The Value of Ultrasound for Detecting and Following Subclinical Interstitial Lung Disease in Systemic Sclerosis
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Study Design
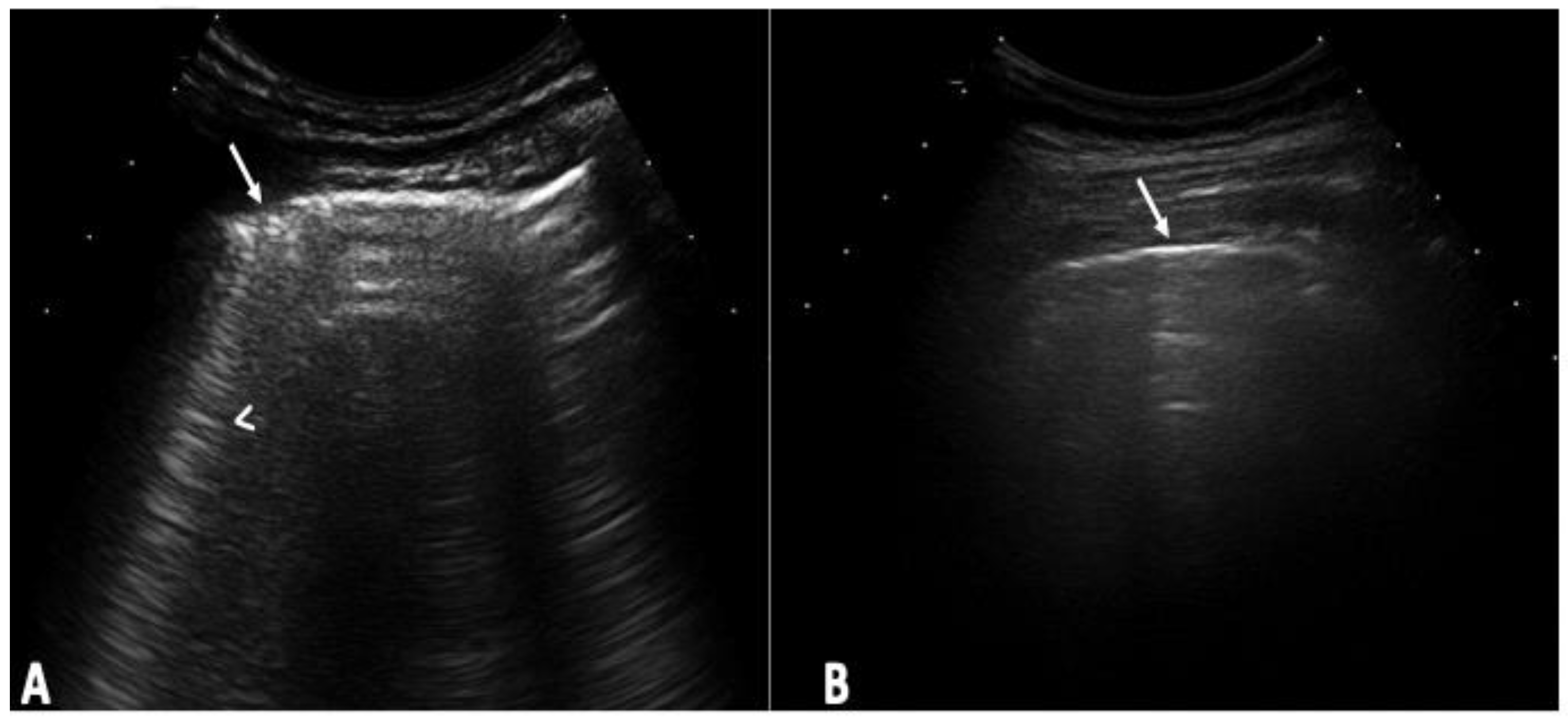
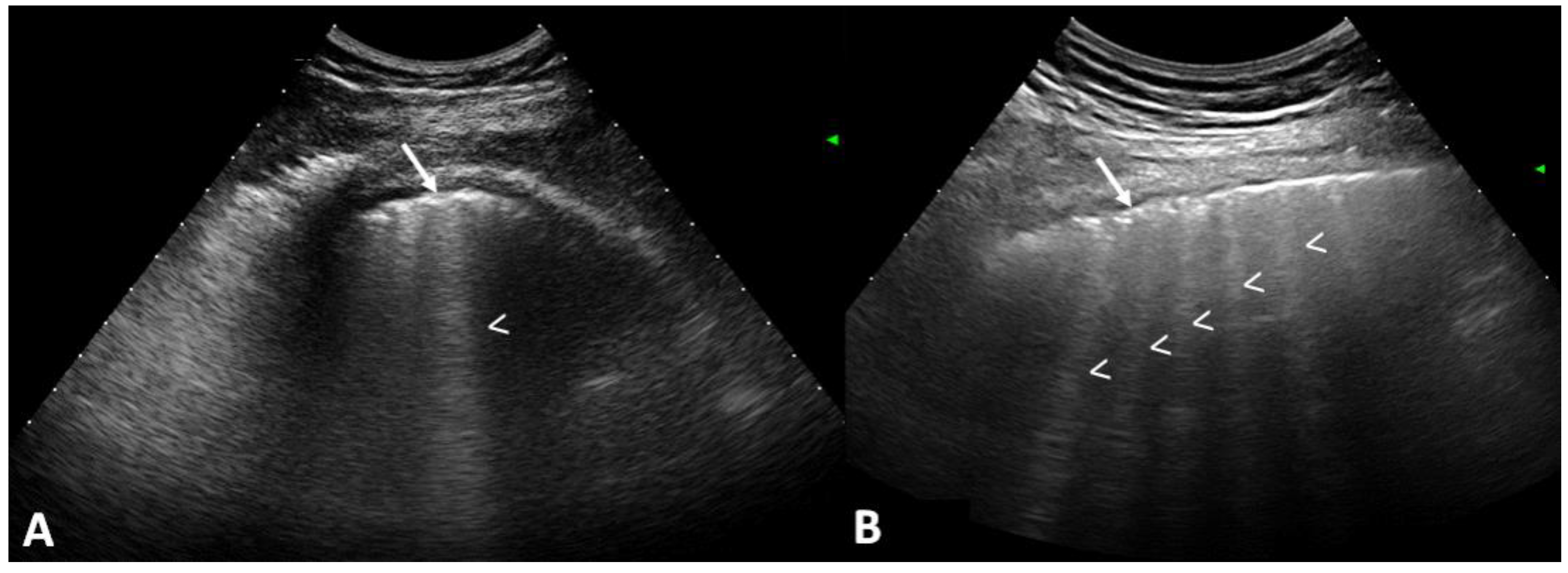
2.3. US Assessment
2.4. US Interpretation
2.5. HRCT Assessment
2.6. Statistical Analysis
3. Results
3.1. Baseline Assessment
3.2. Longitudinal Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cappelli, S.; Bellando Randone, S.; Camiciottoli, G.; De Paulis, A.; Guiducci, S.; Matucci-Cerinic, M. Interstitial lung disease in systemic sclerosis: Where do we stand? Eur. Respir. Rev. 2015, 24, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.; Huscher, D.; Siegert, E.; Allanore, Y.; Czirják, L.; DelGaldo, F.; Denton, C.P.; Distler, O.; Frerix, M.; Matucci-Cerinic, M.; et al. Systemic sclerosis associated interstitial lung disease—Individualized immunosuppressive therapy and course of lung function: Results of the EUSTAR group. Arthritis Res. Ther. 2018, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hoa, S.; Baron, M.; Hudson, M. Screening and management of subclinical interstitial lung disease in systemic sclerosis: An international survey. Rheumatology 2022, 61, 3401–3407. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, P.; Salaffi, F.; Pesci, A. Clinical and subclinical alveolitis in connective tissue diseases assessed by bronchoalveolar lavage. Semin. Arthritis Rheum. 1997, 26, 740–754. [Google Scholar] [CrossRef] [PubMed]
- Roofeh, D.; Brown, K.K.; A Kazerooni, E.; Tashkin, D.; Assassi, S.; Martinez, F.; Wells, A.U.; Raghu, G.; Denton, C.P.; Chung, L.; et al. Systemic sclerosis associated interstitial lung disease: A conceptual framework for subclinical, clinical and progressive disease. Rheumatology 2023, 62, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Le Gouellec, N.; Duhamel, A.; Perez, T.; Hachulla, A.-L.; Sobanski, V.; Faivre, J.-B.; Morell-Dubois, S.; Lambert, M.; Hatron, P.-Y.; Hachulla, E.; et al. Predictors of lung function test severity and outcome in systemic sclerosis-associated interstitial lung disease. PLoS ONE 2017, 12, e0181692. [Google Scholar] [CrossRef] [PubMed]
- Showalter, K.; Hoffmann, A.; Rouleau, G.; Aaby, D.; Lee, J.; Richardson, C.; Dematte, J.; Agrawal, R.; Chang, R.W.; Hinchcliff, M. Performance of Forced Vital Capacity and Lung Diffusion Cutpoints for Associated Radiographic Interstitial Lung Disease in Systemic Sclerosis. J. Rheumatol. 2018, 45, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
