Bedside Small-Bowel Challenge vs. Fluoroscopic Series for SBO: A Cost Effectiveness Analysis
Abstract
1. Introduction
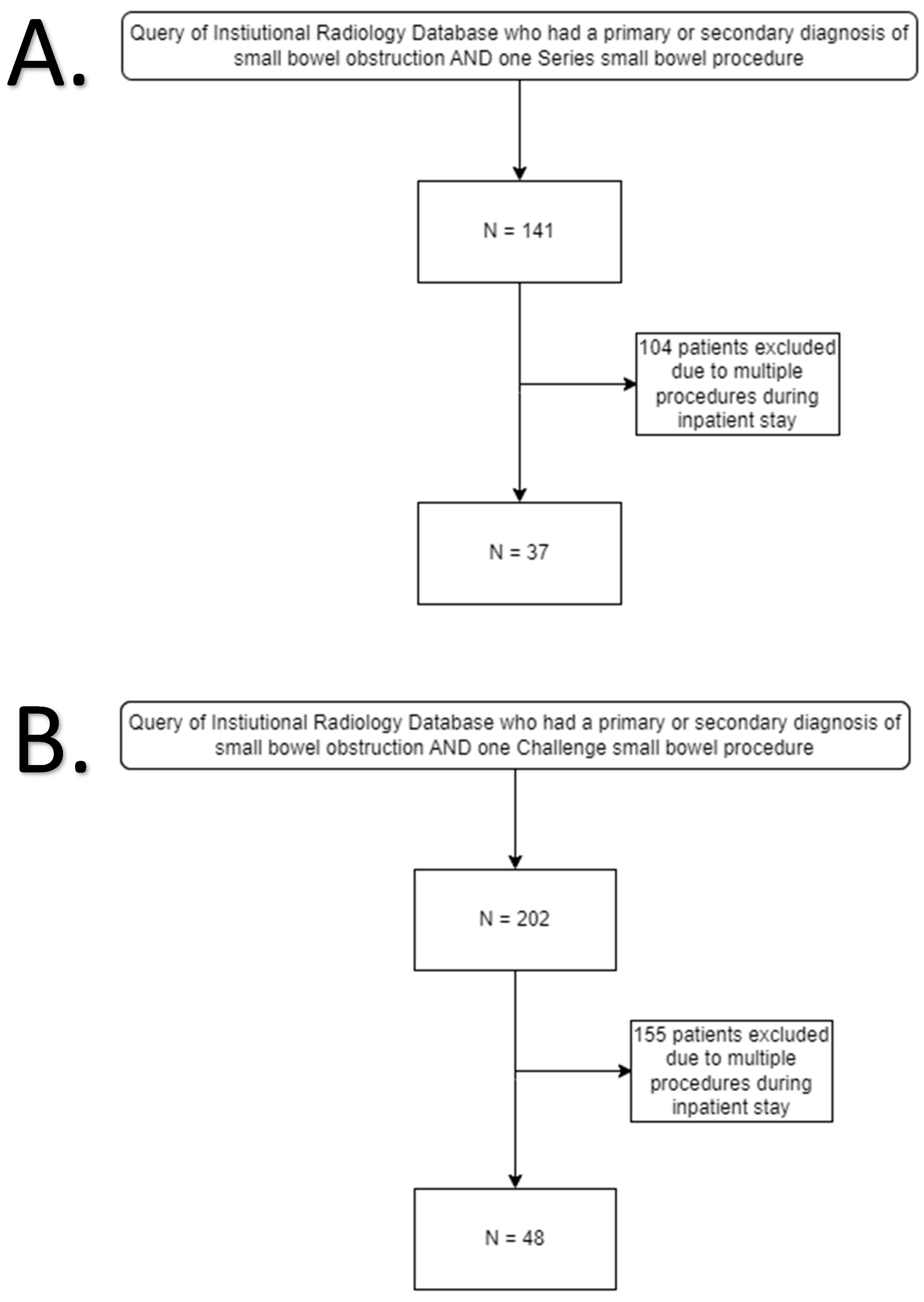
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
2.3. Ethical Considerations
3. Results
3.1. Demographics
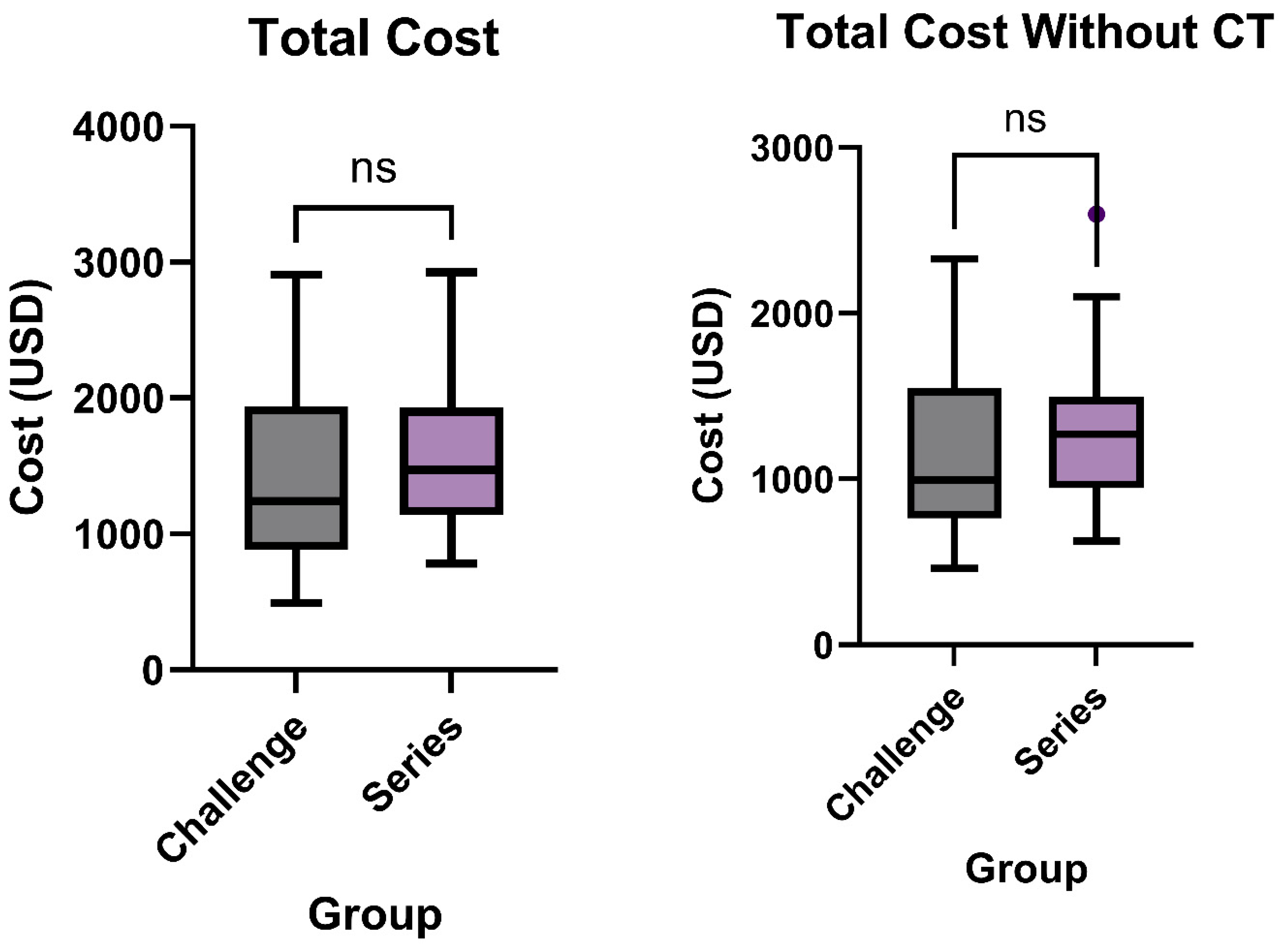
3.2. Total Cost
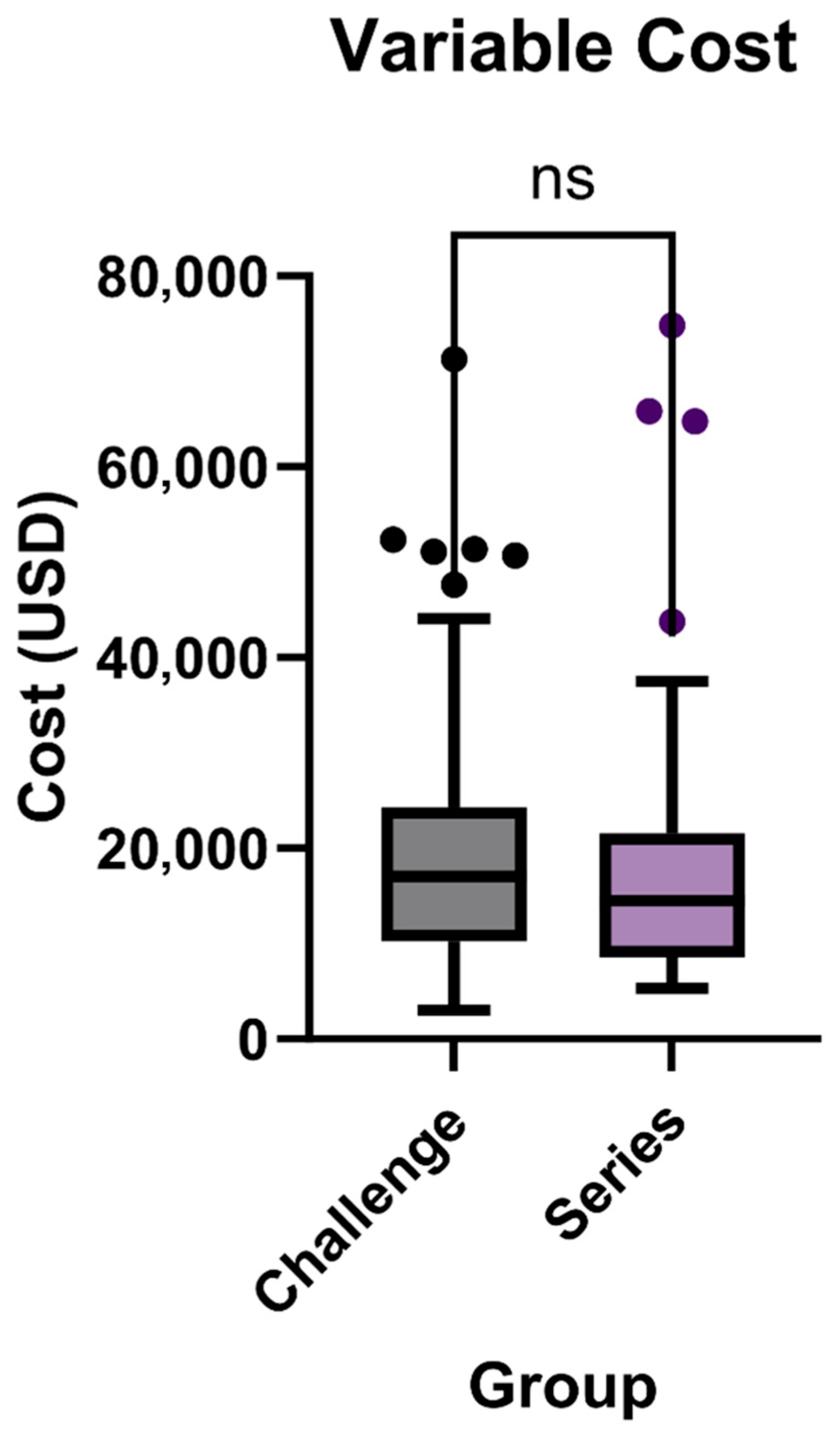
3.3. Variable Cost
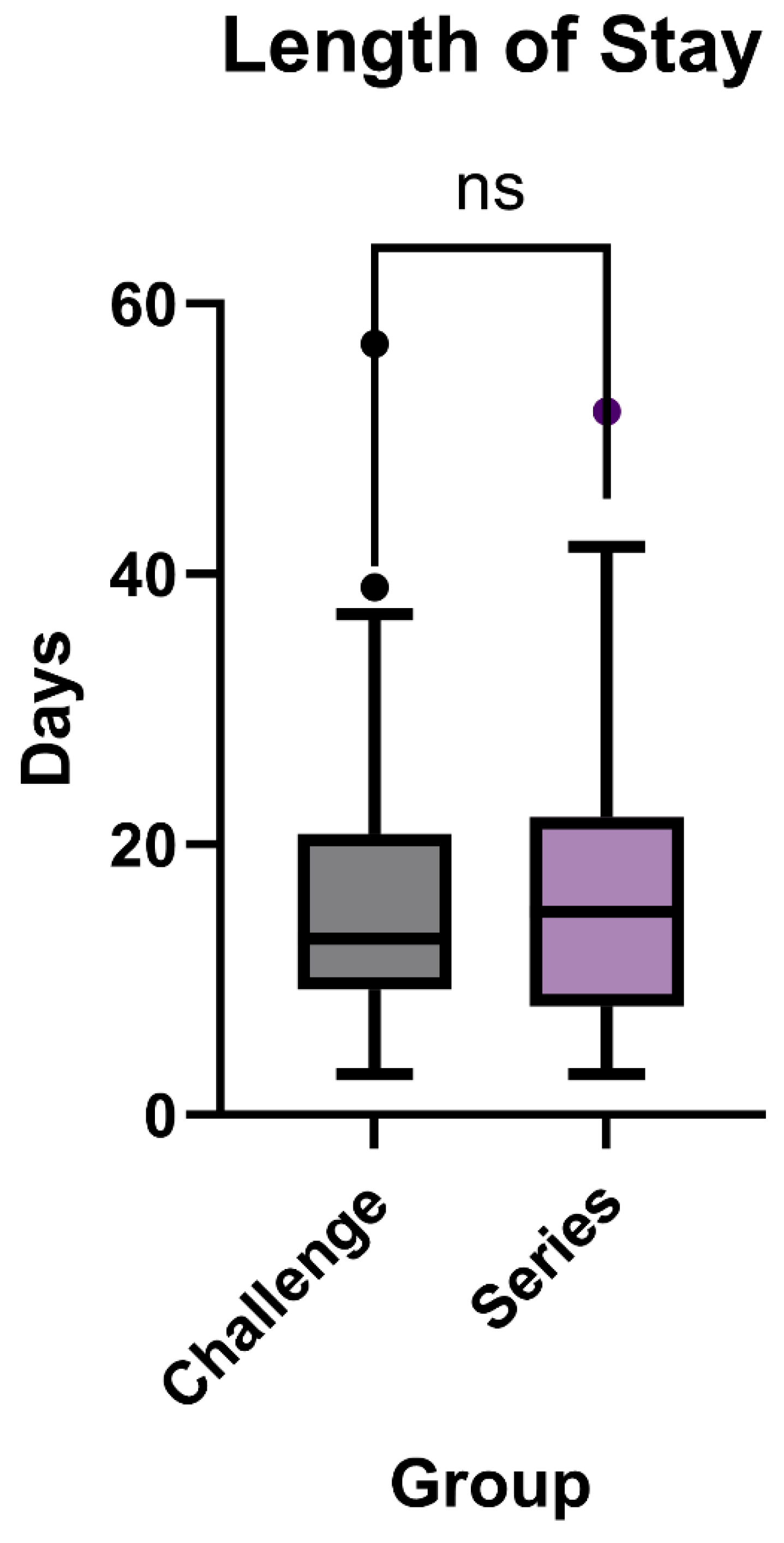
Length of Stay
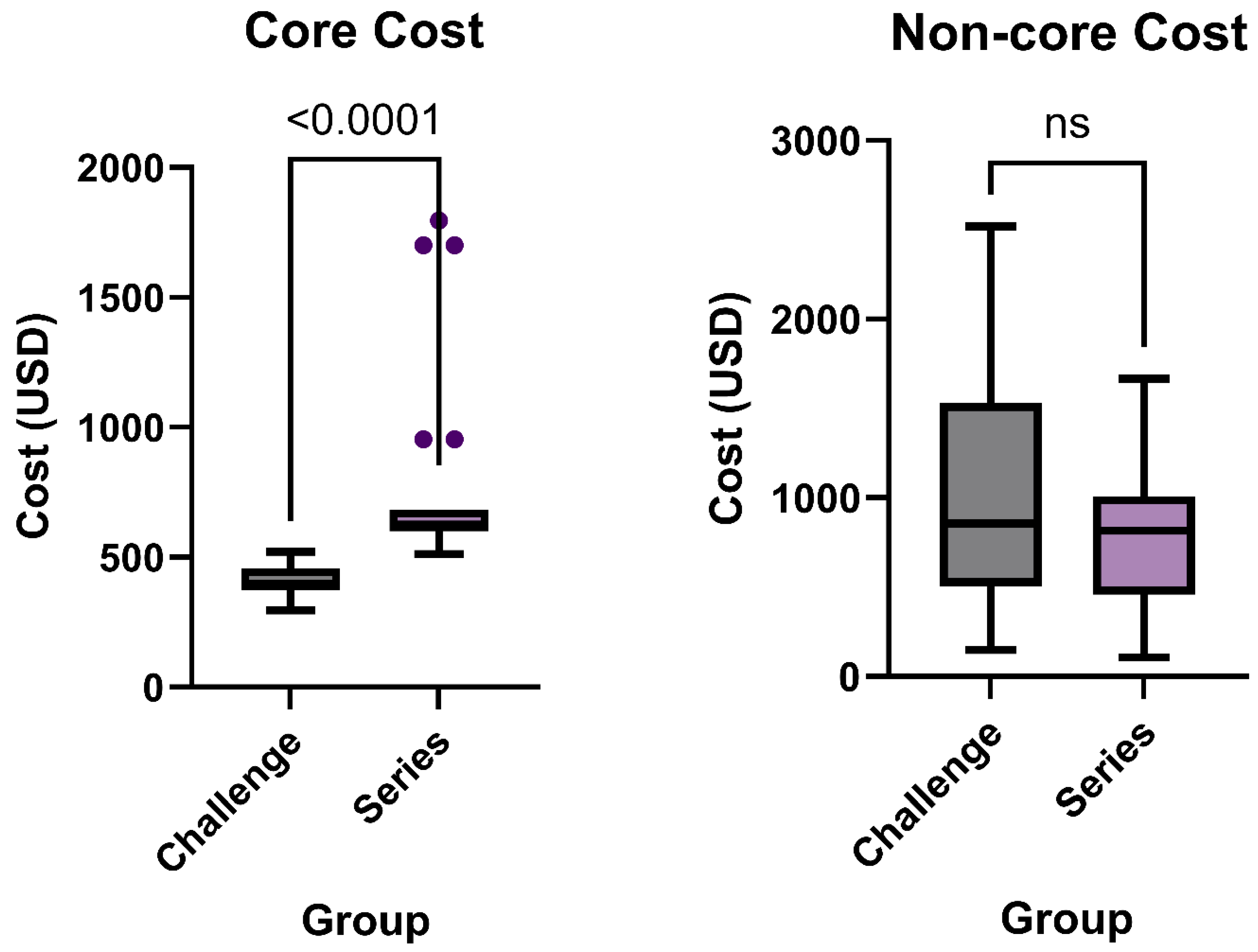
3.4. Core Charges and Non-Core Charges
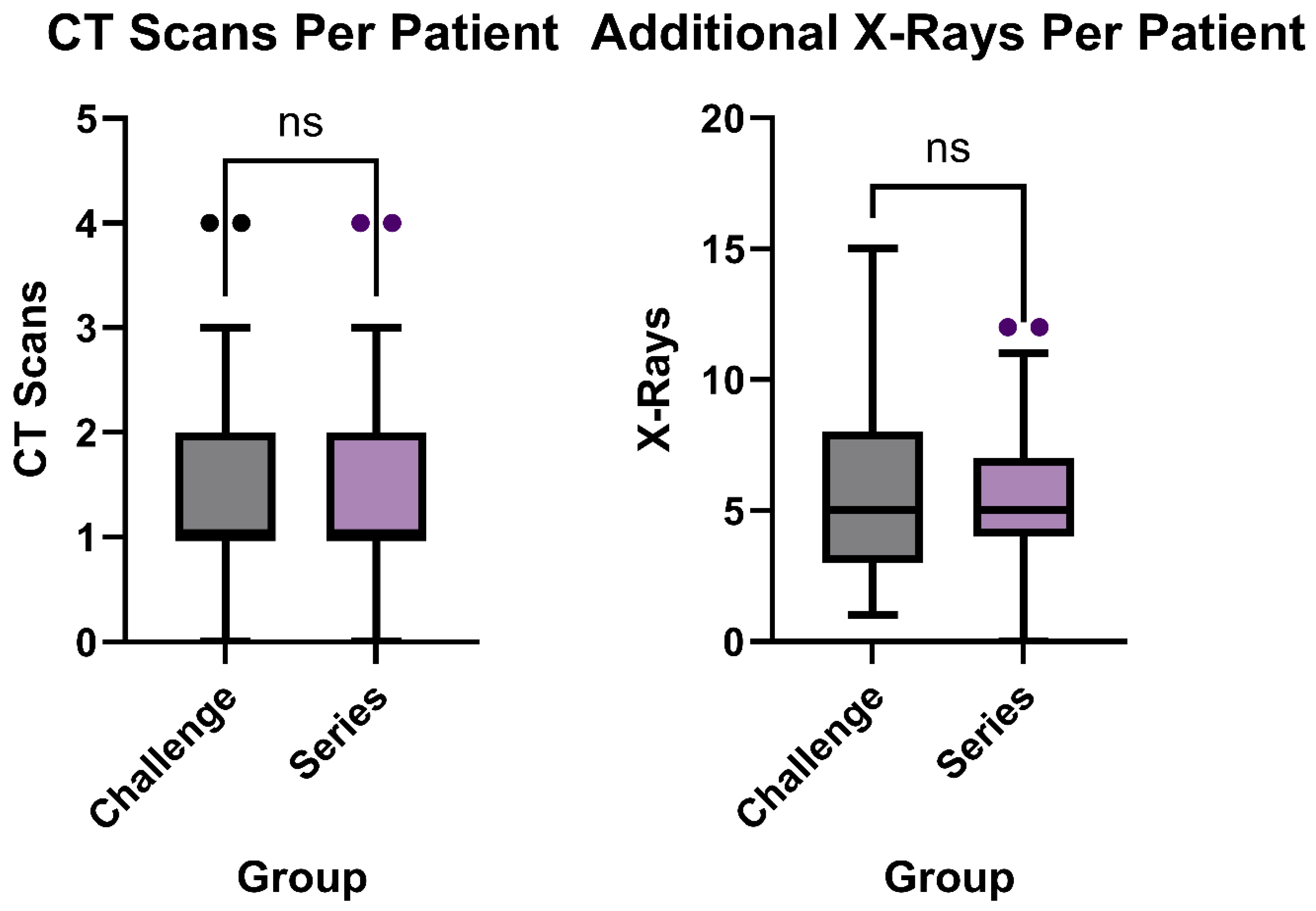
3.5. Additional Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, J.W.V.; Lingam, P.; Shelat, V.G. Adhesive small bowel obstruction—An update. Acute Med. Surg. 2020, 7, e587. [Google Scholar] [CrossRef]
- Catena, F.; De Simone, B.; Coccolini, F.; Di Saverio, S.; Sartelli, M.; Ansaloni, L. Bowel obstruction: A narrative review for all physicians. World J. Emerg. Surg. 2019, 14, 20. [Google Scholar] [CrossRef]
- Smith, D.A.; Kashyap, S.; Nehring, S.M. Bowel Obstruction (Archived); StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Edwards, M.K.; Kuppler, C.S.; Croft, C.A.; Eason-Bates, H.M. Adhesive Closed-loop Small Bowel Obstruction. Clin. Pract. Cases Emerg. Med. 2018, 2, 31–34. [Google Scholar] [CrossRef]
