Dynamic Responses of Human Skin and Fascia to an Innovative Stimulation Device—Shear Wave Stimulation
Abstract
1. Introduction
2. Materials and Methods
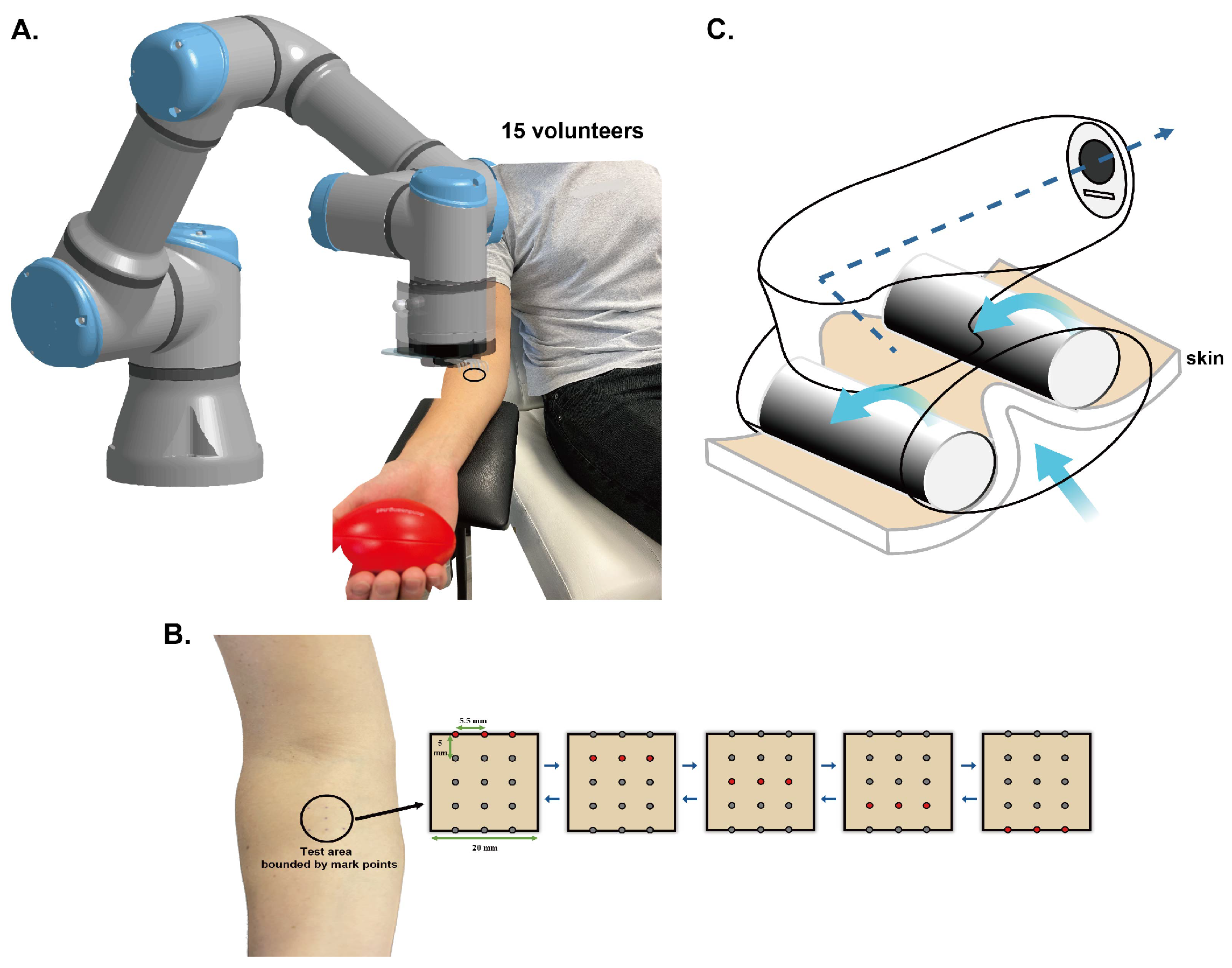
2.1. Participants
2.2. Devices and Protocols
2.2.1. Mechanical Stimulation Devices
- Shear wave stimulation (SWS)
- Traditional massage device (Wellbox)
2.2.2. Characterizations Methods
- The shear modulus of the skin: UNDERSKIN
- The deformation of different layers of skin and fascia: Ultrasound and deformation analysis
- Blood flow: PeriFlux System 5000
2.2.3. Protocols
- Protocol for SWS
- Protocol for Wellbox
2.3. Statistical Analysis
3. Results
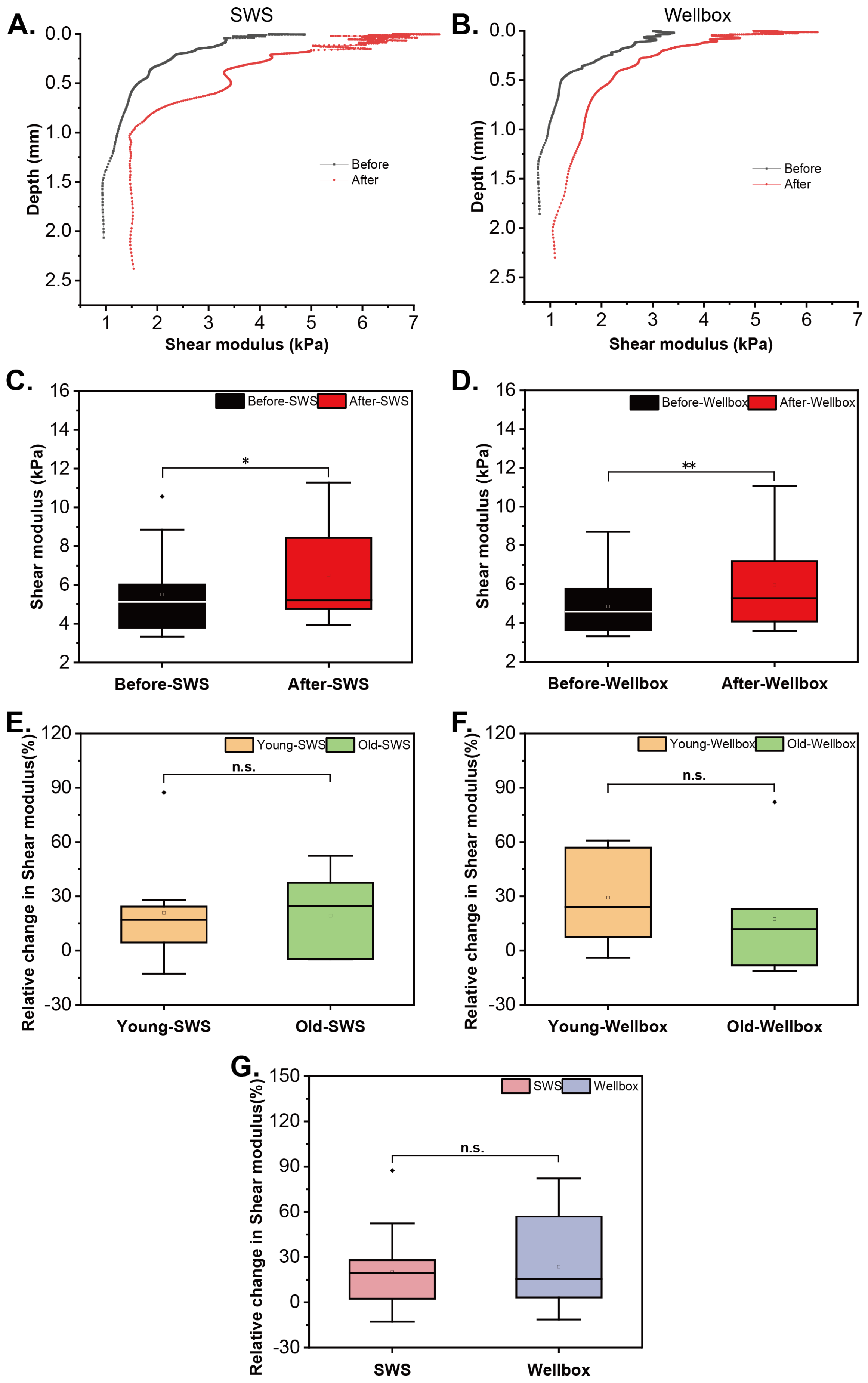
3.1. Response in Skin’s Mechanical Behavior
3.2. Response in Deformation of Skin and Fascia
3.3. Respose in Blood Flow
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and Physiology of the Skin. J. Dermatol. Nurses’ Assoc. 2011, 3, 203. [Google Scholar] [CrossRef]
- Gilbert, S.F. The Epidermis and the Origin of Cutaneous Structures. In Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, UK, 2000. [Google Scholar]
- Calleja-Agius, J.; Brincat, M.; Borg, M. Skin connective tissue and ageing. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, C.E.H.; Duca, L. Elastic fibers: Formation, function, and fate during aging and disease. FEBS J. 2022, 289, 3704–3730. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C. Functional Atlas of the Human Fascial System; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef]
