Flow-Compensated vs. Monopolar Diffusion Encodings: Differences in Lesion Detectability Regarding Size and Position in Liver Diffusion-Weighted MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRI
2.3. Image Analysis
2.3.1. Segmentation
2.3.2. Evaluation
2.4. Statistical Analysis
3. Results
3.1. Study Population
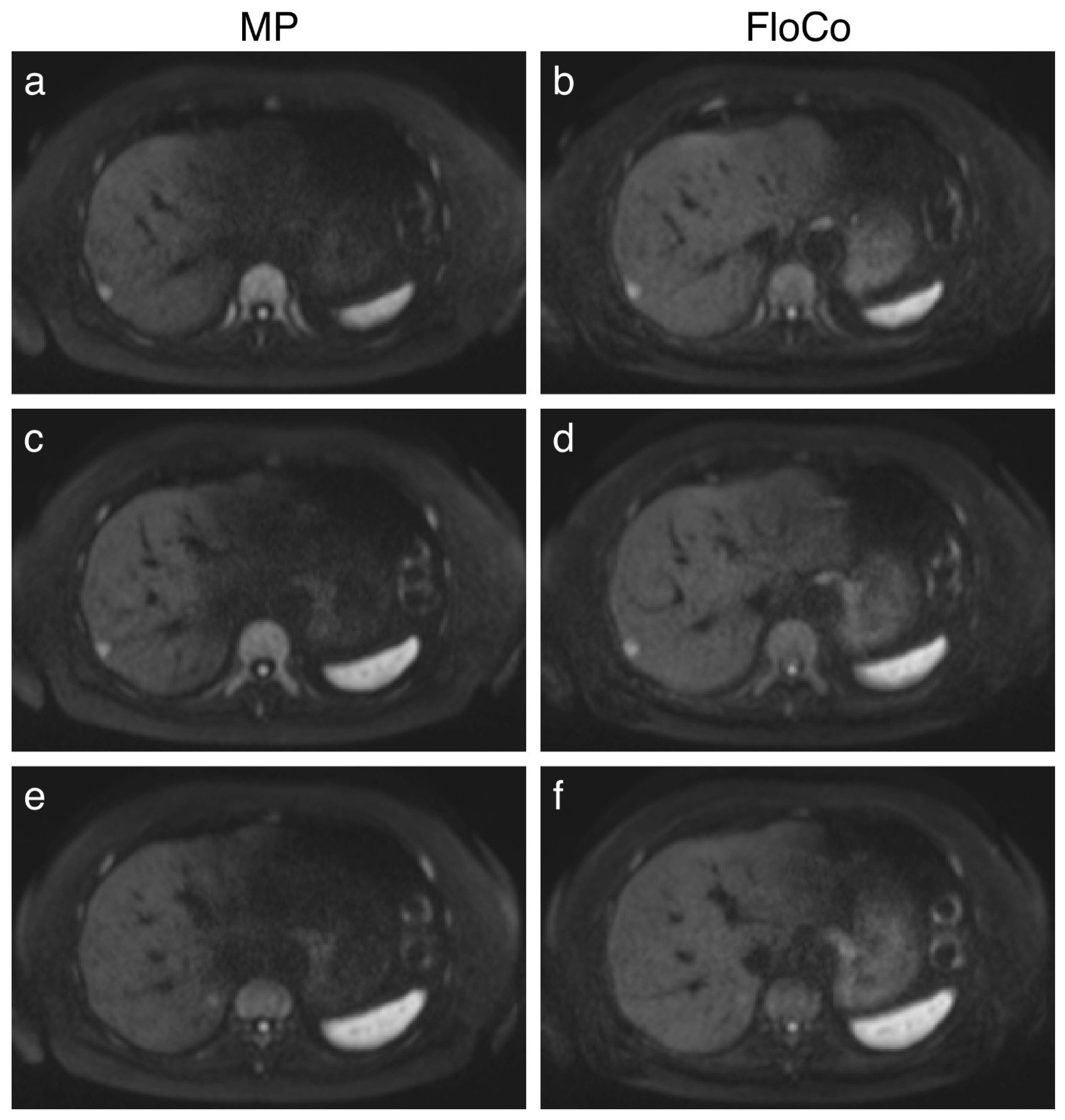
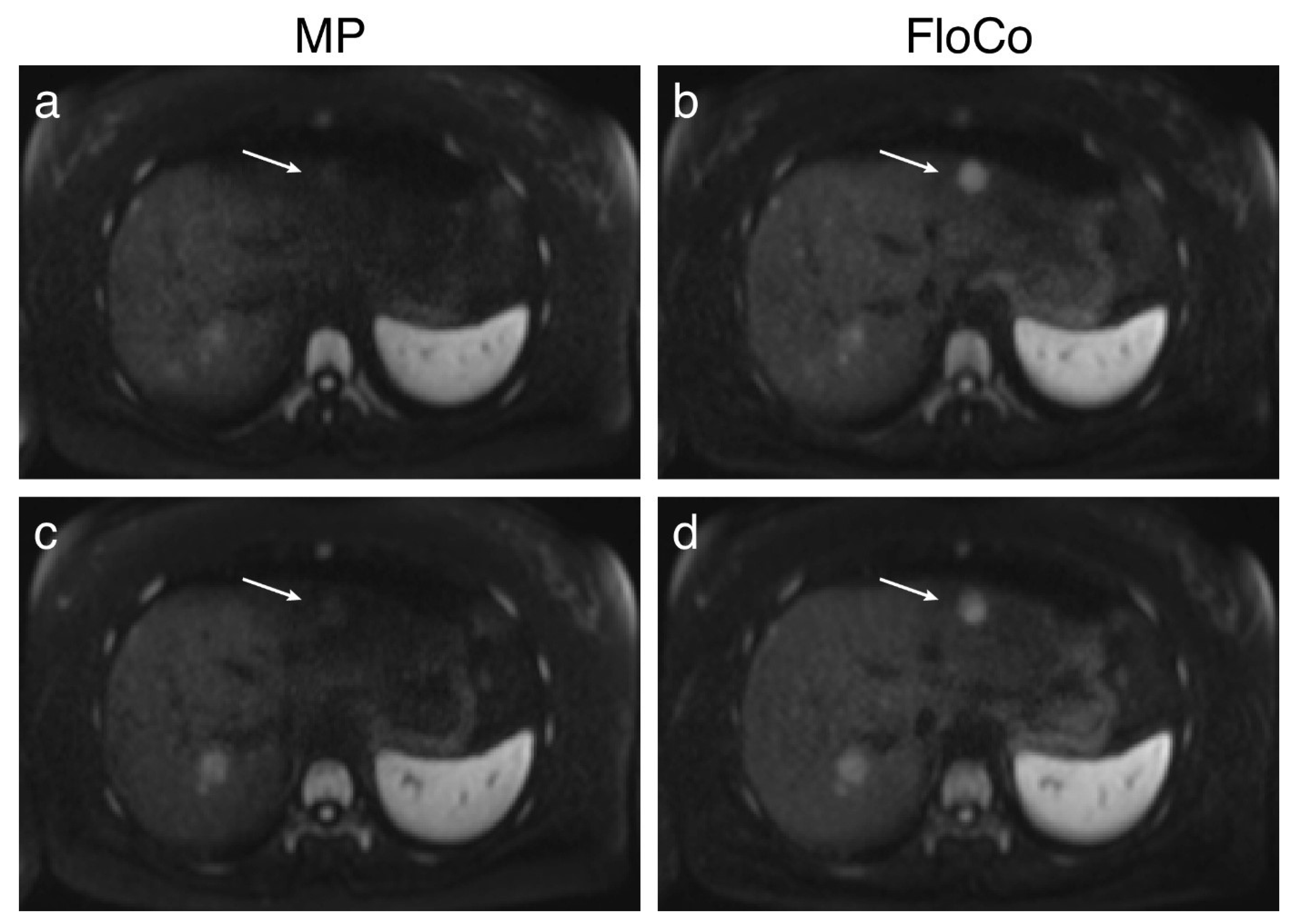
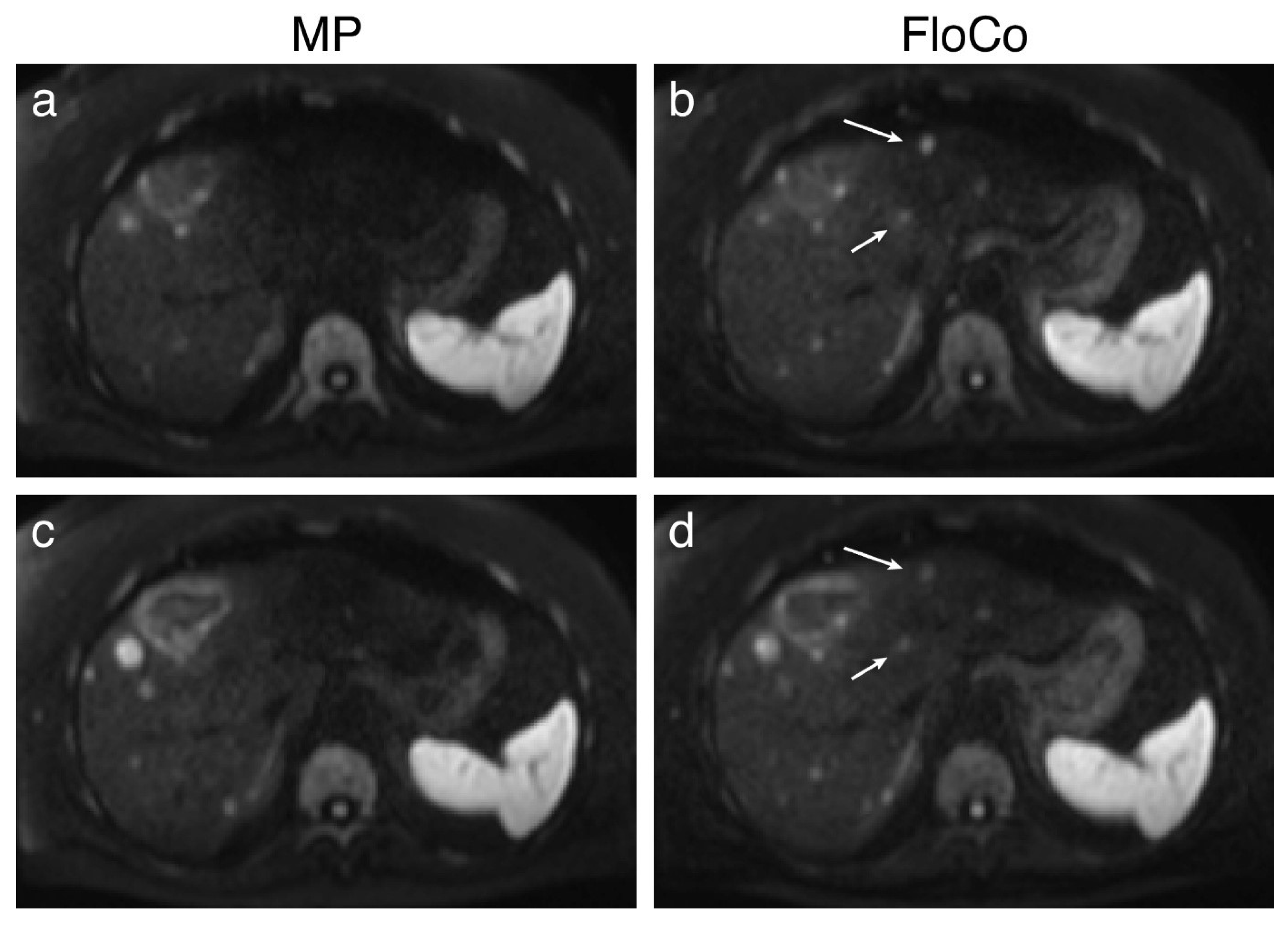
3.2. Representative Images
3.3. Quantitative Analysis
3.3.1. Size-Based Evaluation
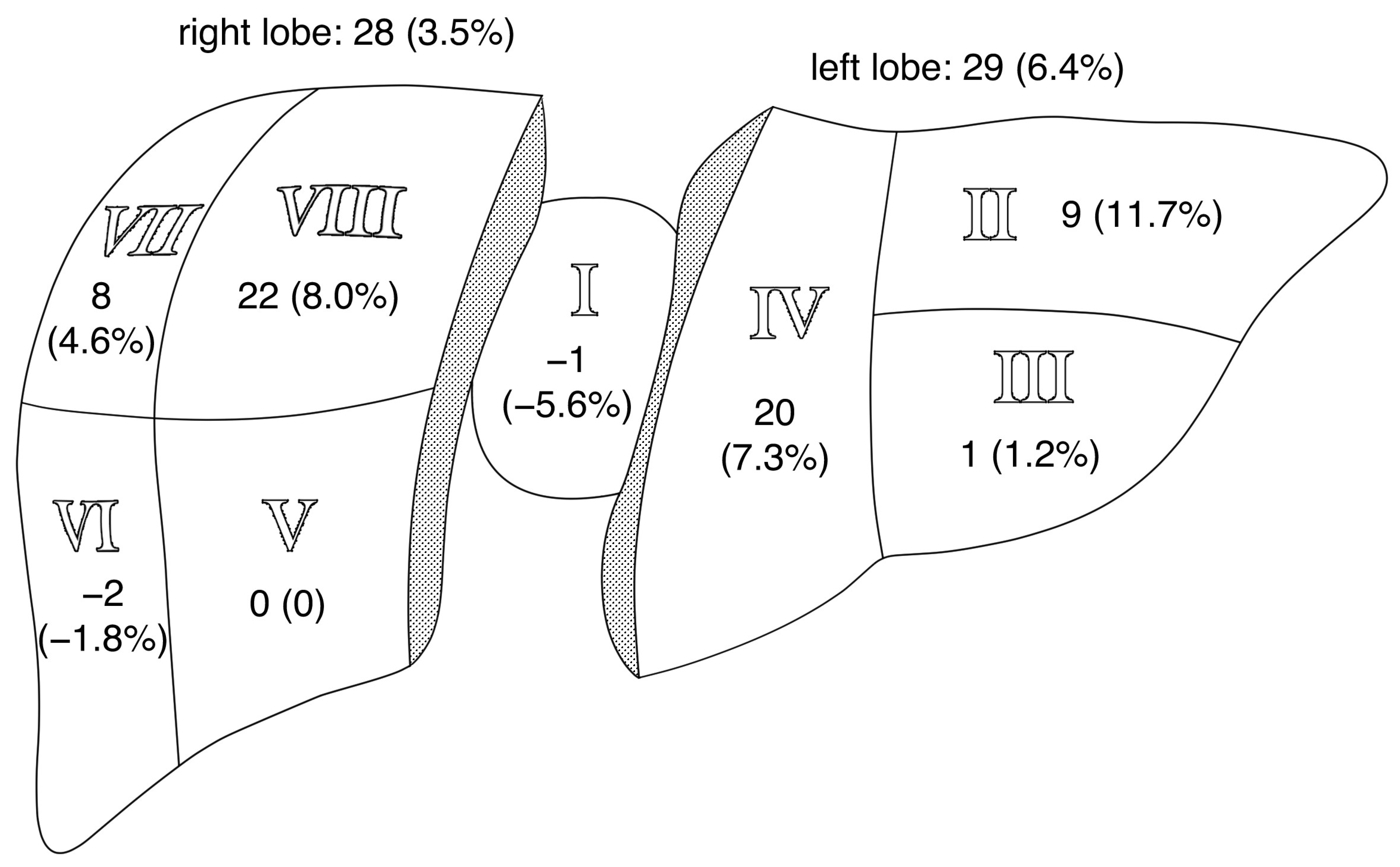
3.3.2. Location-Based Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| CNR | contrast-to-noise ratio |
| DWI | diffusion-weighted MRI |
| FLL | focal liver lesion |
| FloCo | flow-compensated |
| MP | monopolar |
References
- Adam, R.; De Gramont, A.; Figueras, J.; Guthrie, A.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; et al. The oncosurgery approach to managing liver metastases from colorectal cancer: A multidisciplinary international consensus. Oncologist 2012, 17, 1225–1239. [Google Scholar] [CrossRef]
- Chapman, W.C.; Hoff, P.M.; Strasberg, S.M. Selection of patients for resection of hepatic colorectal metastases: Expert consensus statement by Charnsangavej et al. Ann. Surg. Oncol. 2006, 13, 1269–1270. [Google Scholar] [CrossRef]
- Venkatesh, S.K.; Chandan, V.; Roberts, L.R. Liver masses: A clinical, radiologic, and pathologic perspective. Clin. Gastroenterol. Hepatol. 2014, 12, 1414–1429. [Google Scholar] [CrossRef] [PubMed]
- Maino, C.; Vernuccio, F.; Cannella, R.; Cortese, F.; Franco, P.N.; Gaetani, C.; Giannini, V.; Inchingolo, R.; Ippolito, D.; Defeudis, A.; et al. Liver metastases: The role of magnetic resonance imaging. World J. Gastroenterol. 2023, 29, 5180–5197. [Google Scholar] [CrossRef]
- Donato, H.; Franca, M.; Candelaria, I.; Caseiro-Alves, F. Liver MRI: From basic protocol to advanced techniques. Eur. J. Radiol. 2017, 93, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Taron, J.; Johannink, J.; Bitzer, M.; Nikolaou, K.; Notohamiprodjo, M.; Hoffmann, R. Added value of diffusion-weighted imaging in hepatic tumors and its impact on patient management. Cancer Imaging 2018, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Dudeck, O.; Zeile, M.; Wybranski, C.; Schulmeister, A.; Fischbach, F.; Pech, M.; Wieners, G.; Ruhl, R.; Grosser, O.; Amthauer, H.; et al. Early prediction of anticancer effects with diffusion-weighted MR imaging in patients with colorectal liver metastases following selective internal radiotherapy. Eur. Radiol. 2010, 20, 2699–2706. [Google Scholar] [CrossRef]