- Thompson, W.M.; Kilani, R.K.; Smith, B.B.; Thomas, J.; Jaffe, T.A.; Delong, D.M.; Paulson, E.K. Accuracy of abdominal radiography in acute small-bowel obstruction: Does reviewer experience matter? Am. J. Roentgenol. 2007, 188, W233–W238. [Google Scholar] [CrossRef] [PubMed]
- Paulson, E.K.; Thompson, W.M. Review of small-bowel obstruction: The diagnosis and when to worry. Radiology 2015, 275, 332–342. [Google Scholar] [CrossRef]
- Zins, M.; Millet, I.; Taourel, P. Adhesive small bowel obstruction: Predictive radiology to improve patient management. Radiology 2020, 296, 480–492. [Google Scholar] [CrossRef]
- Maung, A.A.; Johnson, D.C.; Piper, G.L.; Barbosa, R.R.; Rowell, S.E.; Bokhari, F.; Collins, J.N.; Gordon, J.R.; Ra, J.H.; Kerwin, A.J. Evaluation and management of small-bowel obstruction: An Eastern Association for the Surgery of Trauma practice management guideline. J. Trauma Acute Care Surg. 2012, 73, S362–S369. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, M.; Galluzzo, M.; Santucci, D.; Trinci, M.; Messina, L.; Laccetti, E.; Faiella, E.; Beomonte Zobel, B. Small bowel obstruction and intestinal ischemia: Emphasizing the role of MDCT in the management decision process. Abdom. Radiol. 