- Molberg, Ø.; Hoffmann-Vold, A.-M. Interstitial lung disease in systemic sclerosis: Progress in screening and early diagnosis. Curr. Opin. Rheumatol. 2016, 28, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Semelka, R.; Ravenel, J.; Matucci-Cerinic, M. Rheumatological diseases and cancer: The hidden variable of radiation exposure. Ann. Rheum. Dis. 2014, 73, 2065–2068. [Google Scholar] [CrossRef]
- Frauenfelder, T.; Winklehner, A.; Nguyen, T.D.L.; Dobrota, R.; Baumueller, S.; Maurer, B.; Distler, O. Screening for interstitial lung disease in systemic sclerosis: Performance of high-resolution CT with limited number of slices: A prospective study. Ann. Rheum. Dis. 2014, 73, 2069–2073. [Google Scholar] [CrossRef]
- Bernstein, E.J.; Khanna, D.; Lederer, D.J. Screening High-Resolution Computed Tomography of the Chest to Detect Interstitial Lung Disease in Systemic Sclerosis: A Global Survey of Rheumatologists. Arthritis Rheumatol. 2018, 70, 971–972. [Google Scholar] [CrossRef] [PubMed]
- Moazedi-Fuerst, F.C.; Kielhauser, S.; Brickmann, K.; Tripolt, N.; Meilinger, M.; Lufti, A.; Graninger, W. Sonographic assessment of interstitial lung disease in patients with rheumatoid arthritis, systemic sclerosis and systemic lupus erythematosus. Clin. Exp. Rheumatol. 2015, 33, S87–S91. [Google Scholar]
- Gargani, L.; Doveri, M.; D’Errico, L.; Frassi, F.; Bazzichi, M.L.; Delle Sedie, A.; Scali, M.C.; Monti, S.; Mondillo, S.; Bombardieri, S.; et al. Ultrasound lung comets in systemic sclerosis: A chest sonography hallmark of pulmonary interstitial fibrosis. Rheumatology 2009, 48, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, M.; De Cata, A.; Molinaro, F.; Trovato, F.M.; Catalano, D.; Simeone, A.; Varriale, A.; Martines, G.F.; Trovato, G. Ultra-sound signs of pulmonary fibrosis in systemic sclerosis as timely indicators for chest computed tomography. Scand. J. Rheumatol. 2015, 44, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Tardella, M.; Gutierrez, M.; Salaffi, F.; Carotti, M.; Ariani, A.; Bertolazzi, C.; Filippucci, E.; Grassi, W. Ultrasound in the assessment of pulmonary fibrosis in connective tissue disorders: Correlation with high-resolution computed tomography. J. Rheumatol. 2012, 39, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Salaffi, F.; Carotti, M.; Tardella, M.; Pineda, C.; Bertolazzi, C.; Bichisecchi, E.; Filippucci, E.; Grassi, W. Utility of a simplified ultrasound assessment to assess interstitial pulmonary fibrosis in connective tissue disorders—Preliminary results. Arthritis Res. Ther. 2011, 13, R134. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Bruni, C.; Cuomo, G.; Sedie, A.D.; Gargani, L.; Gutierrez, M.; Lepri, G.; Ruaro, B.; Santiago, T.; Suliman, Y.; et al. The role of ultrasound in systemic sclerosis: On the cutting edge to foster clinical and research advancement. J. Scleroderma Relat. Disord. 