Machine Learning Assists in the Design and Application of Microneedles
Abstract
1. Introduction
2. Introduction of Machine Learning
2.1. The Rise and Development of Supervised Learning
2.2. The Rise and Development of Unsupervised Learning
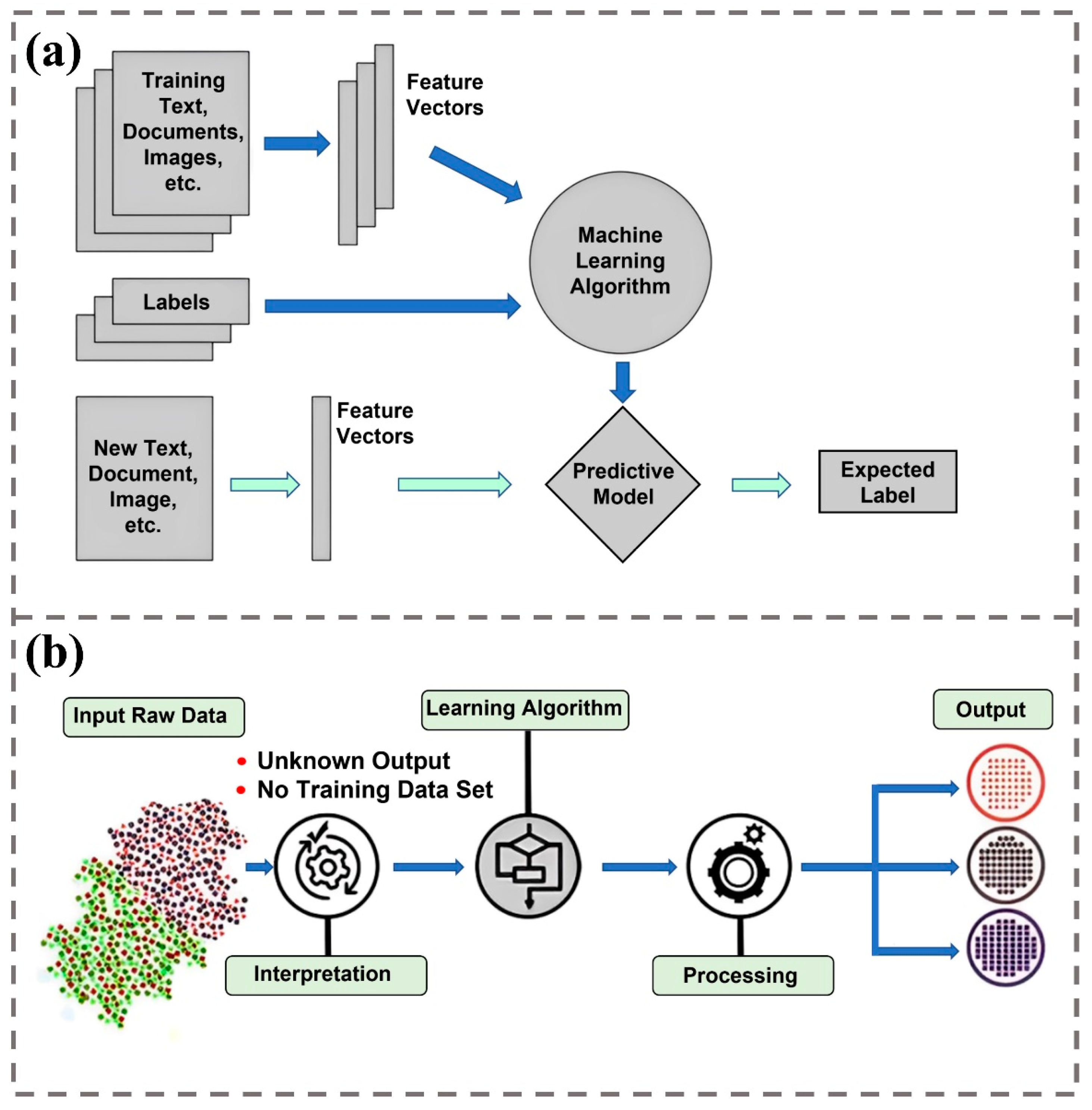
2.3. The Revolution of Deep Learning

3. Machine Learning Assists in the Design of Microneedles
3.1. Microneedle Dimension
3.2. Microneedle Structure
3.3. Material Selection and Fabrication of Microneedles

4. Machine Learning Assisted the Application of Microneedles
4.1. Application in Treatment
4.1.1. Treatment with Microneedles
- 1.
- Skin diseases

- 2.
- Wound healing

- 3.
- Other diseases

4.1.2. Machine Learning-Assisted Microneedle Therapy
4.2. Applications in Biosensing
4.2.1. Microneedle Detection Biomarkers
- 1.
- Microneedles for ISF extraction
- 2.
- Microneedles for capturing biomarkers
- 3.
