight (g) | 20.19 (0.34) | 21.35 (0.32) | 22.36 (0.28) | 23.69 (0.37) | 23.99 (0.29) | - |
| VDP (%) | 10.84 (0.82) | 10.37 (0.45) | 10.22 (0.38) | 8.89 (0.68) | 8.62 (0.32) | 17.59 |
| MSV (mL/mL) | 219.5 (4.3) | 203.6 (7.0) | 202.1 (4.7) | 192.9 (7.2) | 190.8 (3.2) | 8.00 |
| Tidal Volume (mL) | 137.9 (2.0) | 143.5 (4.5) | 144.9 (1.9) | 144.4 (3.2) | 144.0 (1.7) | 5.33 |
| VH (%) | 33.38 (1.64) | 32.49 (1.09) | 31.65 (0.55) | 28.33 (1.20) | 28.40 (0.76) | 11.91 |
| VH–Small Scale (%) | 16.34 (0.86) | 15.96 (0.66) | 16.13 (0.32) | 14.84 (0.75) | 14.22 (0.43) | 11.40 |
| VH–Large Scale (%) | 23.92 (1.20) | 22.71 (1.31) | 21.42 (0.46) | 18.26 (0.96) | 19.81 (0.66) | 15.98 |
| Metric | CCSP/ | Week 0 | Week 4 | Week 8 |
|---|---|---|---|---|
| Body Weight (g) | - | 26.64 (0.94) | 28.93 (1.52) | 30.33 (2.60) |
| TGFα | 24.87 (0.66) | 26.12 (1.01) | 22.25 (1.14) | |
| Ventilation Defect Percentage (VDP %) | - | 11.02 (0.75) | 10.96 (0.70) | 10.01 (0.44) |
| TGFα | 12.32 (0.91) | 11.58 (1.81) | 8.99 (1.25) | |
| Mean Specific Ventilation (MSV, mL/mL) | - | 179.7 (5.0) | 179.7 (5.7) | 175.0 (8.5) |
| TGFα | 125.0 (7.6) | 132.1 (6.4) | 134.6 (16.1) | |
| Tidal Volume (mL) | - | 155.4 (4.7) | 167.6 (6.6) | 187.7 (2.2) |
| TGFα | 159.7 (2.7) | 155.8 (5.8) | 113.8 (21.1) | |
| Ventilation Heterogeneity (%) | - | 34.64 (2.53) | 33.91 (1.98) | 31.65 (1.21) |
| TGFα | 39.61 (2.29) | 40.76 (5.12) | 39.72 (3.06) | |
| Small-Scale Ventilation Heterogeneity (%) | - | 14.96 (0.69) | 14.96 (077) | 14.50 (1.07) |
| TGFα | 15.65 (0.59) | 13.93 (1.00) | 11.44 (0.50) | |
| Large-Scale Ventilation Heterogeneity (%) | - | 24.20 (1.98) | 23.23 (1.58) | 19.76 (0.70) |
| TGFα | 28.72 (2.15) | 29.66 (4.06) | 32.37 (3.61) |
| Scan Rate (PPTP) | Breadth Rate (per min) | X-Ray Filter (mm Mo) | Scan Time (min) | X-Ray Dose (cGY) | VDP (%) | MSV (mL/mL) | Tidal Volume (mL) | VH (%) | VH Small-Scale (%) | VH Large-Scale (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 800 | 176 | 32 | 4:38 | 268.6 | 10.08 (0.53) | 238.4 (6.9) | 159.8 (4.9) | 31.05 (1.00) | 14.80 (0.39) | 21.11 (0.49) |
| 600 | 220 | 32 | 2:47 | 174.5 | 8.55 (0.45) | 177.3 (5.4) | 123.8 (3.6) | 27.18 (1.04) | 12.34 (0.43) | 18.84 (0.51) |
| 450 | 220 | 32 | 2:05 | 144.8 | 8.81 (0.48) | 176.5 (5.4) | 122.8 (3.4) | 27.49 (1.13) | 12.46 (0.40) | 19.24 (0.63) |
| 450 | 220 | 72 | 2:05 | 81.2 | 9.26 (0.47) | 175.9 (4.9) | 121.4 (3.3) | 27.88 (0.92) | 12.85 (0.48) | 19.07 (0.56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heist, K.A.; Bonham, C.A.; Jang, Y.; Bergin, I.L.; Welton, A.; Karnak, D.; Hatt, C.A.; Cooper, M.; Teng, W.; Hardie, W.D.; et al. Murine Functional Lung Imaging Using X-Ray Velocimetry for Longitudinal Noninvasive Quantitative Spatial Assessment of Pulmonary Airflow. Tomography 2025, 11, 112. https://doi.org/10.3390/tomography11100112
Heist KA, Bonham CA, Jang Y, Bergin IL, Welton A, Karnak D, Hatt CA, Cooper M, Teng W, Hardie WD, et al. Murine Functional Lung Imaging Using X-Ray Velocimetry for Longitudinal Noninvasive Quantitative Spatial Assessment of Pulmonary Airflow. Tomography. 2025; 11(10):112. https://doi.org/10.3390/tomography11100112
Chicago/Turabian StyleHeist, Kevin A., Christopher A. Bonham, Youngsoon Jang, Ingrid L. Bergin, Amanda Welton, David Karnak, Charles A. Hatt, Matthew Cooper, Wilson Teng, William D. Hardie, and et al. 2025. "Murine Functional Lung Imaging Using X-Ray Velocimetry for Longitudinal Noninvasive Quantitative Spatial Assessment of Pulmonary Airflow" Tomography 11, no. 10: 112. https://doi.org/10.3390/tomography11100112
APA StyleHeist, K. A., Bonham, C. A., Jang, Y., Bergin, I. L., Welton, A., Karnak, D., Hatt, C. A., Cooper, M., Teng, W., Hardie, W. D., Chenevert, T. L., & Ross, B. D. (2025). Murine Functional Lung Imaging Using X-Ray Velocimetry for Longitudinal Noninvasive Quantitative Spatial Assessment of Pulmonary Airflow. Tomography, 11(10), 112. https://doi.org/10.3390/tomography11100112