- Erturk, S.M.; Ichikawa, T.; Sano, K.; Motosugi, U.; Sou, H.; Araki, T. Diffusion-weighted magnetic resonance imaging for characterization of focal liver masses: Impact of parallel imaging (SENSE) and b value. J. Comput. Assist. Tomogr. 2008, 32, 865–871. [Google Scholar] [CrossRef]
- Lewis, S.; Dyvorne, H.; Cui, Y.; Taouli, B. Diffusion-weighted imaging of the liver: Techniques and applications. Magn. Reson. Imaging Clin. N. Am. 2014, 22, 373–395. [Google Scholar] [CrossRef]
- Kwee, T.C.; Takahara, T.; Niwa, T.; Ivancevic, M.K.; Herigault, G.; Van Cauteren, M.; Luijten, P.R. Influence of cardiac motion on diffusion-weighted magnetic resonance imaging of the liver. MAGMA 2009, 22, 319–325. [Google Scholar] [CrossRef]
- Liau, J.; Lee, J.; Schroeder, M.E.; Sirlin, C.B.; Bydder, M. Cardiac motion in diffusion-weighted MRI of the liver: Artifact and a method of correction. J. Magn. Reson. Imaging 2012, 35, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Van, A.T.; McTavish, S.; Peeters, J.M.; Weiss, K.; Makowski, M.R.; Braren, R.F.; Karampinos, D.C. Motion-induced phase-corrected homodyne reconstruction for partial Fourier single-shot diffusion-weighted echo planar imaging of the liver. NMR Biomed. 2024, 37, e5147. [Google Scholar] [CrossRef] [PubMed]
- Nasu, K.; Kuroki, Y.; Nawano, S.; Kuroki, S.; Tsukamoto, T.; Yamamoto, S.; Motoori, K.; Ueda, T. Hepatic metastases: Diffusion-weighted sensitivity-encoding versus SPIO-enhanced MR imaging. Radiology 2006, 239, 122–130. [Google Scholar] [CrossRef]
- Koh, D.M.; Collins, D.J. Diffusion-weighted MRI in the body: Applications and challenges in oncology. AJR Am. J. Roentgenol. 2007, 188, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, S.S.; Kim, N.; Kim, E.; Kim, Y.J.; Yun, S.C.; Kuhn, B.; Kim, I.S.; Park, S.H.; Kim, S.Y.; et al. Intravoxel incoherent motion diffusion-weighted MR imaging of the liver: Effect of triggering methods on regional variability and measurement repeatability of quantitative parameters. Radiology 2015, 274, 405–415. [Google Scholar] [CrossRef]