- Microneedles combined with biosensors
4.2.2. Machine Learning-Assisted Microneedle Biosensor
- 1.
- Wound health monitoring
- 2.
- Physiological signal measurement
- 3.
- Intrabiological drug monitoring
- 4.
- Freshness monitoring of meat

5. Conclusions
6. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition | Abbreviation | Definition |
| MN | Microneedle | CLIP | Continuous liquid interface production |
| SVM | Support vector machine | CAD | Computer-aided design |
| PCA | Principal component analysis | MNA | Microneedle array |
| ANN | Artificial neural network | TCD | TA-encapsulated candlelit-dissolving microneedle |
| LSTM | Long short-term memory | AGA | Androgenic alopecia |
| CNN | Convolutional neural network | FRM | Fractional radiofrequency microneedle |
| RNN | Recurrent neural network | DMN | Dissolving microneedle |
| GRU | Gated recurrent unit | AZA | Azelaic acid |
| GNN | Graph neural network | MP | Microsphere |
| GCN | Graph convolutional network | BP | Black phosphorus |
| GAT | Graph attention network | NIR | Near-infrared |
| NLP | Natural language processing | GT | Gelatin |
| VQA | Visual question answering | MTX | Methotrexate |
| MoE | Mixture of expert | ROS | Reactive oxygen species |
| MSE | Mean squared error | EGCG | Epigallocatechin gallate |
| ISF | Interstitial fluid | SN | Silicate nanosheet |
| VFR | Volumetric flow rat | GelMA | Gelatin methacryloyl |
| RF | Random forest | PTH | Parathyroid hormone |
| GBoost | Gradient boosting | PBN | Prussian blue nanoenzyme |
| GAT | Graph attention network | VEGF | Vascular endothelial growth factor |
| MEMS | Microelectromechanical system | SPM | Self-plugging microneedle |
| TPP | Two-photon polymerization | RA | Rheumatoid arthritis |
| FDM | fused deposition modeling | TSA | Trichostatin A |
| SLA | Stereolithography | HDAC4 | Histone deacetylase 4 |
| DLP | Digital light processing | MLR | Multiple linear regression |
| PBP | Projection printing | PVA | Polyvinyl alcohol |
| LCD | Liquid crystal display | CGM | Continuous glucose monitoring |
References
- Ma, X.; Zhou, Q.; Gao, B. Recent advances of biosensors on microneedles. Anal. Methods 2023, 15, 5711–5730. [Google Scholar] [CrossRef] [PubMed]
- Le, Z.; Yu, J.; Quek, Y.J.; Bai, B.; Li, X.; Shou, Y.; Myint, B.; Xu, C.; Tay, A. Design principles of microneedles for drug delivery and sampling applications. Mater. Today 2023, 63, 137–169. [Google Scholar] [CrossRef]
- Ma, G.; Wu, C. Microneedle, bio-microneedle and bio-inspired microneedle: A review. J. Control. Release 2017, 251, 11–23. [Google Scholar] [CrossRef]
- Singh, P.; Carrier, A.; Chen, Y.; Lin, S.; Wang, J.; Cui, S.; Zhang, X. Polymeric microneedles for controlled transdermal drug delivery. J. Control. Release 2019, 315, 97–113. [Google Scholar] [CrossRef]
- Yang, D.; Chen, M.; Sun, Y.; Jin, Y.; Lu, C.; Pan, X.; Quan, G.; Wu, C. Microneedle-mediated transdermal drug delivery for treating diverse skin diseases. Acta Biomater. 2021, 121, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Liang, L.; Jing, L.Y.; Liu, Y.; Zhang, Y.H.; Shahbazi, M.A.; Chen, B.Z.; Guo, X.D. Microneedles: A novel strategy for wound management. Biomater. Sci. 2023, 11, 4430–4451. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ge, R.; Lin, K.; Wang, J.; He, Y.; Lu, H.; Dong, H. Advances in the Application of Microneedles in the Treatment of Local Organ Diseases. Small 2024, 20, e2306222. [Google Scholar] [CrossRef]
- Vora, L.K.; Sabri, A.H.; McKenna, P.E.; Himawan, A.; Hutton, A.R.J.; Detamornrat, U.; Paredes, A.J.; Larrañeta, E.; Donnelly, R.F. Microneedle-based biosensing. Nat. Rev. Bioeng. 2024, 2, 64–81. [Google Scholar] [CrossRef]
- Park, H.S.; Nguyen, D.S.; Le-Hong, T.; Van Tran, X. Machine learning-based optimization of process parameters in selective laser melting for biomedical applications. J. Intell. Manuf. 2022, 33, 1843–1858. [Google Scholar] [CrossRef]
- Singh, A.V.; Rosenkranz, D.; Ansari, M.H.D.; Singh, R.; Kanase, A.; Singh, S.P.; Johnston, B.; Tentschert, J.; Laux, P.; Luch, A. Artificial intelligence and machine learning empower advanced biomedical material design to toxicity prediction. Adv. Intell. Syst. 2020, 2, 2000084. [Google Scholar] [CrossRef]