- Stecco, C.; Tiengo, C.; Stecco, A.; Porzionato, A.; Macchi, V.; Stern, R.; De Caro, R. Fascia redefined: Anatomical features and technical relevance in fascial flap surgery. Surg. Radiol. Anat. 2013, 35, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Südel, K.M.; Venzke, K.; Mielke, H.; Breitenbach, U.; Mundt, C.; Jaspers, S.; Koop, U.; Sauermann, K.; Knussman-Hartig, E.; Moll, I.; et al. Novel aspects of intrinsic and extrinsic aging of human skin: Beneficial effects of soy extract. Photochem. Photobiol. 2005, 81, 581–587. [Google Scholar] [CrossRef]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking older: Fibroblast collapse and therapeutic implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Fannin, J.; Rice, K.M.; Wang, B.; Blough, E.R. Effect of aging on cellular mechanotransduction. Ageing Res. Rev. 2011, 10, 1–15. [Google Scholar] [CrossRef]
- Stecco, C.; Schleip, R. A fascia and the fascial system. J. Bodyw. Mov. Ther. 2016, 20, 139–140. [Google Scholar] [CrossRef]
- Adstrum, S.; Hedley, G.; Schleip, R.; Stecco, C.; Yucesoy, C.A. Defining the fascial system. J. Bodyw. Mov. Ther. 2017, 21, 173–177. [Google Scholar] [CrossRef]
- Bordoni, B.; Escher, A.R.; Tobbi, F.; Pianese, L.; Ciardo, A.; Yamahata, J.; Hernandez, S.; Sanchez, O. Fascial Nomenclature: Update 2022. Cureus 2022, 14, e25904. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Akaishi, S.; Longaker, M.T.; Gurtner, G.C. Pushing Back: Wound Mechanotransduction in Repair and Regeneration. J. Investig. Dermatol. 2011, 131, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.L.; Olmedo, M.L. Mechanotransduction: Relevance to Physical Therapist Practice—Understanding Our Ability to Affect Genetic Expression Through Mechanical Forces. Phys. Ther. 2016, 96, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Iskratsch, T.; Wolfenson, H.; Sheetz, M.P. Appreciating