- Metens, T.; Absil, J.; Denolin, V.; Bali, M.A.; Matos, C. Liver apparent diffusion coefficient repeatability with individually predetermined optimal cardiac timing and artifact elimination by signal filtering. J. Magn. Reson. Imaging 2016, 43, 1100–1110. [Google Scholar] [CrossRef]
- Murtz, P.; Flacke, S.; Traber, F.; van den Brink, J.S.; Gieseke, J.; Schild, H.H. Abdomen: Diffusion-weighted MR imaging with pulse-triggered single-shot sequences. Radiology 2002, 224, 258–264. [Google Scholar] [CrossRef]
- Xiang, Z.; Ai, Z.; Liang, J.; Li, G.; Zhu, X.; Yan, X. Evaluation of Regional Variability and Measurement Reproducibility of Intravoxel Incoherent Motion Diffusion Weighted Imaging Using a Cardiac Stationary Phase Based ECG Trigger Method. Biomed. Res. Int. 2018, 2018, 4604218. [Google Scholar] [CrossRef]
- Ichikawa, S.; Motosugi, U.; Tamada, D.; Wakayama, T.; Sato, K.; Funayama, S.; Onishi, H. Improving the Quality of Diffusion-weighted Imaging of the Left Hepatic Lobe Using Weighted Averaging of Signals from Multiple Excitations. Magn. Reson. Med. Sci. 2019, 18, 225–232. [Google Scholar] [CrossRef]
- Rauh, S.S.; Riexinger, A.J.; Ohlmeyer, S.; Hammon, M.; Saake, M.; Stemmer, A.; Uder, M.; Hensel, B.; Laun, F.B. A mixed waveform protocol for reduction of the cardiac motion artifact in black-blood diffusion-weighted imaging of the liver. Magn. Reson. Imaging 2020, 67, 59–68. [Google Scholar] [CrossRef]
- Geng, R.; Zhang, Y.; Rice, J.; Muehler, M.R.; Starekova, J.; Rutkowski, D.R.; Uboha, N.V.; Pirasteh, A.; Roldan-Alzate, A.; Guidon, A.; et al. Motion-robust, blood-suppressed, reduced-distortion diffusion MRI of the liver. Magn. Reson. Med. 2023, 89, 908–921. [Google Scholar] [CrossRef]
- Laun, F.B.; Fuhres, T.; Seuss, H.; Muller, A.; Bickelhaupt, S.; Stemmer, A.; Benkert, T.; Uder, M.; Saake, M. Flow-compensated diffusion encoding in MRI for improved liver metastasis detection. PLoS ONE 2022, 17, e0268843. [Google Scholar] [CrossRef]
- Son, J.S.; Park, H.S.; Park, S.; Kim, Y.J.; Yu, M.H.; Jung, S.I.; Paek, M.; Nickel, M.D. Motion-Corrected versus Conventional Diffusion-Weighted Magnetic Resonance Imaging of the Liver Using Non-Rigid Registration. Diagnostics 2023, 13, 1008. [Google Scholar] [CrossRef]