2022, 47, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Amara, Y.; Leppaniemi, A.; Catena, F.; Ansaloni, L.; Sugrue, M.; Fraga, G.P.; Coccolini, F.; Biffl, W.L.; Peitzman, A.B.; Kluger, Y. Diagnosis and management of small bowel obstruction in virgin abdomen: A WSES position paper. World J. Emerg. Surg. 2021, 16, 36. [Google Scholar] [CrossRef]
- Ten Broek, R.P.; Krielen, P.; Di Saverio, S.; Coccolini, F.; Biffl, W.L.; Ansaloni, L.; Velmahos, G.C.; Sartelli, M.; Fraga, G.P.; Kelly, M.D. Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2017 update of the evidence-based guidelines from the world society of emergency surgery ASBO working group. World J. Emerg. Surg. 2018, 13, 24. [Google Scholar] [CrossRef]
- Eveillard, M.; Quenon, J.L.; Rufat, P.; Mangeol, A.; Fauvelle, F. Association between hospital-acquired infections and patients’ transfers. Infect. Control Hosp. Epidemiol. 2001, 22, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Schaps, D.; Joiner, A.P.; Anderson, D.J. Medical transport-associated infection: Review and commentary making a case for its legitimacy. Infect. Control Hosp. Epidemiol. 2022, 43, 497–503. [Google Scholar] [CrossRef]
