Tumor Morphology for Prediction of Poor Responses Early in Neoadjuvant Chemotherapy for Breast Cancer: A Multicenter Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Image Acquisition
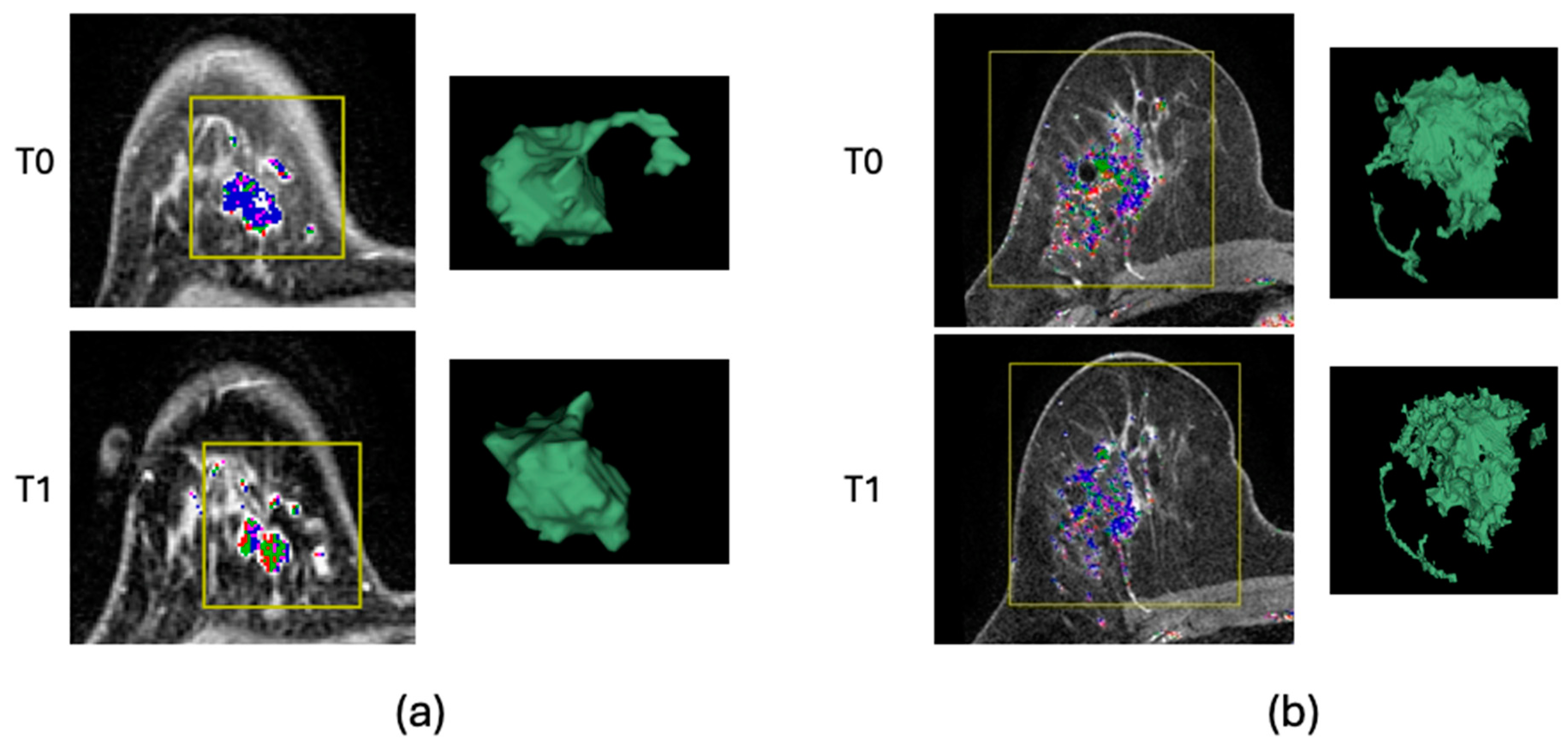
2.3. Image Analysis
2.4. Pathologic Outcome
2.5. Statistical Analysis
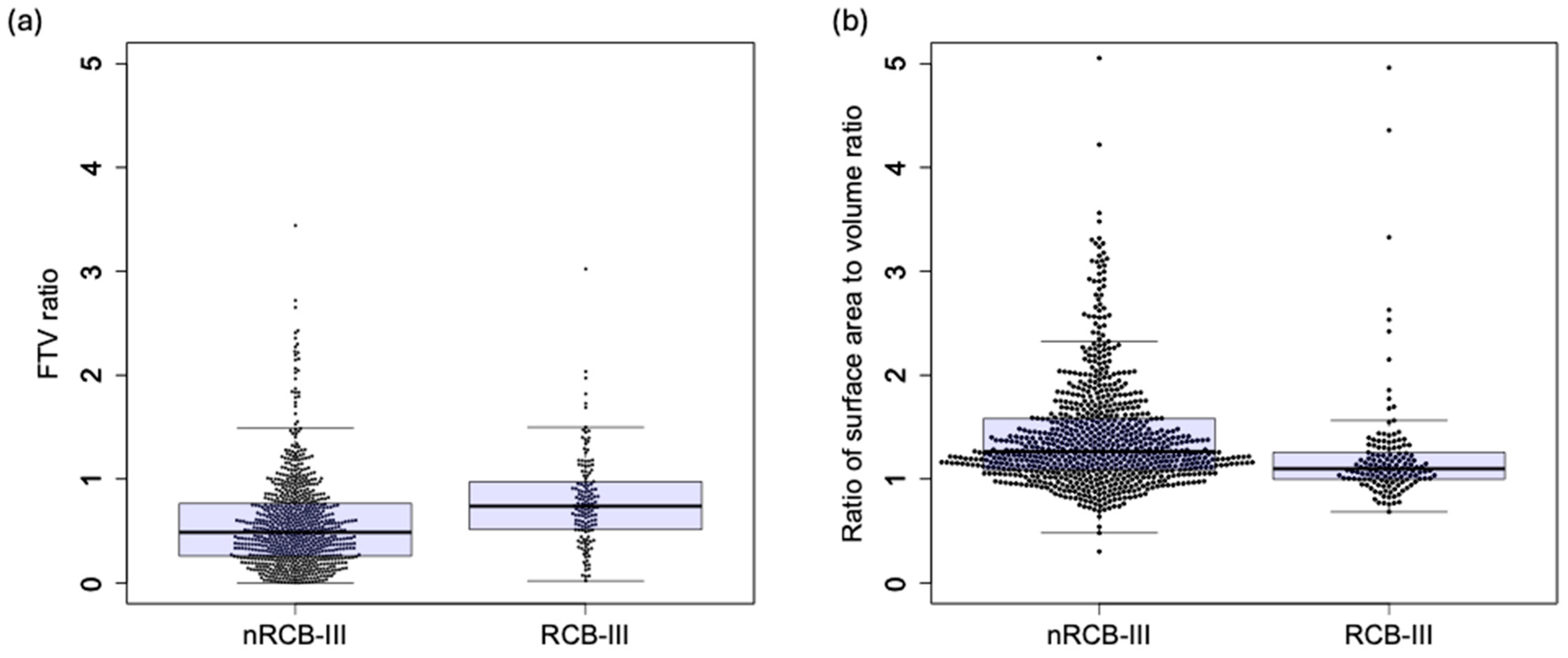
3. Results
3.1. Patient Characteristics
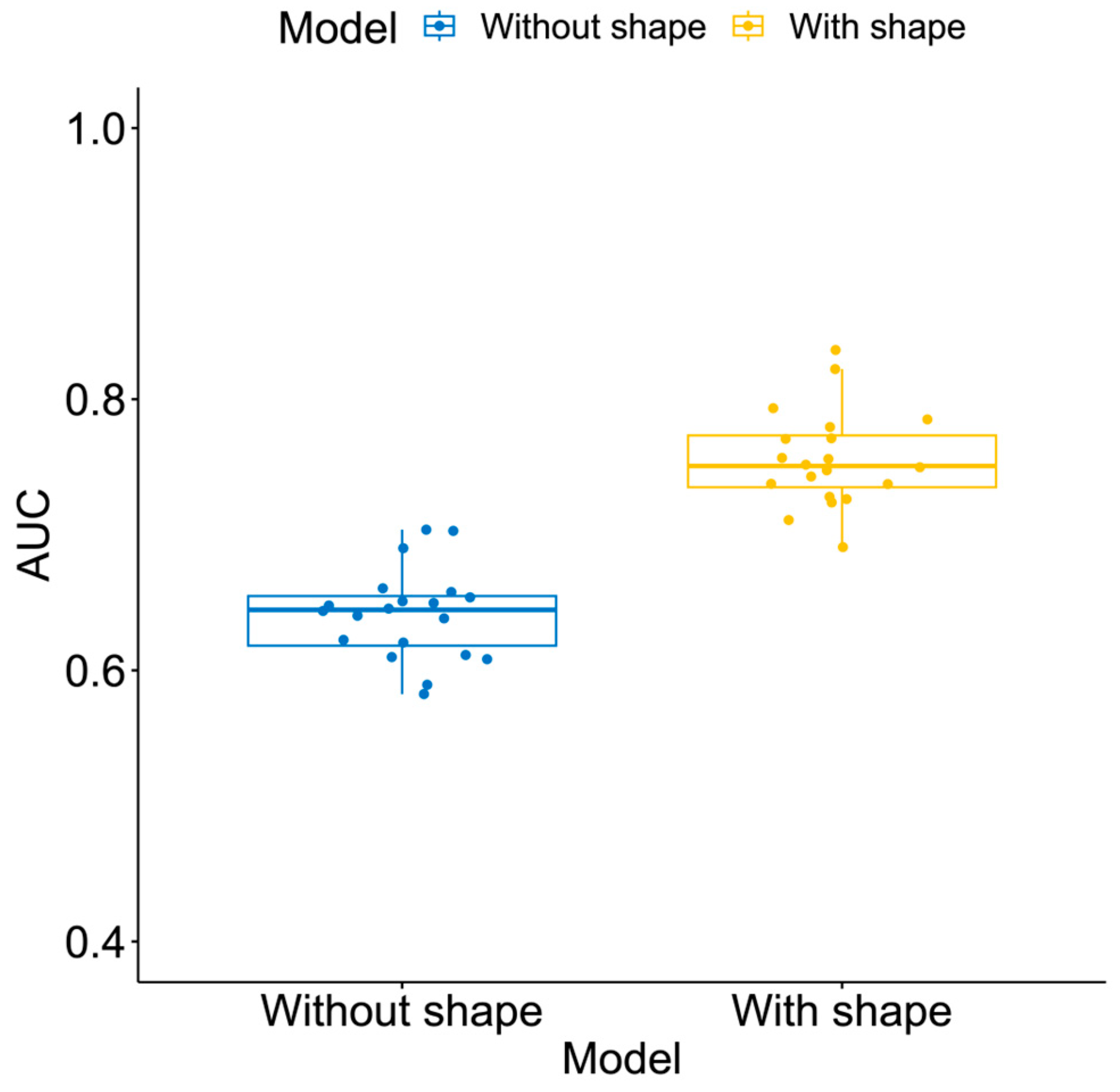
3.2. Additive Value of Shape Features
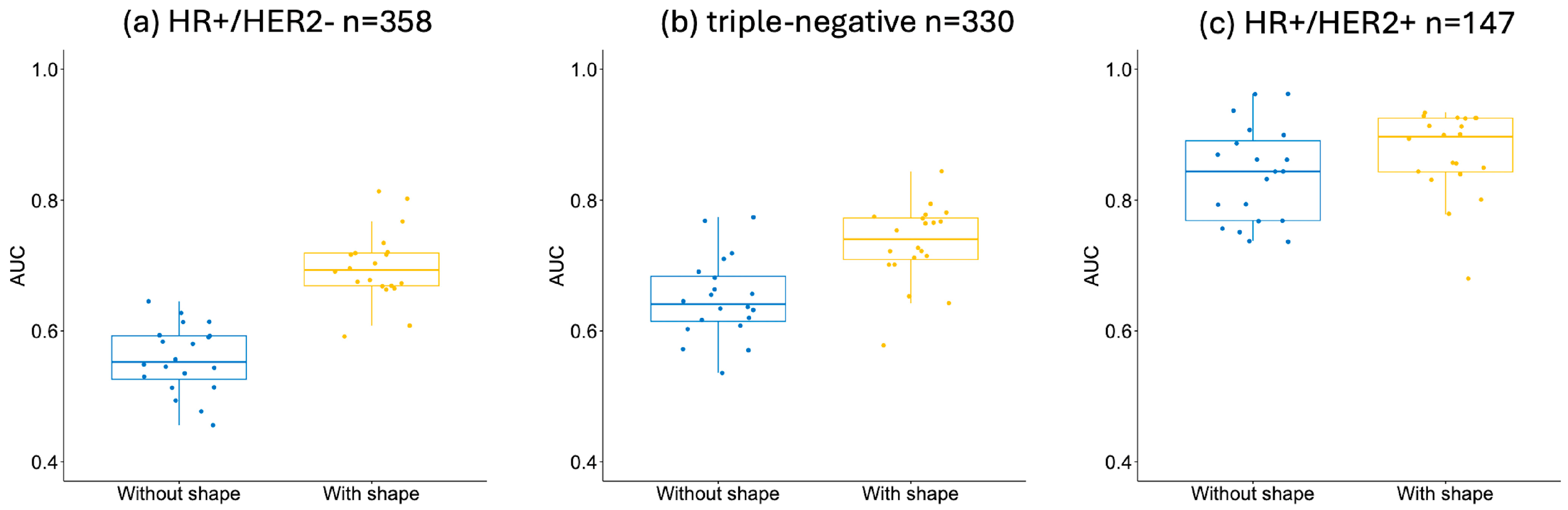
3.3. Additive Value of Shape Features by HR/HER2 Subtype
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-Analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.; DeMichele, A.M.; Yau, C.; Isaacs, C.; Symmans, W.F.; Albain, K.S.; Chen, Y.-Y.; Krings, G.; Wei, S.; Harada, S.; et al. Association of Event-Free and Distant Recurrence-Free Survival With Individual-Level Pathologic Complete Response in Neoadjuvant Treatment of Stages 2 and 3 Breast Cancer: Three-Year Follow-up Analysis for the I-SPY2 Adaptively Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of Residual Breast Cancer Burden to Predict Survival after Neoadjuvant Chemotherapy. J. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, E.; Bossuyt, V.; Viale, G.; Cameron, D.; Badve, S.; Denkert, C.; MacGrogan, G.; Penault-Llorca, F.; Boughey, J.; Curigliano, G.; et al. Standardization of Pathologic Evaluation and Reporting of Postneoadjuvant Specimens in Clinical Trials of Breast Cancer: Recommendations from an International Working Group. Mod. Pathol. 2015, 28, 1185–1201. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, V.; Provenzano, E.; Symmans, W.F.; Boughey, J.C.; Coles, C.; Curigliano, G.; Dixon, J.M.; Esserman, L.J.; Fastner, G.; Kuehn, T.; et al. Recommendations for Standardized Pathological Characterization of Residual Disease for Neoadjuvant Clinical Trials of Breast Cancer by the BIG-NABCG Collaboration. Ann. Oncol. 2015, 26, 1280–1291. [Google Scholar] [CrossRef]
- Symmans, W.F.; Yau, C.; Chen, Y.-Y.; Balassanian, R.; Klein, M.E.; Pusztai, L.; Nanda, R.; Parker, B.A.; Datnow, B.; Krings, G.; et al. Assessment of Residual Cancer Burden and Event-Free Survival in Neoadjuvant Treatment for High-Risk Breast Cancer: An Analysis of Data From the I-SPY2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1654–1663. [Google Scholar] [CrossRef]