- Fuhres, T.; Saake, M.; Lorenz, J.; Seuss, H.; Stemmer, A.; Benkert, T.; Uder, M.; Laun, F.B. Reduction of the cardiac pulsation artifact and improvement of lesion conspicuity in flow-compensated diffusion images in the liver-A quantitative evaluation of postprocessing algorithms. Magn. Reson. Med. 2023, 89, 423–439. [Google Scholar] [CrossRef]
- Cho, K.E.; Yu, J.-S.; Chung, J.-J.; Kim, J.H.; Kim, K.W. Ferucarbotran-Enhanced Hepatic MRI at 3T Unit: Quantitative and Qualitative Comparison of Fast Breath-hold Imaging Sequences. J. Korean Soc. Magn. Reson. Med. 2010, 14, 31–40. [Google Scholar] [CrossRef]
- Ozaki, M.; Inoue, Y.; Miyati, T.; Hata, H.; Mizukami, S.; Komi, S.; Matsunaga, K.; Woodhams, R. Motion artifact reduction of diffusion-weighted MRI of the liver: Use of velocity-compensated diffusion gradients combined with tetrahedral gradients. J. Magn. Reson. Imaging 2013, 37, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Gorgec, B.; Hansen, I.S.; Kemmerich, G.; Syversveen, T.; Abu Hilal, M.; Belt, E.J.T.; Bosscha, K.; Burgmans, M.C.; Cappendijk, V.C.; D’Hondt, M.; et al. MRI in addition to CT in patients scheduled for local therapy of colorectal liver metastases (CAMINO): An international, multicentre, prospective, diagnostic accuracy trial. Lancet Oncol. 2024, 25, 137–146. [Google Scholar] [CrossRef]
- Michael, E.S.; Hennel, F.; Pruessmann, K.P. Motion-compensated diffusion encoding in multi-shot human brain acquisitions: Insights using high-performance gradients. Magn. Reson. Med. 2024, 92, 556–572. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Fan, Z.; Sharif, B.; He, Y.; Dharmakumar, R.; Berman, D.S.; Li, D. In vivo three-dimensional high resolution cardiac diffusion-weighted MRI: A motion compensated diffusion-prepared balanced steady-state free precession approach. Magn. Reson. Med. 2014, 72, 1257–1267. [Google Scholar] [CrossRef]
- Fuhres, T.; Saake, M.; Szczepankiewicz, F.; Bickelhaupt, S.; Uder, M.; Laun, F.B. Impact of velocity- and acceleration-compensated encodings on signal dropout and black-blood state in diffusion-weighted magnetic resonance liver imaging at clinical TEs. PLoS ONE 2023, 18, e0291273. [Google Scholar] [CrossRef]
- Saito, K.; Tajima, Y.; Harada, T.L. Diffusion-weighted imaging of the liver: Current applications. World J. Radiol. 2016, 8, 857–867. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schima, W.; Koh, D.M.; Baron, R. Focal Liver Lesions. In Diseases of the Abdomen and Pelvis 2018–2021: Diagnostic Imaging—IDKD Book; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; IDKD Springer Series; Springer: Cham, Switzerland, 2018; pp. 173–196. [Google Scholar]