2021, 6, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Long, S.; Gutierrez, M.; Clavijo-Cornejo, D.; Alfaro-Rodríguez, A.; González-Sámano, K.; Cortes-Altamirano, J.L.; Muñoz-Louis, R.; Cruz-Arenas, E.; Camargo, K.; Gonzalez, F.; et al. Subclinical interstitial lung disease in patients with systemic sclerosis. A pilot study on the role of ultrasound. Reumatol. Clin. 2021, 17, 144–149. [Google Scholar] [CrossRef]
- Gutierrez, M.; Soto-Fajardo, C.; Pineda, C.; Alfaro-Rodriguez, A.; Terslev, L.; Bruyn, G.; Iagnocco, A.; Bertolazzi, C.; D’agostino, M.A.; Sedie, A.D. Ultrasound in the Assessment of Interstitial Lung Disease in Systemic Sclerosis: A Systematic Literature Review by the OMERACT Ultrasound Group. J. Rheumatol. 2020, 47, 991–1000. [Google Scholar] [CrossRef]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Mezière, G.; Biderman, P.A.; Gepner, A. The comet-tail artifact: An ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999, 25, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, K.; Galanski, M.; Peters, E.; Hansen, J. Pneumothorax: Evaluation by ultrasound Preliminary results. J. Thorac. Imaging 1987, 2, 76–78. [Google Scholar] [CrossRef]
- Warrick, J.H.; Bhalla, M.; Schabel, S.I.; Siver, R.M. High resolution computed tomography in early scleroderma lung disease. J. Rheumatol. 1991, 18, 1520–1528. [Google Scholar] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Carotti, M.; Di Donato, E.; Di Carlo, M.; Ceccarelli, L.; Giuseppetti, G. Computer-Aided Tomographic Analysis of Interstitial Lung Disease (ILD) in Patients with Systemic Sclerosis (SSc). Correlation with Pulmonary Physiologic Tests and Patient-Centred Measures of Perceived Dyspnea and Functional Disability. PLoS ONE 2016, 11, e0149240. [Google Scholar]
- Salaffi, F.; Carotti, M.; Baldelli, S.; Secchi, E.B.; Manganelli, P.; Subiaco, S.; Salvolini, L. Subclinical interstitial lung involvement in rheumatic diseases. Correlation of high resolution computerized tomography and functional and cytologic findings. Radiol. Med. 1999, 97, 33–41. [Google Scholar] [PubMed]
- Beigi, D.M.R.; Pellegrino, G.; Loconte, M.; Landini, N.; Mattone, M.; Paone, G.; Truglia, S.; Di Ciommo, F.R.; Bisconti, I.; Cadar, M.; et al. Lung ultrasound compared to computed tomography detection and automated quantification of systemic sclerosis-associated interstitial lung disease: Preliminary study. Rheumatology 2023. [Google Scholar] [CrossRef]
- Gomes Guerra, M.; Machado Pinto, T.; Águeda, A.; Rodrigues, J.; Marona, J.; Violante, A.; Oliveira, M. The Role of Lung Ultrasound in Systemic Sclerosis: A Systematic Review. J. Clin. Rheumatol. 2023, 29, e32–e39. [Google Scholar] [CrossRef] [PubMed]