- Kay, C.; Wozniak, E.M.; Szabo, A.; Jackson, J.L. Examining Invasive Bedside Procedure Performance at an Academic Medical Center. South. Med. J. 2016, 109, 402–407. [Google Scholar] [CrossRef]
- Barsuk, J.H.; Kozmic, S.E.; Scher, J.; Feinglass, J.; Hoyer, A.; Wayne, D.B. Are we providing patient-centered care? Preferences about paracentesis and thoracentesis procedures. Patient Exp. J. 2014, 1, 94–103. [Google Scholar] [CrossRef]
- Peterson, J.; Cox, C.; Unrue, E.; Steed, R.; Mentzer, C.; Currence, C.; Mount, M. Utility of abbreviated small bowel follow through study in the management of small bowel obstruction. Am. Surg. 2023, 89, 3444–3448. [Google Scholar] [CrossRef]
- Lyu, H.G.; Castillo-Angeles, M.; Bruno, M.; Cooper, Z.; Nehra, D.; Nitzschke, S.L.; Askari, R.; Kelly, E.; Shimizu, N.; Riviello, R. Outcomes of a low-osmolar water-soluble contrast pathway in small bowel obstruction. J. Trauma Acute Care Surg. 2019, 87, 630–635. [Google Scholar] [CrossRef]