- Sutherland, T.; Seale, M.; Yap, K. Part 2: MRI of hypervascular focal liver lesions using liver specific contrast agents. J. Med. Imaging Radiat. Oncol. 2014, 58, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Galea, N.; Cantisani, V.; Taouli, B. Liver lesion detection and characterization: Role of diffusion-weighted imaging. J. Magn. Reson. Imaging 2013, 37, 1260–1276. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, M.J.; Chung, Y.E.; Kim, K.A.; Choi, J.Y.; Lim, J.S.; Park, M.S.; Kim, K.W. Comparison of breathhold, navigator-triggered, and free-breathing diffusion-weighted MRI for focal hepatic lesions. J. Magn. Reson. Imaging 2013, 38, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Dyvorne, H.A.; Galea, N.; Nevers, T.; Fiel, M.I.; Carpenter, D.; Wong, E.; Orton, M.; de Oliveira, A.; Feiweier, T.; Vachon, M.L.; et al. Diffusion-weighted imaging of the liver with multiple b values: Effect of diffusion gradient polarity and breathing acquisition on image quality and intravoxel incoherent motion parameters--a pilot study. Radiology 2013, 266, 920–929. [Google Scholar] [CrossRef]
- Lee, P.K.; Zhou, X.; Wang, N.; Syed, A.B.; Brunsing, R.L.; Vasanawala, S.S.; Hargreaves, B.A. Distortionless, free-breathing, and respiratory resolved 3D diffusion weighted imaging of the abdomen. Magn. Reson. Med. 2024, 92, 586–604. [Google Scholar] [CrossRef]
- Saake, M.; Seuss, H.; Riexinger, A.; Bickelhaupt, S.; Hammon, M.; Uder, M.; Laun, F.B. Image Quality and Detection of Small Focal Liver Lesions in Diffusion-Weighted Imaging: Comparison of Navigator Tracking and Free-Breathing Acquisition. Investig. Radiol. 2021, 56, 579–590. [Google Scholar] [CrossRef]
- Szklaruk, J.; Son, J.B.; Wei, W.; Bhosale, P.; Javadi, S.; Ma, J. Comparison of free breathing and respiratory triggered diffusion-weighted imaging sequences for liver imaging. World J. Radiol. 2019, 11, 134–143. [Google Scholar] [CrossRef]
- Taron, J.; Martirosian, P.; Erb, M.; Kuestner, T.; Schwenzer, N.F.; Schmidt, H.; Honndorf, V.S.; Weibeta, J.; Notohamiprodjo, M.; Nikolaou, K.; et al. Simultaneous multislice diffusion-weighted MRI of the liver: Analysis of different breathing schemes in comparison to standard sequences. J. Magn. Reson. Imaging 2016, 44, 865–879. [Google Scholar] [CrossRef]