force and shape—The rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 2014, 15, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.D.; Ogborn, D.I.; Cupido, C.; Melov, S.; Hubbard, A.; Bourgeois, J.M.; Tarnopolsky, M.A. Massage Therapy Attenuates Inflammatory Signaling After Exercise-Induced Muscle Damage. Sci. Transl. Med. 2012, 4, 119ra13. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.O.; Cooke, P.H.; Goodship, A.E. The effect of exercise and anabolic steroids on the mechanical properties and crimp morphology of the rat tendon. Am. J. Sports Med. 1988, 16, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.A.H.; Józsa, L.; Kannus, P.; Järvinen, T.L.N.; Järvinen, M. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. An immunohistochemical, polarization and scanning electron microscopic study. J. Muscle Res. Cell Motil. 2002, 23, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M.; Langberg, H.; Heinemeier, K.; Bayer, M.L.; Hansen, M.; Holm, L.; Doessing, S.; Kongsgaard, M.; Krogsgaard, M.R.; Magnusson, S.P. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand. J. Med. Sci. Sports 2009, 19, 500–510. [Google Scholar] [CrossRef]
- Qiao, N.; Dumas, V.; Bergheau, A.; Ouillon, L.; Laroche, N.; Privet-Thieulin, C.; Perrot, J.L.; Zahouani, H. Contactless mechanical stimulation of the skin using shear waves. J. Mech. Behav. Biomed. Mater. 2024, 156, 106597. [Google Scholar] [CrossRef]