- Symmans, W.F.; Yau, C.; Chen, Y.-Y.; Datnow, B.; Wei, S.; Feldman, M.D.; Ritter, J.; Duan, X.; Chen, B.; Tickman, R.; et al. Residual Cancer Burden (RCB) as Prognostic in the I-SPY 2 TRIAL. J. Clin. Oncol. 2018, 36 (Suppl. S15), 520. [Google Scholar] [CrossRef]
- Symmans, W.F.; Wei, C.; Gould, R.; Yu, X.; Zhang, Y.; Liu, M.; Walls, A.; Bousamra, A.; Ramineni, M.; Sinn, B.; et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J. Clin. Oncol. 2017, 35, 1049–1060. [Google Scholar] [CrossRef]
- Hylton, N.M.; Blume, J.D.; Bernreuter, W.K.; Pisano, E.D.; Rosen, M.A.; Morris, E.A.; Weatherall, P.T.; Lehman, C.D.; Newstead, G.M.; Polin, S.; et al. Locally Advanced Breast Cancer: MR Imaging for Prediction of Response to Neoadjuvant Chemotherapy—Results from ACRIN 6657/I-SPY TRIAL. Radiology 2012, 263, 663–672. [Google Scholar] [CrossRef]
- Reig, B.; Lewin, A.A.; Du, L.; Heacock, L.; Toth, H.K.; Heller, S.L.; Gao, Y.; Moy, L. Breast MRI for Evaluation of Response to Neoadjuvant Therapy. Radiographics 2021, 41, 665–679. [Google Scholar] [CrossRef]