- Riexinger, A.; Laun, F.B.; Bickelhaupt, S.; Seuss, H.; Uder, M.; Hensel, B.; Saake, M. On the dependence of the cardiac motion artifact on the breathing cycle in liver diffusion-weighted imaging. PLoS ONE 2020, 15, e0239743. [Google Scholar] [CrossRef]
- Fuhres, T.; Saake, M.; Lorenz, J.; Seuss, H.; Bickelhaupt, S.; Uder, M.; Laun, F.B. Feature-guided deep learning reduces signal loss and increases lesion CNR in diffusion-weighted imaging of the liver. Z. Med. Phys. 2023, 34, 258–269. [Google Scholar] [CrossRef]
- Lee, P.K.; Zhou, X.; Hargreaves, B.A. Robust multishot diffusion-weighted imaging of the abdomen with region-based shot rejection. Magn. Reson. Med. 2024, 92, 519–531. [Google Scholar] [CrossRef]
- Bruegel, M.; Holzapfel, K.; Gaa, J.; Woertler, K.; Waldt, S.; Kiefer, B.; Stemmer, A.; Ganter, C.; Rummeny, E.J. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur. Radiol. 2008, 18, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Kwee, T.C.; Takahara, T.; Koh, D.M.; Nievelstein, R.A.; Luijten, P.R. Comparison and reproducibility of ADC measurements in breathhold, respiratory triggered, and free-breathing diffusion-weighted MR imaging of the liver. J. Magn. Reson. Imaging 2008, 28, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, S.; Kawai, H.; Fukatsu, H.; Sakurai, Y.; Aoki, I.; Miura, S.; Mimura, T.; Kanazawa, H.; Ishigaki, T. Diffusion-weighted imaging of the liver: Technical challenges and prospects for the future. Magn. Reson. Med. Sci. 2005, 4, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Namimoto, T.; Yamashita, Y.; Sumi, S.; Tang, Y.; Takahashi, M. Focal liver masses: Characterization with diffusion-weighted echo-planar MR imaging. Radiology 1997, 204, 739–744. [Google Scholar] [CrossRef]

| Parameter | MP | FloCo |
|---|---|---|
| Sequence | DWI EPI | |
| Repetition time (ms) | 12,400 | |
| Echo time (ms) | 70 | |
| Echo spacing (ms) | 0.49 | |
| Maximum gradient strength (mT/m) | 45 | |
| Maximum slew rate (T/m/s) | 200 | |
| Voxel size (mm3) | 3.125 × 3.125 × 5 interpolated to 1.56 × 1.56 × 5 | |
| Field of view (read × phase; mm2) | 400 × 325 | |
| Phase direction | anterior–posterior | |
| Phase resolution | 100% | |
| b-values (s/mm2) | 50, 800 | |
| Averages (b50, b800) | 1, 4 | |