- Sarmadi, M.; Behrens, A.M.; McHugh, K.J.; Contreras, H.T.; Tochka, Z.L.; Lu, X.; Langer, R.; Jaklenec, A. Modeling, design, and machine learning-based framework for optimal injectability of microparticle-based drug formulations. Sci. Adv. 2020, 6, eabb6594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Bamakan, S.M.H.; Qu, Q.; Li, S. Learning for personalized medicine: A comprehensive review from a deep learning perspective. IEEE Rev. Biomed. Eng. 2018, 12, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Chekroud, A.M.; Bondar, J.; Delgadillo, J.; Doherty, G.; Wasil, A.; Fokkema, M.; Cohen, Z.; Belgrave, D.; DeRubeis, R.; Iniesta, R. The promise of machine learning in predicting treatment outcomes in psychiatry. World Psychiatry 2021, 20, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.K.; Singh, K. A review on conventional machine learning vs deep learning. In Proceedings of the 2018 International Conference on Computing, Power and Communication Technologies (GUCON), Greater Noida, India, 28–29 September 2018; pp. 347–352. [Google Scholar]
- Jiang, T.; Gradus, J.L.; Rosellini, A.J. Supervised machine learning: A brief primer. Behav. Ther. 2020, 51, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Usama, M.; Qadir, J.; Raza, A.; Arif, H.; Yau, K.-L.A.; Elkhatib, Y.; Hussain, A.; Al-Fuqaha, A. Unsupervised machine learning for networking: Techniques, applications and research challenges. IEEE Access 2019, 7, 65579–65615. [Google Scholar] [CrossRef]
- Hotelling, H. Analysis of a complex of statistical variables with principal components. J. Educ. Psychol. 1933, 24, 498–520. [Google Scholar] [CrossRef]
- MacQueen, J. Some methods for classification and analysis of multivariate observations. In Proceedings of the fifth Berkeley Symposium on Mathematical Statistics and Probability, Berkeley, CA, USA, 21 June–18 July 1965; pp. 281–297. [Google Scholar]
- Öztürk, O.; Özcan, A. Ideology Detection Using Transformer-Based Machine Learning Models; Academic Press: Cambridge, MA, USA, 2022; pp. 1–23. [Google Scholar] [CrossRef]
- Mahesh, B. Machine learning algorithms—A review. Int. J. Sci. Res. IJSR 2020, 9, 381–386. [Google Scholar] [CrossRef]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- LeCun, Y.; Boser, B.; Denker, J.S.; Henderson, D.; Howard, R.E.; Hubbard, W.; Jackel, L.D. Backpropagation applied to handwritten zip code recognition. Neural Comput. 1989, 1, 541–551. [Google Scholar] [CrossRef]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Hinton, G.E.; Salakhutdinov, R.R. Reducing the dimensionality of data with neural networks. Science 2006, 313, 504–507. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Adv. Neural Inf. Process. Syst. 2012, 25, 1–9. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Cho, K.; Van Merriënboer, B.; Gulcehre, C.; Bahdanau, D.; Bougares, F.; Schwenk, H.; Bengio, Y. Learning phrase representations using RNN encoder-decoder for statistical machine translation. arXiv 2014, arXiv:1406.1078. [Google Scholar]
- Scarselli, F.; Gori, M.; Tsoi, A.C.; Hagenbuchner, M.; Monfardini, G. The graph neural network model. IEEE Trans. Neural Netw. 2008, 20, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Bruna, J.; Zaremba, W.; Szlam, A.; LeCun, Y. Spectral networks and locally connected networks on graphs. arXiv 2013, arXiv:1312.6203. [Google Scholar]
- Velickovic, P.; Cucurull, G.; Casanova, A.; Romero, A.; Lio, P.; Bengio, Y. Graph attention networks, international conference on learning representations. In Proceedings of the International Conference on Learning Representations, Vancouver, BC, Canada, 30 April–3 May 2018; pp. 1–2. [Google Scholar]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, L.; Polosukhin, I. Attention is all you need. Adv. Neural Inf. Process. Syst. 2017, 30, 1–11. [Google Scholar]
- Lu, J.; Batra, D.; Parikh, D.; Lee, S. Vilbert: Pretraining task-agnostic visiolinguistic representations for vision-and-language tasks. Adv. Neural Inf. Process. Syst. 2019, 32, 1–11. [Google Scholar]
- Li, L.H.; Yatskar, M.; Yin, D.; Hsieh, C.-J.; Chang, K.-W. Visualbert: A simple and performant baseline for vision and language. arXiv 2019, arXiv:1908.03557. [Google Scholar]
- Fedus, W.; Zoph, B.; Shazeer, N. Switch transformers: Scaling to trillion parameter models with simple and efficient sparsity. J. Mach. Learn. Res. 2022, 23, 1–39. [Google Scholar]