- Speck, K.E.; Shah, N.R.; Van Arendonk, K.; Rubalcava, N.S.; Hirschl, R.B.; Goldstein, S.D.; Leys, C.; Markel, T.A.; Rymeski, B.; Wright, T. Implementation of a Contrast Challenge Algorithm for Adhesive Small Bowel Obstructions in Children: A Prospective, Multi-Institutional Study. Ann. Surg. 2025. [Google Scholar] [CrossRef] [PubMed]
- Mahony, C.R.; Traynor, M.D., Jr.; Knight, A.W.; Hughes, J.D.; Hernandez, M.C.; Finnesgard, E.J.; Musa, J.; Selby, S.L.; Rivera, M.; Kim, B.D. Small bowel obstruction managed without hospital admission: A safe way to reduce both cost and time in the hospital? Surgery 2022, 171, 1665–1670. [Google Scholar] [CrossRef]
- Moskowitz, E.; Campion, E.M.; Burlew, C.C.; Helmkamp, L.J.; Peltz, E.D.; Gansar, B.L.; McIntyre, R.C. Obstruction reduction: Use of water-soluble contrast challenge to differentiate between partial and complete small bowel obstruction. Am. J. Surg. 2019, 218, 913–917. [Google Scholar] [CrossRef]
- Azizoglu, M.; Arslan, S.; Kamci, T.O.; Basuguy, E.; Aydogdu, B.; Karabel, M.A.; Okur, M.H. Can direct bilirubin-to-lymphocyte ratio predict surgery for pediatric adhesive small bowel obstruction? Cirugía Y Cirujanos 2024, 92, 307–313. [Google Scholar] [PubMed]