- Chen, T.L.W.; Agresta, C.E.; Lipps, D.B.; Provenzano, S.G.; Hafer, J.F.; Wong, D.W.C.; Zhang, M.; Zernicke, R.F. Ultrasound elastographic assessment of plantar fascia in runners using rearfoot strike and forefoot strike. J. Biomech. 2019, 89, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Fox, J.R.; Koptiuch, C.; Badger, G.J.; Greenan-Naumann, A.C.; Bouffard, N.A.; Konofagou, E.E.; Lee, W.N.; Triano, J.J.; Henry, S.M. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet. Disord. 2011, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Luomala, T.; Pihlman, M.; Heiskanen, J.; Stecco, C. Case study: Could ultrasound and elastography visualized densified areas inside the deep fascia? J. Bodyw. Mov. Ther. 2014, 18, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Burns, S.; Jan, Y.K. Skin blood flow dynamics and its role in pressure ulcers. J. Tissue Viability 2013, 22, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Monteiro Rodrigues, L.; Rocha, C.; Ferreira, H.T.; Silva, H.N. Lower limb massage in humans increases local perfusion and impacts systemic hemodynamics. J. Appl. Physiol. 2020, 128, 1217–1226. [Google Scholar] [CrossRef]
- Ayadh, M.; Abellan, M.A.; Helfenstein-Didier, C.; Bigouret, A.; Zahouani, H. Methods for characterizing the anisotropic behavior of the human skin’s relief and its mechanical properties In Vivo Linked to Age Effects. Surf. Topogr. Metrol. Prop. 2020, 8, 014002. [Google Scholar] [CrossRef]
- Bergheau, A.; Perrot, J.L.; Vargiolu, R.; Zahouani, H. Viscoelasticity assessment of tumoral skin with the use of a novel contact-free palpation methodology based upon surface waves. Sci. Rep. 2022, 12, 18716. [Google Scholar] [CrossRef] [PubMed]
- Aüllo-Rasser, G.; Dousset, E.; Roffino, S.; Zahouani, H.; Lecurieux-Clerville, R.; Argenson, J.N.; Chabrand, P. Early-stage knee OA induced by MIA and MMT compared in the murine model via histological and topographical approaches. Sci. Rep. 2020, 10, 15430. [Google Scholar] [CrossRef] [PubMed]