- Bruni, C.; Mattolini, L.; Tofani, L.; Gargani, L.; Landini, N.; Roma, N.; Lepri, G.; Orlandi, M.; Guiducci, S.; Bellando-Randone, S.; et al. Lung Ultrasound B-Lines in the Evaluation of the Extent of Interstitial Lung Disease in Systemic Sclerosis. Diagnostics 2022, 12, 1696. [Google Scholar] [CrossRef]
- Gargani, L.; Bruni, C.; Romei, C.; Frumento, P.; Moreo, A.; Agoston, G.; Guiducci, S.; Bellando-Randone, S.; Lepri, G.; Belloli, L.; et al. Prognostic Value of Lung Ultrasound B-Lines in Systemic Sclerosis. Chest 2020, 158, 1515–1525. [Google Scholar] [CrossRef]
- Aghdashi, M.; Broofeh, B.; Mohammadi, A. Diagnostic performances of high resolution trans-thoracic lung ultrasonography in pulmonary alveoli-interstitial involvement of rheumatoid lung disease. Int. J. Clin. Exp. Med. 2013, 6, 562–566. [Google Scholar] [PubMed]
- Hassan, R.I.; Lubertino, L.I.; Barth, M.A.; Quaglia, M.F.; Montoya, S.F.; Kerzberg, E.; Binda, M.D.C. Lung Ultrasound as a Screening Method for Interstitial Lung Disease in Patients with Systemic Sclerosis. J. Clin. Rheumatol. 2019, 25, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Moazedi-Fuerst, F.C.; Zechner, P.M.; Tripolt, N.J.; Kielhauser, S.M.; Brickmann, K.; Scheidl, S.; Lutfi, A.; Graninger, W.G. Pulmonary echography in systemic sclerosis. Clin. Rheumatol. 2012, 31, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Gigante, A.; Fanelli, F.R.; Lucci, S.; Barilaro, G.; Quarta, S.; Barbano, B.; Giovannetti, A.; Amoroso, A.; Rosato, E. Lung ultrasound in systemic sclerosis: Correlation with high-resolution computed tomography, pulmonary function tests and clinical variables of disease. Intern. Emerg. Med. 2016, 11, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Oshnoei, S.; Ghasemi-Rad, M. Comparison of a new, modified lung ultrasonography technique with high-resolution CT in the diagnosis of the alveolo-interstitial syndrome of systemic scleroderma. Med. Ultrason. 2014, 16, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Sedie, A.D.; Doveri, M.; Frassi, F.; Gargani, L.; D’Errico, G.; Pepe, P.; Bazzichi, L.; Riente, L.; Caramella, D.; Bombardieri, S. Ultrasound lung comets in systemic sclerosis: A useful tool to detect lung interstitial fibrosis. Clin. Exp. Rheumatol. 2010, 28, S54. [Google Scholar]
- Barskova, T.; Gargani, L.; Guiducci, S.; Randone, S.B.; Bruni, C.; Carnesecchi, G.; Conforti, M.L.; Porta, F.; Pignone, A.; Caramella, D.; et al. Lung ultrasound for the screening of interstitial lung disease in very early systemic sclerosis. Ann. Rheum. Dis. 2013, 72, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Rivas, M.; Royo, C.; Simeón-Aznar, C.P.; Corbella, X.; Fonollosa, V. Mortality and survival in systemic sclerosis: Systematic review and meta-analysis. Semin. Arthritis Rheum. 2014, 44, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Mouthon, L.; Berezné, A.; Brauner, M.; Kambouchner, M.; Guillevin, L.; Valeyre, D. Interstitial lung disease in systemic sclerosis. Rev. Mal. Respir. 2007, 24, 1035–1046. [Google Scholar] [CrossRef]