- Öztürk, V.S.; Polat, Y.D.; Soyder, A.; Tanyeri, A.; Karaman, C.Z.; Taşkın, F. The Relationship Between MRI Findings and Molecular Subtypes in Women With Breast Cancer. Curr. Probl. Diagn. Radiol. 2020, 49, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Lunkiewicz, M.; Forte, S.; Freiwald, B.; Singer, G.; Leo, C.; Kubik-Huch, R.A. Interobserver Variability and Likelihood of Malignancy for Fifth Edition BI-RADS MRI Descriptors in Non-Mass Breast Lesions. Eur. Radiol. 2020, 30, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Newitt, D.C.; La Yun, B.; Jones, E.F.; Arasu, V.; Wilmes, L.; Gibbs, J.; Nguyen, A.; Onishi, N.; Kornak, J.; et al. Tumor Sphericity Predicts Response in Neoadjuvant Chemotherapy for Invasive Breast Cancer. Tomography 2020, 6, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Newitt, D.C.; Gibbs, J.; Wilmes, L.J.; Jones, E.F.; Arasu, V.A.; Strand, F.; Onishi, N.; Nguyen, A.A.T.; Kornak, J.; et al. I-SPY 2 Breast Dynamic Contrast Enhanced MRI Trial (ISPY2) (Version 1). Available online: https://doi.org/10.7937/TCIA.D8Z0-9T85 (accessed on 1 October 2023).