- Worret, W.I.; Jessberger, B. Effectiveness of LPG® treatment in morphea. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 527–530. [Google Scholar] [CrossRef]
- Moortgat, P.; Meirte, J.; Van Daele, U.; Anthonissen, M.; Vanhullebusch, T.; Maertens, K. Vacuum Massage in the Treatment of Scars. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 475–483. [Google Scholar] [CrossRef]
- Schulze, C.; Wetzel, F.; Kueper, T.; Malsen, A.; Muhr, G.; Jaspers, S.; Blatt, T.; Wittern, K.P.; Wenck, H.; Käs, J.A. Stiffening of Human Skin Fibroblasts with Age. Biophys. J. 2010, 99, 2434–2442. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Lelonkiewicz, M.; Wieczorowski, M. Age-dependent biomechanical properties of the skin. Adv. Dermatol. Allergol. Dermatol. I Alergol. 2013, 30, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.J.; Jones, J.D.; Woessner, A.E.; Quinn, K.P. Skin Structure–Function Relationships and the Wound Healing Response to Intrinsic Aging. Adv. Wound Care 2020, 9, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Cafarelli, E.; Flint, F. The Role of Massage in Preparation For and Recovery From Exercise: An Overview. Sports Med. 1992, 14, 1–9. [Google Scholar] [CrossRef]
- Watson, J.; Fodor, P.B.; Cutcliffe, B.; Sayah, D.; Shaw, W. Physiological Effects of Endermologie®: A Preliminary Report. Aesthetic Surg. J. 1999, 19, 27–33. [Google Scholar] [CrossRef]
- Plakornkul, V.; Vannabhum, M.; Viravud, Y.; Roongruangchai, J.; Mutirangura, P.; Akarasereenont, P.; Laohapand, T. The effects of the court-type Thai traditional massage on anatomical relations, blood flow, and skin temperature of the neck, shoulder, and arm. BMC Complement. Altern. Med. 2016, 16, 363. [Google Scholar] [CrossRef] [PubMed]