- Steen, V.D.; Conte, C.; Owens, G.R.; Medsger, T.A. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994, 37, 1283–1289. [Google Scholar] [CrossRef]
- Hatabu, H.; Hunninghake, G.M.; Richeldi, L.; Brown, K.K.; Wells, A.U.; Remy-Jardin, M.; Verschakelen, J.; Nicholson, A.G.; Beasley, M.B.; Christiani, D.C.; et al. Interstitial lung abnormalities detected incidentally on CT: A Position Paper from the Fleischner Society. Lancet Respir. Med. 2020, 8, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, E.R.; A Sparks, J.; Hoffmann-Vold, A.-M.; Doyle, T.J.; Emery, P.; Dieudé, P. Preclinical or subclinical rheumatoid arthritis-associated interstitial lung disease: Misleading terms with potentially deleterious consequences. Lancet Rheumatol. 2023, 5, e116–e118. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Radiation Protection 118: Referral Guidelines for Imaging. Available online: https://op.europa.eu/en/publication-detail/-/publication/ac475fa0-09b6-4430-a3a3-6edef21df2e6 (accessed on 18 April 2010).
- Picano, E.; Matucci-Cerinic, M. Unnecessary radiation exposure from medical imaging in the rheumatology patient. Rheumatology 2011, 50, 1537–1539. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.Y.; Hill, C.L.; Pontifex, E.K.; Roberts-Thompsom, P.J. Breast cancer and systemic sclerosis: A clinical description of 21 patients in a population-based cohort study. Rheumatol. Int. 2008, 28, 895–899. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Pallisa-Nuñez, E.; Selva-O’Callaghan, A.; Castella-Fierro, E.; SimeOn-Aznar, C.P.; Fonollosa-Pla, V.; Vilardell-Tarres, M. Pleural irregularity, a new ultrasound sign for the study of interstitial lung disease in systemic sclerosis and antisynthetase syndrome. Clin. Exp. Rheumatol. 2015, 33, S136–S141. [Google Scholar]

| Variable | Mean ± SD or (%) |
|---|---|
| Sex | |
| Male | 13 (9.77) |
| Female | 120 (90.23) |
| Age (years) | 51.2 ± 10.2 |
| Disease duration (years) | 4.09 ± 2.3 |
| Type of SSc | |
| Limited | 57 (42.86) |
| Diffuse | 76 (57.14) |
| Current treatment | |
| None | 51 (38.35) |
| Methotrexate | 35 (26.32) |
| Mycophenolatemofetil | 40 (30.08) |
| Sildenafil | 0 (0.00) |
| Azathioprine | 2 (1.50) |
| Bosentan | 2 (1.50) |
| Cyclosporine | 1 (0.75) |
| Cyclophosphamide | 2 (1.50) |
| Raynaud’s phenomenon | |
| Yes | 115 (86.47) |
| No | 18 (13.53) |
| Rodnan skin score | 10.9 ± 7.9 |