- Mu, R.; Zeng, X. A review of deep learning research. KSII Trans. Internet Inf. Syst. TIIS 2019, 13, 1738–1764. [Google Scholar]
- Sung, S.-H.; Kim, J.-M.; Park, B.-K.; Kim, S. A Study on Cryptocurrency Log-Return Price Prediction Using Multivariate Time-Series Model. Axioms 2022, 11, 448. [Google Scholar] [CrossRef]
- Zhou, J.; Cui, G.; Hu, S.; Zhang, Z.; Yang, C.; Liu, Z.; Wang, L.; Li, C.; Sun, M. Graph neural networks: A review of methods and applications. AI Open 2020, 1, 57–81. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Planz, V.; Lehr, C.-M.; Windbergs, M. In vitro models for evaluating safety and efficacy of novel technologies for skin drug delivery. J. Control. Release 2016, 242, 89–104. [Google Scholar] [CrossRef]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain 2008, 24, 585–594. [Google Scholar] [CrossRef]
- Abdullah, A.C.; Tasoglu, S. ML-Augmented Bayesian Optimization of Pain Induced by Microneedles. Adv. Sensor Res. 2023, 3, 2300181. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Tarar, C.; Aydın, E.; Yetisen, A.K.; Tasoglu, S. Bayesian machine learning optimization of microneedle design for biological fluid sampling. Sens. Diagn. 2023, 2, 858–866. [Google Scholar] [CrossRef]
- Park, J.-H.; Allen, M.G.; Prausnitz, M.R. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J. Control. Release 2005, 104, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- van der Maaden, K.; Sekerdag, E.; Jiskoot, W.; Bouwstra, J. Impact-insertion applicator improves reliability of skin penetration by solid microneedle arrays. AAPS J. 2014, 16, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Choi, S.-O.; Tong, N.T.; Aiyar, A.R.; Patel, S.R.; Prausnitz, M.R.; Allen, M.G. Hollow microneedles for intradermal injection fabricated by sacrificial micromolding and selective electrodeposition. Biomed. Microdevices 2013, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tao, Y.; Zhou, Y.; Gui, S. Development of sinomenine hydrochloride-loaded polyvinylalcohol/maltose microneedle for transdermal delivery. J. Drug Deliv. Sci. Technol. 2016, 35, 1–7. [Google Scholar] [CrossRef]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-forming microneedles: Current advancements and future trends. Macromol. Biosci. 2021, 21, 2000307. [Google Scholar] [CrossRef]
- Lee, K.J.; Jeong, S.S.; Roh, D.H.; Kim, D.Y.; Choi, H.-K.; Lee, E.H. A practical guide to the development of microneedle systems–In clinical trials or on the market. Int. J. Pharm. 2020, 573, 118778. [Google Scholar] [CrossRef]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Verbaan, F.; Bal, S.; Van den Berg, D.; Groenink, W.; Verpoorten, H.; Lüttge, R.; Bouwstra, J. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J. Control. Release 2007, 117, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Martanto, W.; Moore, J.S.; Kashlan, O.; Kamath, R.; Wang, P.M.; O’Neal, J.M.; Prausnitz, M.R. Microinfusion using hollow microneedles. Pharm. Res. 2006, 23, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Ceramic microneedles and hollow microneedles for transdermal drug delivery: Two decades of research. J. Drug Deliv. Sci. Technol. 2018, 44, 314–322. [Google Scholar] [CrossRef]
- Cai, B.; Xia, W.; Bredenberg, S.; Engqvist, H. Self-setting bioceramic microscopic protrusions for transdermal drug delivery. J. Mater. Chem. B 2014, 2, 5992–5998. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Mehta, P.; Arshad, M.; Kucuk, I.; Chang, M.; Ahmad, Z. Transdermal microneedles—A materials perspective. AAPS PharmSciTech 2020, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Hagiwara, E.; Saeki, A.; Sugioka, N.; Takada, K. Feasibility of microneedles for percutaneous absorption of insulin. Eur. J. Pharm. Sci. 2006, 29, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Chumpu, R.; Chu, C.-L.; Treeratanaphitak, T.; Marukatat, S.; Hsu, S.-H. Physics-informed graph neural networks accelerating microneedle simulations towards novelty of micro-nano scale materials discovery. Eng. Appl. Artif. Intell. 2023, 126, 106894. [Google Scholar] [CrossRef]