- Newitt, D.C.; Aliu, S.O.; Witcomb, N.; Sela, G.; Kornak, J.; Esserman, L.; Hylton, N.M. Real-Time Measurement of Functional Tumor Volume by MRI to Assess Treatment Response in Breast Cancer Neoadjuvant Clinical Trials: Validation of the Aegis SER Software Platform. Transl. Oncol. 2014, 7, 94–100. [Google Scholar] [CrossRef]
- Whitaker, R.T. Reducing Aliasing Artifacts in Iso-Surfaces of Binary Volumes. In Proceedings of the 2000 IEEE Symposium on Volume Visualization (VV 2000), Salt Lake City, UT, USA, 9–10 October 2000; pp. 23–32. [Google Scholar] [CrossRef]
- Yaniv, Z.; Lowekamp, B.C.; Johnson, H.J.; Beare, R. SimpleITK Image-Analysis Notebooks: A Collaborative Environment for Education and Reproducible Research. J. Digit. Imaging 2018, 31, 290–303. [Google Scholar] [CrossRef]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Stekhoven, D.J.; Bühlmann, P. MissForest—Non-Parametric Missing Value Imputation for Mixed-Type Data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef]
- MDAnderson. Residual Cancer Burden Calculator. Available online: https://www.mdanderson.org/breastcancer_RCB (accessed on 23 April 2024).
- Rydzewski, N.R.; Peterson, E.; Lang, J.M.; Yu, M.; Laura Chang, S.; Sjöström, M.; Bakhtiar, H.; Song, G.; Helzer, K.T.; Bootsma, M.L.; et al. Predicting Cancer Drug TARGETS -TreAtment Response Generalized Elastic-NeT Signatures. NPJ Genom. Med. 2021, 6, 76. [Google Scholar] [CrossRef]
- Brown, T.J.; Gimotty, P.A.; Mamtani, R.; Karasic, T.B.; Yang, Y.-X. Classification and Regression Trees to Predict for Survival for Patients With Hepatocellular Carcinoma Treated With Atezolizumab and Bevacizumab. JCO Clin. Cancer Inform. 2024, 8, e2300220. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Zhang, H.; Duan, T.; Zhang, C.; Zhao, T. Deep Learning Radiomics Based on Two-Dimensional Ultrasound for Predicting the Efficacy of Neoadjuvant Chemotherapy in Breast Cancer. Ultrason. Imaging 2024, 46, 357–366. [Google Scholar] [CrossRef]
- Rodríguez-Tomàs, E.; Arenas, M.; Baiges-Gaya, G.; Acosta, J.; Araguas, P.; Malave, B.; Castañé, H.; Jiménez-Franco, A.; Benavides-Villarreal, R.; Sabater, S.; et al. Gradient Boosting Machine Identified Predictive Variables for Breast Cancer Patients Pre- and Post-Radiotherapy: Preliminary Results of an 8-Year Follow-Up Study. Antioxidants 2022, 11, 2394. [Google Scholar] [CrossRef] [PubMed]
- Gennatas, E.D. Towards Precision Psychiatry: Gray Matter Development and Cognition in Adolescence. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 2017. [Google Scholar]