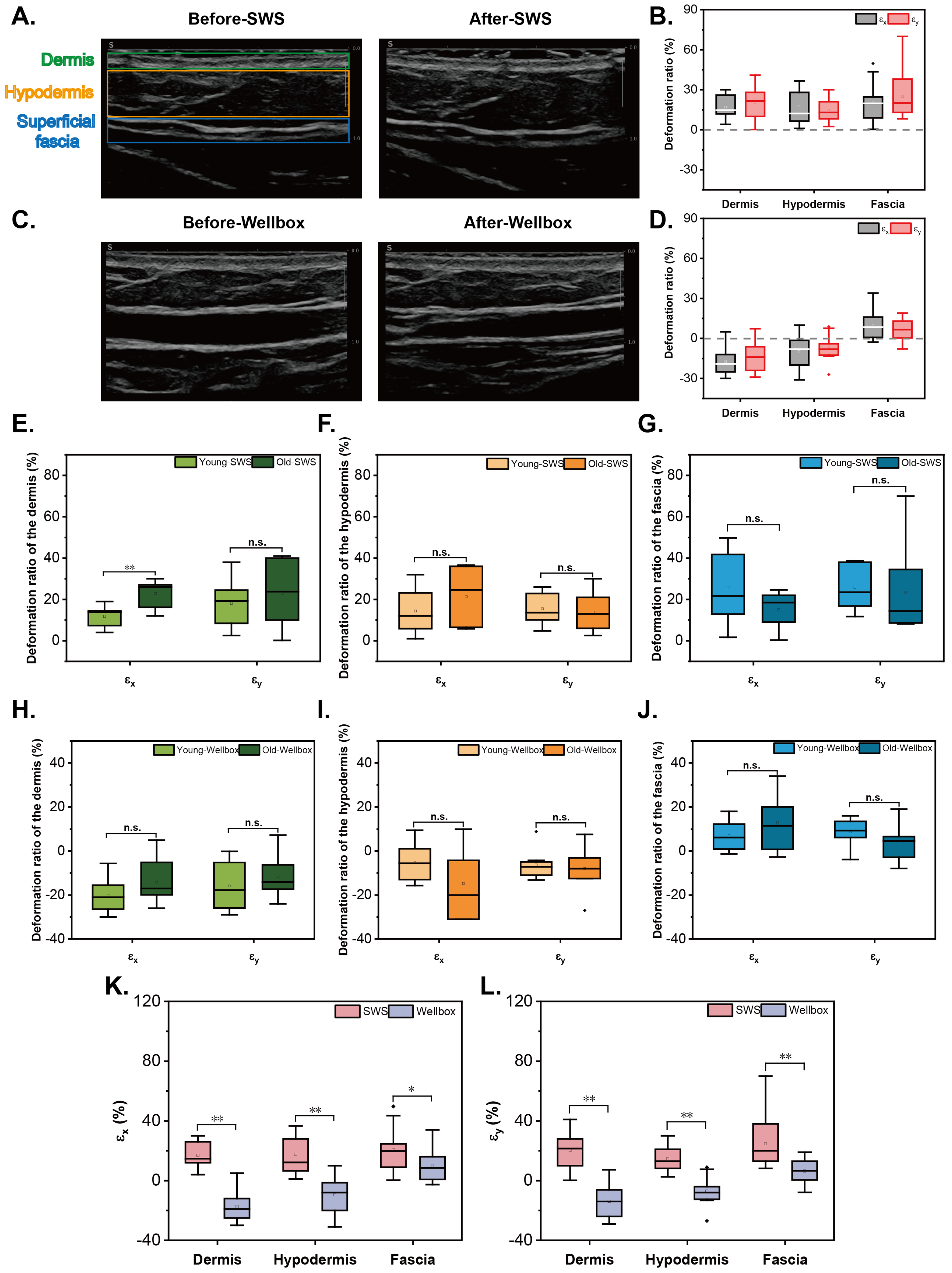

| Dermis | Hypodermis | Fasica | |
|---|---|---|---|
| 16.98 ± 2.06 | 17.63 ± 3.16 | 20.65 ± 3.76 | |
| 20.37 ± 3.38 | 14.69 ± 2.16 | 24.78 ± 4.3 |
| Dermis | Hypodermis | Fasica | |
|---|---|---|---|
| −17.33 ± 2.46 | −9.61 ± 3.32 | 9.67 ± 2.56 | |
| −13.81 ± 2.75 | −7.05 ± 2.19 | 6.42 ± 1.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, N.; Ouillon, L.; Bergheau, A.; Dumas, V.; Privet-Thieulin, C.; Perrot, J.-L.; Zahouani, H. Dynamic Responses of Human Skin and Fascia to an Innovative Stimulation Device—Shear Wave Stimulation. Biomimetics 2024, 9, 475. https://doi.org/10.3390/biomimetics9080475
Qiao N, Ouillon L, Bergheau A, Dumas V, Privet-Thieulin C, Perrot J-L, Zahouani H. Dynamic Responses of Human Skin and Fascia to an Innovative Stimulation Device—Shear Wave Stimulation. Biomimetics. 2024; 9(8):475. https://doi.org/10.3390/biomimetics9080475
Chicago/Turabian StyleQiao, Na, Lucas Ouillon, Alexandre Bergheau, Virginie Dumas, Coralie Privet-Thieulin, Jean-Luc Perrot, and Hassan Zahouani. 2024. "Dynamic Responses of Human Skin and Fascia to an Innovative Stimulation Device—Shear Wave Stimulation" Biomimetics 9, no. 8: 475. https://doi.org/10.3390/biomimetics9080475
APA StyleQiao, N., Ouillon, L., Bergheau, A., Dumas, V., Privet-Thieulin, C., Perrot, J.-L., & Zahouani, H. (2024). Dynamic Responses of Human Skin and Fascia to an Innovative Stimulation Device—Shear Wave Stimulation. Biomimetics, 9(8), 475. https://doi.org/10.3390/biomimetics9080475