- Tarbox, T.N.; Watts, A.B.; Cui, Z.; Williams, R.O. An update on coating/manufacturing techniques of microneedles. Drug Deliv. Transl. Res. 2018, 8, 1828–1843. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, B.; Gao, Y. Controlled transdermal delivery of model drug compounds by MEMS microneedle array. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 184–190. [Google Scholar] [CrossRef]
- Wang, M.; Hu, L.; Xu, C. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab. Chip 2017, 17, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- Ramöller, I.K.; McAlister, E.; Bogan, A.; Cordeiro, A.S.; Donnelly, R.F. Novel design approaches in the fabrication of polymeric microarray patches via micromoulding. Micromachines 2020, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.A. Micro Injection Moulding: Tooling and Process Factors; Cardiff University: Cardiff, UK, 2008. [Google Scholar]
- Lee, K.; Park, S.H.; Lee, J.; Ryu, S.; Joo, C.; Ryu, W. Three-step thermal drawing for rapid prototyping of highly customizable microneedles for vascular tissue insertion. Pharmaceutics 2019, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Yang, D.Y.; Park, S.H.; Kim, R.H. Recent developments in the use of two-photon polymerization in precise 2D and 3D microfabrications. Polym. Adv. Technol. 2006, 17, 72–82. [Google Scholar] [CrossRef]
- Gittard, S.D.; Ovsianikov, A.; Chichkov, B.N.; Doraiswamy, A.; Narayan, R.J. Two-photon polymerization of microneedles for transdermal drug delivery. Expert. Opin. Drug Deliv. 2010, 7, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Plamadeala, C.; Gosain, S.R.; Hischen, F.; Buchroithner, B.; Puthukodan, S.; Jacak, J.; Bocchino, A.; Whelan, D.; O’Mahony, C.; Baumgartner, W. Bio-inspired microneedle design for efficient drug/vaccine coating. Biomed. Microdevices 2020, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, M.A.; Berry, D.R.; Reagan, J.C.; Smaldone, R.A.; Gassensmith, J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab. Chip 2018, 18, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Lai, L.; Li, Y. Preparation of microneedle array mold based on MEMS lithography technology. Micromachines 2020, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.; Fei, J.; Liu, H.; Chen, W.; Liu, R. Multilayered pyramidal dissolving microneedle patches with flexible pedestals for improving effective drug delivery. J. Control. Release. 2017, 265, 113–119. [Google Scholar] [CrossRef]
- Vecchione, R.; Coppola, S.; Esposito, E.; Casale, C.; Vespini, V.; Grilli, S.; Ferraro, P.; Netti, P.A. Electro-drawn drug-loaded biodegradable polymer microneedles as a viable route to hypodermic injection. Adv. Funct. Mater. 2014, 24, 3515–3523. [Google Scholar] [CrossRef]
- Yang, H.; Kim, S.; Kang, G.; Lahiji, S.F.; Jang, M.; Kim, Y.M.; Kim, J.M.; Cho, S.N.; Jung, H. Centrifugal lithography: Self-shaping of polymer microstructures encapsulating biopharmaceutics by centrifuging polymer drops. Adv. Healthc. Mater. 2017, 6, 1700326. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Maleki, A.; Shabani, M.; Hutton, A.R.; Kirkby, M.; Jamaledin, R.; Fang, T.; He, J.; Lee, J.; Mazzolai, B. Bioinspired microneedle patches: Biomimetic designs, fabrication, and biomedical applications. Matter 2022, 5, 390–429. [Google Scholar] [CrossRef]
- Elahpour, N.; Pahlevanzadeh, F.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. 3D printed microneedles for transdermal drug delivery: A brief review of two decades. Int. J. Pharm. 2021, 597, 120301. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, Y.; Baek, S.; De Backer, W.; Harik, R. Manufacturability analysis for additive manufacturing using a novel feature recognition technique. Comput. Aided Des. Appl. 2018, 15, 941–952. [Google Scholar] [CrossRef]
- Gan, Z.; Li, H.; Wolff, S.J.; Bennett, J.L.; Hyatt, G.; Wagner, G.J.; Cao, J.; Liu, W.K. Data-driven microstructure and microhardness design in additive manufacturing using a self-organizing map. Engineering 2019, 5, 730–735. [Google Scholar] [CrossRef]
- Garg, A.; Lam, J.S.L.; Savalani, M. A new variant of genetic programming in formulation of laser energy consumption model of 3D printing process. In Handbook of Sustainability in Additive Manufacturing; Springer: Singapore, 2016; Volume 1, pp. 31–50. [Google Scholar]
- Sarlo, R.; Tarazaga, P.A. A neural network approach to 3D printed surrogate systems. In Proceedings of the Topics in Modal Analysis & Testing, Volume 10: Conference Proceedings of the Society for Experimental Mechanics Series; Springer: Cham, Switzerland, 2016; pp. 215–222. [Google Scholar] [CrossRef]