- Onishi, N.; Gibbs, J.E.; Li, W.; Newitt, D.C.; Price, E.R.; LeStage, B.; Symmans, W.F.; DeMichele, A.M.; Yau, C.; The I-SPY 2 TRIAL Imaging Working Group; et al. Functional Tumor Volume at 3 and 6-Week MRI as an Indicator of Patients with Inferior Outcome after Neoadjuvant Chemotherapy. Cancer Res. 2022, 82 (Suppl. S4), P3-03-01. [Google Scholar] [CrossRef]
- Li, W.; Newitt, D.C.; Gibbs, J.; Wilmes, L.J.; Jones, E.F.; Onishi, N.; Joe, B.N.; Price, E.; Kornak, J.; Yau, C.; et al. Subtype-Specific MRI Models to Guide Selection of Candidates for de-Escalation of Neoadjuvant Therapy. Cancer Res. 2021, 81 (Suppl. S4), PD6-05. [Google Scholar] [CrossRef]
- Li, W.; Newitt, D.C.; Gibbs, J.; Wilmes, L.J.; Jones, E.F.; Arasu, V.A.; Strand, F.; Onishi, N.; Nguyen, A.A.T.; Kornak, J.; et al. Predicting Breast Cancer Response to Neoadjuvant Treatment Using Multi-Feature MRI: Results from the I-SPY 2 TRIAL. NPJ Breast Cancer 2020, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Musall, B.C.; Abdelhafez, A.H.; Adrada, B.E.; Candelaria, R.P.; Mohamed, R.M.M.; Boge, M.; Le-Petross, H.; Arribas, E.; Lane, D.L.; Spak, D.A.; et al. Functional Tumor Volume by Fast Dynamic Contrast-Enhanced MRI for Predicting Neoadjuvant Systemic Therapy Response in Triple-Negative Breast Cancer. J. Magn. Reson. Imaging 2021, 54, 251–260. [Google Scholar] [CrossRef]
- Onishi, N.; Bareng, T.J.; Gibbs, J.; Li, W.; Price, E.R.; Joe, B.N.; Kornak, J.; Esserman, L.J.; Newitt, D.C.; Hylton, N.M.; et al. Effect of Longitudinal Variation in Tumor Volume Estimation for MRI-Guided Personalization of Breast Cancer Neoadjuvant Treatment. Radiol. Imaging Cancer 2023, 5, e220126. [Google Scholar] [CrossRef]
- Granzier, R.W.Y.; Ibrahim, A.; Primakov, S.P.; Samiei, S.; van Nijnatten, T.J.A.; de Boer, M.; Heuts, E.M.; Hulsmans, F.-J.; Chatterjee, A.; Lambin, P.; et al. MRI-Based Radiomics Analysis for the Pretreatment Prediction of Pathologic Complete Tumor Response to Neoadjuvant Systemic Therapy in Breast Cancer Patients: A Multicenter Study. Cancers 2021, 13, 2447. [Google Scholar] [CrossRef]
- Huynh, E.; Coroller, T.P.; Narayan, V.; Agrawal, V.; Romano, J.; Franco, I.; Parmar, C.; Hou, Y.; Mak, R.H.; Aerts, H.J.W.L. Associations of Radiomic Data Extracted from Static and Respiratory-Gated CT Scans with Disease Recurrence in Lung Cancer Patients Treated with SBRT. PLoS ONE 2017, 12, e0169172. [Google Scholar] [CrossRef][Green Version]
- Rakshit, S.; Orooji, M.; Beig, N.; Alilou, M.; Pennell, N.A.; Stevenson, J.; Shapiro, M.A.; Madabhushi, A.; Velcheti, V. Evaluation of Radiomic Features on Baseline CT Scan to Predict Clinical Benefit for Pemetrexed Based Chemotherapy in Metastatic Lung Adenocarcinoma. J. Clin. Oncol. 2016, 34 (Suppl. S15), 11582. [Google Scholar] [CrossRef]