- Li, Z.; Zhang, L.; Liu, X.; Yuan, F.; Song, B. Diagnostic utility of CT for small bowel obstruction: Systematic review and meta-analysis. PLoS ONE 2019, 14, e0226740. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Gupta, S.; Sudhakar, P.; Venkataramu, N.; Sood, B.; Wig, J. Comparative evaluation of plain films, ultrasound and CT in the diagnosis of intestinal obstruction. Acta Radiol. 1999, 40, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Mettler, F.A., Jr.; Huda, W.; Yoshizumi, T.T.; Mahesh, M. Effective doses in radiology and diagnostic nuclear medicine: A catalog. Radiology 2008, 248, 254–263. [Google Scholar] [CrossRef]
- Chang, K.J.; Marin, D.; Kim, D.H.; Fowler, K.J.; Camacho, M.A.; Cash, B.D.; Garcia, E.M.; Hatten, B.W.; Kambadakone, A.R.; Levy, A.D. ACR Appropriateness Criteria® suspected small-bowel obstruction. J. Am. Coll. Radiol. 2020, 17, S305–S314. [Google Scholar] [CrossRef]
- Lawrence, E.M.; Pickhardt, P.J. Water-soluble contrast challenge for suspected small-bowel obstruction: Technical success rate, accuracy, and clinical outcomes. Am. J. Roentgenol. 2021, 217, 1365–1366. [Google Scholar] [CrossRef]