- Castro, B.M.; Elbadawi, M.; Ong, J.J.; Pollard, T.; Song, Z.; Gaisford, S.; Pérez, G.; Basit, A.W.; Cabalar, P.; Goyanes, A. Machine learning predicts 3D printing performance of over 900 drug delivery systems. J. Control. Release 2021, 337, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Rezapour Sarabi, M.; Alseed, M.M.; Karagoz, A.A.; Tasoglu, S. Machine learning-enabled prediction of 3D-printed microneedle features. Biosensors 2022, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 26 June–1 July 2016; pp. 770–778. [Google Scholar]
- Sandler, M.; Howard, A.; Zhu, M.; Zhmoginov, A.; Chen, L.-C. Mobilenetv2: Inverted residuals and linear bottlenecks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 4510–4520. [Google Scholar]
- Liu, Z.; Mao, H.; Wu, C.-Y.; Feichtenhofer, C.; Darrell, T.; Xie, S. A convnet for the 2020s. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, New Orleans, LA, USA, 18–24 June 2022; pp. 11976–11986. [Google Scholar]
- Yu, J.; Zhang, Y.; Ye, Y.; DiSanto, R.; Sun, W.; Ranson, D.; Ligler, F.S.; Buse, J.B.; Gu, Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 8260–8265. [Google Scholar] [CrossRef] [PubMed]
- Serup, J.; Jemec, G.B.E.; Grove, G. Handbook of Non-Invasive Methods and the Skin, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Chandrashekar, B.; Yepuri, V.; Mysore, V. Alopecia areata-successful outcome with microneedling and triamcinolone acetonide. J. Cutan. Aesthet. Surg. 2014, 7, 63–64. [Google Scholar] [CrossRef]
- Asad, U.; Wallis, D.; Tarbox, M. Ophiasis alopecia areata treated with microneedling. Bayl. Univ. Med. Cent. Proc. 2020, 33, 413–414. [Google Scholar] [CrossRef]
- Wei, F.; Cheng Jun, C.; Cheng, S.; Fang, L. Effect of minoxidil combined with triamcinolone acetonide on alopecia areata by microneedle injection. Skin. Res. Technol. 2024, 30, e13713. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jang, M.; Ahn, H.; Kang, B.M.; Yang, H.; Kang, G.; Kwon, O.; Jung, H. Novel treatment of alopecia areata with shooting-type candlelit-dissolving microneedle. Appl. Mater. Today 2023, 35, 101946. [Google Scholar] [CrossRef]
- Almohanna, H.M.; Perper, M.; Tosti, A. Safety concerns when using novel medications to treat alopecia. Expert. Opin. Drug Saf. 2018, 17, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chen, Q.; Wen, D.; Chen, Z.; Wang, J.; Chen, G.; Wang, Z.; Zhang, X.; Zhang, Y.; Hu, Q.; et al. A Therapeutic Microneedle Patch Made from Hair-Derived Keratin for Promoting Hair Regrowth. ACS Nano 2019, 13, 4354–4360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, W.; Chang, D.; Wei, Z.; Wang, E.; Yu, J.; Xu, Y.; Que, Y.; Chen, Y.; Fan, C.; et al. A combination therapy for androgenic alopecia based on quercetin and zinc/copper dual-doped mesoporous silica nanocomposite microneedle patch. Bioact. Mater. 2023, 24, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.R.; Lee, E.G.; Lee, H.J.; Yoon, M.S. Assessment of treatment efficacy and sebosuppressive effect of fractional radiofrequency microneedle on acne vulgaris. Lasers Surg. Med. 2013, 45, 639–647. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, P.; Yu, J.; Yang, J.; Zhao, J.; Wang, J.; Shen, Q.; Gu, Z. ROS-Responsive Microneedle Patch for Acne Vulgaris Treatment. Adv. Ther. 2018, 1, 1800035. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, S.; Ma, Y.; Chen, Y.; Yang, G.; Zhou, Z.; Gao, Y. Preparation and evaluation of dissolving microneedle loaded with azelaic acid for acne vulgaris therapy. J. Drug Deliv. Sci. Technol. 2022, 75, 103667. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, J.; Mao, C.; Zhu, Y.; Wang, C.; Wu, J.; Liu, X.; Wu, S.; Kwan, K.Y.H.; Cheung, K.M.C.; et al. Ultrasound-triggered interfacial engineering-based microneedle for bacterial infection acne treatment. Sci. Adv. 2023, 9, eadf0854. [Google Scholar] [CrossRef]
- Pamornpathomkul, B.; Ngawhirunpat, T.; Tekko, I.A.; Vora, L.; McCarthy, H.O.; Donnelly, R.F. Dissolving polymeric microneedle arrays for enhanced site-specific acyclovir delivery. Eur. J. Pharm. Sci. 2018, 121, 200–209. [Google Scholar] [CrossRef]