| Pulmonary auscultation | |
| Positive | 0 |
| Negative | 133 (100) |
| Borg Dyspnea Scale | |
| 0 | 130 (97.74) |
| 0.5 | 3 (2.26) |
| Anti-topoisomerase (anti-Scl-70) | |
| Positive | 123 (92.48) |
| Negative | 10 (7.52) |
| Anti-centromere | |
| Positive | 74 (55.64) |
| Negative | 59 (44.36) |
| Chest X-ray | |
| Positive | 3 (2.26) |
| Negative | 130 (97.74) |
| Pulmonary US (semi-quantitative scale) | |
| 0 | 54 (40.6) |
| 1 | 51 (38.35) |
| 2 | 28 (21.05) |
| 3 | 0 (0) |
| HRTC (semi-quantitative scoring) | |
| 0 | 53 (39.85) |
| 1 | 58 (43.61) |
| 2 | 19 (14.29) |
| 3 | 3 (2.26) |
| FEV 1% predicted | 93 ± 24.5 |
| FVC% predicted | 96.9 ± 26.2 |
| Variable | OR (CI 95%) | p |
|---|---|---|
| Sex | 2.46 (0.64–9.41) | 0.178 |
| Age (years) | 1.03 (0.99–1.07) | 0.107 |
| Anti-topoisomerase (anti-Scl-70) | 2.34 (0.63–8.74) | 0.205 |
| Anti-centromere | 2.80 (1.37–5.72) | 0.005 |
| Disease duration (years) | 1.14 (0.98–1.34) | 0.087 |
| Raynaud’s phenomenon | 0.70 (0.24–1.99) | 0.501 |
| Rodnan skin score | 1.07 (1.02–1.13) | 0.003 |
| Borg Dyspnea scale (Borg score) | 77.68 (3.86–1562.08) | 0.086 |
| Chest X-ray | 1.37 (0.12–15.57) | 0.796 |
| RFT | 0.98 (0.96–1.01) | 0.144 |
| Sensitivity | Specificity | PPV | NPV | AUC | 95%IC | |
|---|---|---|---|---|---|---|
| HRCT | ||||||
| Chest X-ray | 2.5 | 98.1 | 66.6 | 40 | 0.503 | 0.477–0.528 |
| Pulmonary auscultation | 8.7 | 98.1 | 87.5 | 41.6 | 0.503 | 0.498–0.570 |
| RFT | 27.5 | 77.3 | 64.7 | 41.4 | 0.524 | 0.449–0.599 |
| Pulmonary US | 91.2 | 88.6 | 92.4 | 87 | 0.899 | 0.846–0.952 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez, M.; Bertolazzi, C.; Zozoaga-Velazquez, E.; Clavijo-Cornejo, D. The Value of Ultrasound for Detecting and Following Subclinical Interstitial Lung Disease in Systemic Sclerosis. Tomography 2024, 10, 521-532. https://doi.org/10.3390/tomography10040041
Gutierrez M, Bertolazzi C, Zozoaga-Velazquez E, Clavijo-Cornejo D. The Value of Ultrasound for Detecting and Following Subclinical Interstitial Lung Disease in Systemic Sclerosis. Tomography. 2024; 10(4):521-532. https://doi.org/10.3390/tomography10040041
Chicago/Turabian StyleGutierrez, Marwin, Chiara Bertolazzi, Edgar Zozoaga-Velazquez, and Denise Clavijo-Cornejo. 2024. "The Value of Ultrasound for Detecting and Following Subclinical Interstitial Lung Disease in Systemic Sclerosis" Tomography 10, no. 4: 521-532. https://doi.org/10.3390/tomography10040041
APA StyleGutierrez, M., Bertolazzi, C., Zozoaga-Velazquez, E., & Clavijo-Cornejo, D. (2024). The Value of Ultrasound for Detecting and Following Subclinical Interstitial Lung Disease in Systemic Sclerosis. Tomography, 10(4), 521-532. https://doi.org/10.3390/tomography10040041