- Banerjee, I.; Malladi, S.; Lee, D.; Depeursinge, A.; Telli, M.; Lipson, J.; Golden, D.; Rubin, D.L. Assessing Treatment Response in Triple-Negative Breast Cancer from Quantitative Image Analysis in Perfusion Magnetic Resonance Imaging. J. Med. Imaging 2018, 5, 11008. [Google Scholar] [CrossRef]
- Park, H.; Kim, K.A.; Jung, J.-H.; Rhie, J.; Choi, S.Y. MRI Features and Texture Analysis for the Early Prediction of Therapeutic Response to Neoadjuvant Chemoradiotherapy and Tumor Recurrence of Locally Advanced Rectal Cancer. Eur. Radiol. 2020, 30, 4201–4211. [Google Scholar] [CrossRef] [PubMed]
- Yau, C.; Osdoit, M.; van der Noordaa, M.; Shad, S.; Wei, J.; de Croze, D.; Hamy, A.-S.; Laé, M.; Reyal, F.; Sonke, G.S.; et al. Residual Cancer Burden after Neoadjuvant Chemotherapy and Long-Term Survival Outcomes in Breast Cancer: A Multicentre Pooled Analysis of 5161 Patients. Lancet Oncol. 2022, 23, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Fukada, I.; Araki, K.; Kobayashi, K.; Shibayama, T.; Takahashi, S.; Gomi, N.; Kokubu, Y.; Oikado, K.; Horii, R.; Akiyama, F.; et al. Pattern of Tumor Shrinkage during Neoadjuvant Chemotherapy Is Associated with Prognosis in Low-Grade Luminal Early Breast Cancer. Radiology 2017, 286, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Du, S.; Gao, S.; Zhao, R.; Liu, S.; Jiang, W.; Peng, C.; Chai, R.; Zhang, L. MRI-Based Tumor Shrinkage Patterns after Early Neoadjuvant Therapy in Breast Cancer: Correlation with Molecular Subtypes and Pathological Response after Therapy. Breast Cancer Res. 2024, 26, 26. [Google Scholar] [CrossRef]
- Onishi, N.; Li, W.; Gibbs, J.; Wilmes, L.J.; Nguyen, A.; Jones, E.F.; Arasu, V.; Kornak, J.; Joe, B.N.; Esserman, L.J.; et al. Impact of MRI Protocol Adherence on Prediction of Pathological Complete Response in the I-SPY 2 Neoadjuvant Breast Cancer Trial. Tomography 2020, 6, 77–85. [Google Scholar] [CrossRef]

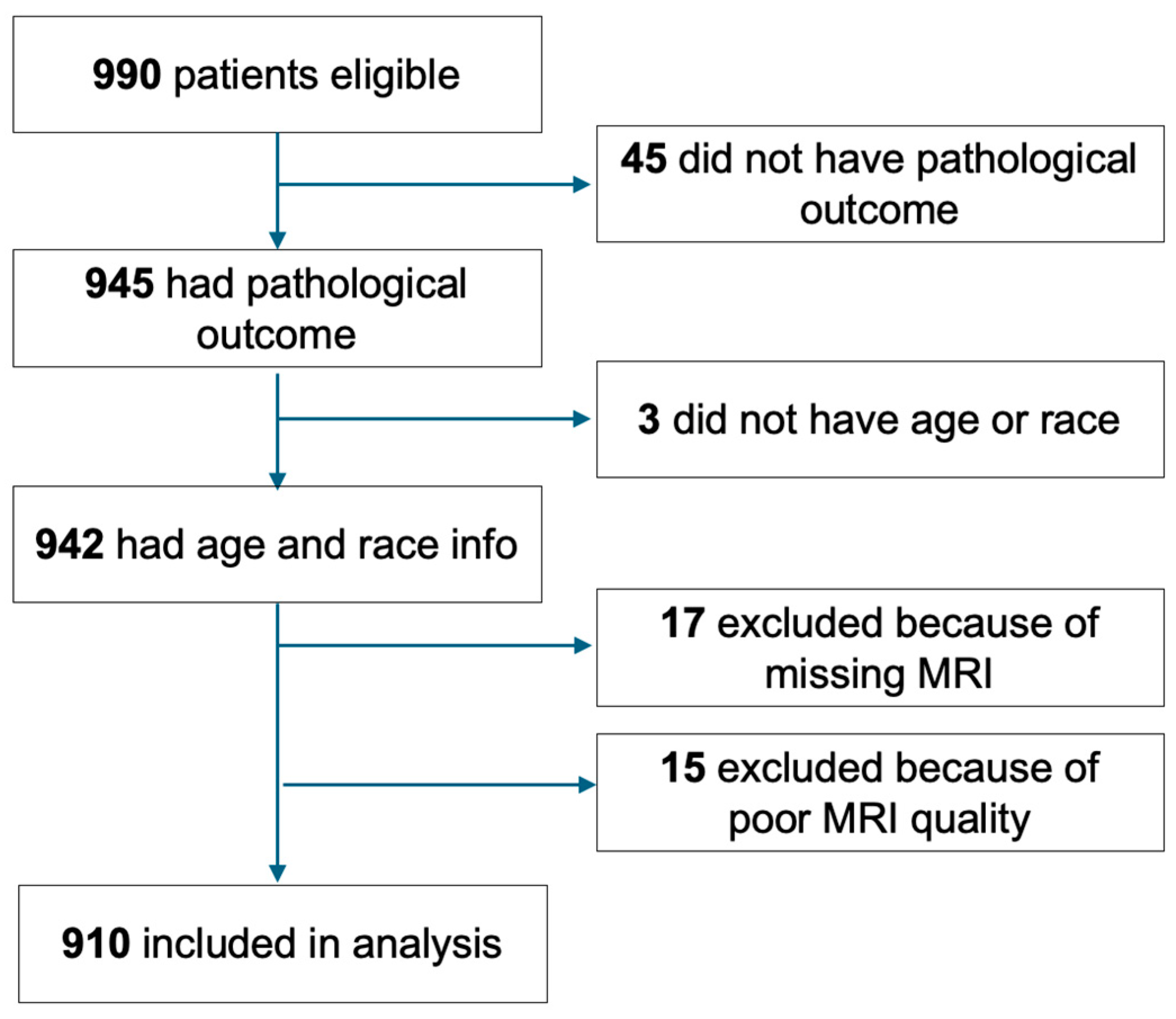

| Characteristic | Eligible Cohort n = 990 | Analysis Cohort n = 910 | Excluded Cohort n = 80 | p-Value (Analysis versus Excluded) |
|---|---|---|---|---|
| Age (mean ± standard deviation) | 48.8 ± 10.5 | 48.8 ± 10.5 | 47.8 ± 11.0 | 0.41 |
| HR/HER2 subtype (n, %) | 0.024 | |||
| HR+/HER2- | 382 (39%) | 358 (39%) | 24 (30%) | |
| HR+/HER2+ | 156 (16%) | 147 (16%) | 9 (11%) | |
| HR-/HER2+ | 89 (9%) | 75 (8%) | 14 (18%) | |
| HR-/HER2- (triple-negative) | 363 (37%) | 330 (36%) | 33 (41%) | |