- Nagra, U.; Barkat, K.; Ashraf, M.U.; Shabbir, M. Feasibility of Enhancing Skin Permeability of Acyclovir through Sterile Topical Lyophilized Wafer on Self-Dissolving Microneedle-Treated Skin. Dose Response 2022, 20, 15593258221097594. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, X.; Nie, M.; Xu, Y.; Wang, Y.; Shang, L.; Zhao, Y.; Zhao, Y. Photothermal Responsive Microspheres-Triggered Separable Microneedles for Versatile Drug Delivery. Adv. Funct. Mater. 2022, 32, 2110746. [Google Scholar] [CrossRef]
- Bi, D.; Qu, F.; Xiao, W.; Wu, J.; Liu, P.; Du, H.; Xie, Y.; Liu, H.; Zhang, L.; Tao, J.; et al. Reactive Oxygen Species-Responsive Gel-Based Microneedle Patches for Prolonged and Intelligent Psoriasis Management. ACS Nano 2023, 17, 4346–4357. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Zhao, Z.; Zheng, H.; Wang, S.; Liao, Z.; Sheng, T.; Zhao, S.; Hou, W.; Yu, X.; et al. Adoptive T(reg) therapy with metabolic intervention via perforated microneedles ameliorates psoriasis syndrome. Sci. Adv. 2023, 9, eadg6007. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, Y.; He, X.; Yang, F.; Han, R.; Yang, C.; Li, W.; Qian, Z. Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy. Bioact. Mater. 2020, 5, 542–552. [Google Scholar] [CrossRef]
- Shao, J.; Li, X.; Li, Y.; Lin, J.; Huang, P. Self-Heating Multistage Microneedle Patch for Topical Therapy of Skin Cancer. Adv. Mater. 2024, 36, e2308217. [Google Scholar] [CrossRef]
- Joo, S.-H.; Kim, J.; Hong, J.; Fakhraei Lahiji, S.; Kim, Y.-H. Dissolvable Self-Locking Microneedle Patches Integrated with Immunomodulators for Cancer Immunotherapy. Adv. Mater. 2023, 35, 2209966. [Google Scholar] [CrossRef]
- Chen, B.Z.; Zhao, Z.Q.; Shahbazi, M.-A.; Guo, X.D. Microneedle-based technology for cell therapy: Current status and future directions. Nanoscale Horiz. 2022, 7, 715–728. [Google Scholar] [CrossRef]
- Ahmed Saeed Al-Japairai, K.; Mahmood, S.; Hamed Almurisi, S.; Reddy Venugopal, J.; Rebhi Hilles, A.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Chi, J.; Zhao, Y. Smart Microneedles for Therapy and Diagnosis. Research 2020, 2020, 7462915. [Google Scholar] [CrossRef]
- Enoch, S.; Leaper, D.J. Basic science of wound healing. Surgery 2005, 23, 37–42. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Jeon, E.Y.; Lee, J.; Kim, B.J.; Joo, K.I.; Kim, K.H.; Lim, G.; Cha, H.J. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials 2019, 222, 119439. [Google Scholar] [CrossRef] [PubMed]
- Haghniaz, R.; Kim, H.J.; Montazerian, H.; Baidya, A.; Tavafoghi, M.; Chen, Y.; Zhu, Y.; Karamikamkar, S.; Sheikhi, A.; Khademhosseini, A. Tissue adhesive hemostatic microneedle arrays for rapid hemorrhage treatment. Bioact. Mater. 2023, 23, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Anjani, Q.K.; Sartini; Utomo, E.; Volpe-Zanutto, F.; Paredes, A.J.; Evary, Y.M.; Mardikasari, S.A.; Pratama, M.R.; Tuany, I.N.; et al. Selective delivery of silver nanoparticles for improved treatment of biofilm skin infection using bacteria-responsive microparticles loaded into dissolving microneedles. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111786. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Chi, J.; Wang, Y.; Zhao, Y.; Luo, Y.; Wang, Y. Zn-MOF Encapsulated Antibacterial and Degradable Microneedles Array for Promoting Wound Healing. Adv. Healthc. Mater. 2021, 10, e2100056. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Danehy, R.; Cai, H.; Ao, Z.; Pu, M.; Nusawardhana, A.; Rowe-Magnus, D.; Guo, F. Microneedle Patch-Mediated Treatment of Bacterial Biofilms. ACS Appl. Mater. Interfaces 2019, 11, 14640–14646. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, X.; Yan, Z.; Wang, T.; Chen, Z.; Song, H.; Zheng, Y. Microneedle Patches with Antimicrobial and Immunomodulating Properties for Infected Wound Healing. Adv. Sci. 2023, 10, e2300576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, G.; Liu, Y.; Sun, L.; Sun, L.; Zhao, Y. Black Phosphorus-Loaded Separable Microneedles as Responsive Oxygen Delivery Carriers for Wound Healing. ACS Nano 2020, 14, 5901–5908. [Google Scholar] [CrossRef]
- Yao, Z.; Xue, T.; Xiong, H.; Cai, C.; Liu, X.; Wu, F.; Liu, S.; Fan, C. Promotion of collagen deposition during skin healing through Smad3/mTOR pathway by parathyroid hormone-loaded microneedle. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111446. [Google Scholar] [CrossRef]