| Diffusion mode | 3-scan trace | |
| Diffusion scheme | Monopolar (zeroth-order gradient moment nulling) | velocity-compensated (zeroth- and first-order gradient moment nulling) |
| Matrix | 128 × 104 | |
| Number of slices | 39 (axial) | |
| Slice distance | 20% | |
| Parallel imaging | GRAPPA × 2, 24 reference lines | |
| Partial Fourier | 6/8 | |
| Acquisition time (min:s) | 3:43 | |
| Bandwidth (Hz/pixel) | 2790 | |
| Surface coil intensity correction | Yes, the “pre-scan normalize” option was used | |
| Fat saturation | SPAIR and gradient reversal | |
| Acquisition mode | Free breathing | |
| Size | Ntotal_all | Nboth | Nonly_mp | Nonly_fl | NΔ | Sen. (95% CI) MP | Sen. (95% CI) FloCo | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| In Voxels | ⌀ in mm | ||||||||||
| 1–10 | ≤6.5 | 446 | 317 | 43 | 9.6% | 86 | 19.3% | 43 | 9.6% | 80.7% (76.7–84.3%) | 90.4% (87.2–92.9%) |
| 11–25 | 6.6–8.8 | 272 | 252 | 4 | 1.5% | 16 | 5.9% | 12 | 4.4% | 94.1% (90.6–96.6%) | 98.5% (96.3–99.6%) |
| 26–50 | 8.9–11.1 | 146 | 144 | 0 | 0 | 2 | 1.4% | 2 | 1.4% | 98.6% (95.1–99.8%) | 100% (97.5–100%) |
| 51–100 | 11.2–14.0 | 105 | 105 | 0 | 0 | 0 | 0 | 0 | 0 | 100% (96.6–100%) | 100% (96.6–100%) |
| 101–250 | 14.1–19.1 | 122 | 122 | 0 | 0 | 0 | 0 | 0 | 0 | 100% (97.0–100%) | 100% (97.0–100%) |
| 251–500 | 19.2–24.0 | 62 | 62 | 0 | 0 | 0 | 0 | 0 | 0 | 100% (94.2–100%) | 100% (94.2–100%) |
| ≥501 | ≥24.1 | 105 | 105 | 0 | 0 | 0 | 0 | 0 | 0 | 100% (96.6–100%) | 100% (96.6–100%) |
| Summary | 1258 | 1107 | 47 | 3.7% | 104 | 8.3% | 57 | 4.5% | 91.7% (90.1–93.2%) | 96.3% (95.1–97.2%) | |
| Liver Segment | Ntotal_all | Nboth | Nonly_mp | Nonly_fl | NΔ | Sen. (95% CI) MP | Sen. (95% CI) FloCo | |||
|---|---|---|---|---|---|---|---|---|---|---|
| I | 18 | 17 | 1 | 5.6% | 0 | 0 | −1 | −5.6% | 100% (81.5–100%) | 94.4% (72.7–99.9%) |
| II | 77 | 68 | 0 | 0 | 9 | 11.7% | 9 | 11.7% | 88.3% (79.0–94.5%) | 100% (95.3–100%) |
| III | 82 | 71 | 5 | 6.1% | 6 | 7.3% | 1 | 1.2% | 92.7% (84.8–97.3%) | 93.9% (86.3–98.0%) |
| IV | 274 | 240 | 7 | 2.6% | 27 | 9.6% | 20 | 7.3% | 90.1% (86.0–93.4%) | 97.4% (94.8–99.0%) |
| V | 247 | 215 | 16 | 6.5% | 16 | 6.5% | 0 | 0 | 93.5% (89.7–96.3%) | 93.5% (89.7–96.3%) |
| VI | 110 | 96 | 8 | 7.2% | 6 | 5.5% | −2 | −1.8% | 94.5% (88.5–98.0%) | 92.7% (86.2–96.8%) |