| Menopausal status (n, %) | 0.63 | |||
| Premenopausal | 481 (49%) | 438 (48%) | 43 (54%) | |
| Perimenopausal | 35 (4%) | 33 (4%) | 2 (3%) | |
| Postmenopausal | 301 (30%) | 282 (31%) | 19 (24%) | |
| Not applicable | 135 (14%) | 123 (14%) | 12 (15%) | |
| Unknown | 38 (4%) | 34 (4%) | 4 (5%) | |
| Race (n, %) | 0.018 | |||
| White | 783 (79%) | 730 (80%) | 53 (66%) | |
| Black or African American | 120 (12%) | 104 (11%) | 16 (20%) | |
| Asian | 68 (7%) | 61 (7%) | 7 (9%) | |
| Mixed | 7 (0.7%) | 7 (0.8%) | 0 (0%) | |
| Native Hawaiian or Pacific Islander | 5 (0.5%) | 5 (0.5%) | 0 (0%) | |
| American Indian or Alaska Native | 4 (0.4%) | 3 (0.3%) | 1 (1%) | |
| Unknown | 1 (0.1%) | 0 (0%) | 1 (1%) | |
| Residual cancer burden (n, %) | <0.001 | |||
| RCB-0 (pCR) | 325 (33%) | 315 (35%) | 10 (13%) | |
| RCB-I | 135 (14%) | 127 (14%) | 8 (10%) | |
| RCB-II | 339 (34%) | 327 (36%) | 12 (15%) | |
| RCB-III | 146 (15%) | 141 (15%) | 5 (6%) | |
| Unknown | 45 (5%) | 0 (0%) | 45 (56%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Le, N.N.; Nadkarni, R.; Onishi, N.; Wilmes, L.J.; Gibbs, J.E.; Price, E.R.; Joe, B.N.; Mukhtar, R.A.; Gennatas, E.D.; et al. Tumor Morphology for Prediction of Poor Responses Early in Neoadjuvant Chemotherapy for Breast Cancer: A Multicenter Retrospective Study. Tomography 2024, 10, 1832-1845. https://doi.org/10.3390/tomography10110134
Li W, Le NN, Nadkarni R, Onishi N, Wilmes LJ, Gibbs JE, Price ER, Joe BN, Mukhtar RA, Gennatas ED, et al. Tumor Morphology for Prediction of Poor Responses Early in Neoadjuvant Chemotherapy for Breast Cancer: A Multicenter Retrospective Study. Tomography. 2024; 10(11):1832-1845. https://doi.org/10.3390/tomography10110134
Chicago/Turabian StyleLi, Wen, Nu N. Le, Rohan Nadkarni, Natsuko Onishi, Lisa J. Wilmes, Jessica E. Gibbs, Elissa R. Price, Bonnie N. Joe, Rita A. Mukhtar, Efstathios D. Gennatas, and et al. 2024. "Tumor Morphology for Prediction of Poor Responses Early in Neoadjuvant Chemotherapy for Breast Cancer: A Multicenter Retrospective Study" Tomography 10, no. 11: 1832-1845. https://doi.org/10.3390/tomography10110134
APA StyleLi, W., Le, N. N., Nadkarni, R., Onishi, N., Wilmes, L. J., Gibbs, J. E., Price, E. R., Joe, B. N., Mukhtar, R. A., Gennatas, E. D., Kornak, J., Magbanua, M. J. M., van’t Veer, L. J., LeStage, B., Esserman, L. J., & Hylton, N. M. (2024). Tumor Morphology for Prediction of Poor Responses Early in Neoadjuvant Chemotherapy for Breast Cancer: A Multicenter Retrospective Study. Tomography, 10(11), 1832-1845. https://doi.org/10.3390/tomography10110134