- Alnachoukati, O.; Ray-Zack, M.; Godin, S.; Apodaca, T.; Zielinski, M.; Dunn, J. Optimal timing of first abdominal radiography after gastrografin administration for small bowel obstruction. J. Surg. Res. 2020, 256, 193–197. [Google Scholar] [CrossRef]
- Mansoori, B.; Vasan, V.; Xi, Y.; Fielding, J.R. Variations in use of “water soluble contrast challenge” for small bowel obstruction among academic radiologists: Results of a national survey. Abdom. Radiol. 2020, 45, 1050–1056. [Google Scholar] [CrossRef]
- Lawrence, E.M.; Pickhardt, P.J. Evaluating suspected small bowel obstruction with the water-soluble contrast challenge. Br. J. Radiol. 2022, 95, 20210791. [Google Scholar] [CrossRef] [PubMed]
| Demographics | Challenge Group | Series Group | p Value |
|---|---|---|---|
| Gender | 0.268 | ||
| Female | 60.4% (29) | 45.9% (17) | |
| Male | 39.6% (19) | 54.1% (20) | |
| Race | 0.808 | ||
| Asian | 0% (0) | 2.7% (1) | |
| Black or African American | 35.4% (17) | 40.5% (15) | |
| Native Hawaiian or Other Pacific Islander | 2.1% (1) | 0% (0) | |
| Unknown | 2.1% (1) | 0% (0) | |
| White | 60.4% (29) | 56.8% (21) | |