| VII | 175 | 161 | 3 | 1.7% | 11 | 6.2% | 8 | 4.6% | 93.7% (89.0–96.8%) | 98.3% (95.1–99.7%) |
| VIII | 275 | 239 | 7 | 2.5% | 29 | 10.6% | 22 | 8.0% | 89.5% (85.2–92.8%) | 97.5% (94.8–99.0%) |
| Summary | 1258 | 1107 | 47 | 3.7% | 104 | 8.3% | 57 | 4.5% | 91.7% (90.1–93.2%) | 96.3% (95.1–97.2%) |
| Liver Segment | CNR (Mean ± Standard Deviation) | Liver Lobe | |||||
|---|---|---|---|---|---|---|---|
| MP | FloCo | p-Value | MP | FloCo | p-Value | ||
| I | 17.6 ± 10.2 | 22.8 ± 12.7 | 0.02 | 12.3 ± 8.9 | 15.9 ± 9.9 | <0.001 | left |
| II | 9.4 ± 8.7 | 13.5 ± 8.8 | <0.001 | ||||
| III | 11.8 ± 8.0 | 14.1 ± 8.9 | <0.001 | ||||
| IV | 13.0 ± 8.9 | 16.7 ± 10.0 | <0.001 | ||||
| V | 16.7 ± 9.9 | 17.4 ± 12.1 | 0.02 | 15.9 ± 10.1 | 18.1 ± 11.8 | <0.001 | right |
| VI | 19.1 ± 12.6 | 21.7 ± 13.9 | <0.001 | ||||
| VII | 13.3 ± 8.9 | 17.6 ± 11.2 | <0.001 | ||||
| VIII | 15.6 ± 9.4 | 17.6 ± 10.9 | <0.001 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldenhauer, A.; Laun, F.B.; Seuss, H.; Bickelhaupt, S.; Reithmeier, B.; Benkert, T.; Uder, M.; Saake, M.; Führes, T. Flow-Compensated vs. Monopolar Diffusion Encodings: Differences in Lesion Detectability Regarding Size and Position in Liver Diffusion-Weighted MRI. Tomography 2025, 11, 106. https://doi.org/10.3390/tomography11100106
Moldenhauer A, Laun FB, Seuss H, Bickelhaupt S, Reithmeier B, Benkert T, Uder M, Saake M, Führes T. Flow-Compensated vs. Monopolar Diffusion Encodings: Differences in Lesion Detectability Regarding Size and Position in Liver Diffusion-Weighted MRI. Tomography. 2025; 11(10):106. https://doi.org/10.3390/tomography11100106
Chicago/Turabian StyleMoldenhauer, Alessandra, Frederik B. Laun, Hannes Seuss, Sebastian Bickelhaupt, Bianca Reithmeier, Thomas Benkert, Michael Uder, Marc Saake, and Tobit Führes. 2025. "Flow-Compensated vs. Monopolar Diffusion Encodings: Differences in Lesion Detectability Regarding Size and Position in Liver Diffusion-Weighted MRI" Tomography 11, no. 10: 106. https://doi.org/10.3390/tomography11100106
APA StyleMoldenhauer, A., Laun, F. B., Seuss, H., Bickelhaupt, S., Reithmeier, B., Benkert, T., Uder, M., Saake, M., & Führes, T. (2025). Flow-Compensated vs. Monopolar Diffusion Encodings: Differences in Lesion Detectability Regarding Size and Position in Liver Diffusion-Weighted MRI. Tomography, 11(10), 106. https://doi.org/10.3390/tomography11100106