| Age (years) | 64.7 ± 13.6 | 63.5 ± 17.3 | 0.870 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganapathy, A.K.; Cunningham, L.; Lanier, M.H.; Nakhaima, S.; Thiel, M.; Hoffman, D.; Ilahi, O.; Ballard, D.H.; Mellnick, V.M. Bedside Small-Bowel Challenge vs. Fluoroscopic Series for SBO: A Cost Effectiveness Analysis. Tomography 2025, 11, 107. https://doi.org/10.3390/tomography11100107
Ganapathy AK, Cunningham L, Lanier MH, Nakhaima S, Thiel M, Hoffman D, Ilahi O, Ballard DH, Mellnick VM. Bedside Small-Bowel Challenge vs. Fluoroscopic Series for SBO: A Cost Effectiveness Analysis. Tomography. 2025; 11(10):107. https://doi.org/10.3390/tomography11100107
Chicago/Turabian StyleGanapathy, Aravinda Krishna, Liam Cunningham, M. Hunter Lanier, Selasi Nakhaima, Madelyn Thiel, Daniel Hoffman, Obeid Ilahi, David H. Ballard, and Vincent M. Mellnick. 2025. "Bedside Small-Bowel Challenge vs. Fluoroscopic Series for SBO: A Cost Effectiveness Analysis" Tomography 11, no. 10: 107. https://doi.org/10.3390/tomography11100107
APA StyleGanapathy, A. K., Cunningham, L., Lanier, M. H., Nakhaima, S., Thiel, M., Hoffman, D., Ilahi, O., Ballard, D. H., & Mellnick, V. M. (2025). Bedside Small-Bowel Challenge vs. Fluoroscopic Series for SBO: A Cost Effectiveness Analysis. Tomography, 11(10), 107. https://doi.org/10.3390/tomography11100107