- Long, L.Y.; Liu, W.; Li, L.; Hu, C.; He, S.; Lu, L.; Wang, J.; Yang, L.; Wang, Y.B. Dissolving microneedle-encapsulated drug-loaded nanoparticles and recombinant humanized collagen type III for the treatment of chronic wound via anti-inflammation and enhanced cell proliferation and angiogenesis. Nanoscale 2022, 14, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Liu, K.; Jiang, T.; Li, S.; Chen, J.; Wu, Z.; Li, W.; Tan, R.; Wei, W.; Yang, X.; et al. GelMA/PEGDA microneedles patch loaded with HUVECs-derived exosomes and Tazarotene promote diabetic wound healing. J. Nanobiotechnol. 2022, 20, 147. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Zhang, Q.; Jiang, Z.; Liu, J.; Wan, J.; Jin, P.; Lv, Q. Multifunctional Silk Fibroin Methacryloyl Microneedle for Diabetic Wound Healing. Small 2022, 18, e2203064. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Dong, Y.; Shen, Y.; Hou, A.; Quan, G.; Pan, X.; Wu, C. Bilayer dissolving microneedle array containing 5-fluorouracil and triamcinolone with biphasic release profile for hypertrophic scar therapy. Bioact. Mater. 2021, 6, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, E.H.; Kim, J.H.; Park, S.; Kang, Y.; Park, S.; Lee, K.; Kim, J.-H.; Youn, Y.-N.; Ryu, W. Highly flexible and porous silk fibroin microneedle wraps for perivascular drug delivery. J. Control. Release 2021, 340, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Fang, H.; Zhang, T.; Hu, B.; Liu, S.; Lv, F.; Zeng, Z.; Liu, H.; Zhou, W.; Wang, X. Drug-loaded balloon with built-in NIR controlled tip-separable microneedles for long-effective arteriosclerosis treatment. Bioact. Mater. 2023, 23, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Li, Y.; Meng, Y.; Pu, X.Q.; Qin, J.W.; Xie, R.; Wang, W.; Liu, Z.; Jiang, L.; Ju, X.J.; et al. Composite dissolvable microneedle patch for therapy of oral mucosal diseases. Biomater. Adv. 2022, 139, 213001. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhu, T.; Yu, X.; Yi, X.; Li, L.; Qu, X.; Zhang, Z.; Hao, Y.; Wang, W. Betamethasone-loaded dissolvable microneedle patch for oral ulcer treatment. Colloids Surf. B Biointerfaces 2023, 222, 113100. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Li, X.J.; Li, Y.; Zhang, T.Y.; Liu, D.; Wu, Y.Q.; Hou, F.F.; Ye, L.; Wu, C.J.; Feng, X.D.; et al. Novel Double-Layer Dissolving Microneedles for Transmucosal Sequential Delivery of Multiple Drugs in the Treatment of Oral Mucosa Diseases. ACS Appl. Mater. Interfaces 2023, 15, 13892–13906. [Google Scholar] [CrossRef]
- Manimaran, R.; Patel, K.D.; Lobo, V.M.; Kumbhar, S.S.; Venuganti, V.V.K. Buccal mucosal application of dissolvable microneedle patch containing photosensitizer provides effective localized delivery and phototherapy against oral carcinoma. Int. J. Pharm. 2023, 640, 122991. [Google Scholar] [CrossRef]
- Than, A.; Liu, C.; Chang, H.; Duong, P.K.; Cheung, C.M.G.; Xu, C.; Wang, X.; Chen, P. Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery. Nat. Commun. 2018, 9, 4433. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, J.; Wang, Y.; Zhu, Y.; Lin, D.; Lei, L.; Vakal, S.; Wang, J.; Li, X. A Rapid Corneal Healing Microneedle for Efficient Ocular Drug Delivery. Small 2022, 18, e2104657. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, S.; Jo, D.H.; Cho, C.S.; Jang, H.Y.; Yi, J.; Kang, M.; Kim, J.; Jung, H.Y.; Kim, J.H.; et al. Self-Plugging Microneedle (SPM) for Intravitreal Drug Delivery. Adv. Healthc. Mater. 2022, 11, e2102599. [Google Scholar] [CrossRef] [PubMed]
- Mahfufah, U.; Sya’ban Mahfud, M.A.; Saputra, M.D.; Abd Azis, S.B.; Salsabila, A.; Asri, R.M.; Habibie, H.; Sari, Y.; Yulianty, R.; Alsayed, A.R.; et al. Incorporation of Inclusion Complexes in the Dissolvable Microneedle Ocular Patch System for the Efficiency of Fluconazole in the Therapy of Fungal Keratitis. ACS Appl. Mater. Interfaces 2024, 16, 25637–25651. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, S.; Batra, U.; Puppala, E.R.; Waghule, T.; Naidu, V.G.M.; Singhvi, G. Emerging trends in microneedle-based drug delivery strategies for the treatment of rheumatoid arthritis. Expert. Opin. Drug Deliv. 2022, 19, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; He, P.; Zhao, J.; He, C.; Jiang, M.; Zhang, Z.; Zhang, Z.; Sun, X. Polymeric microneedle-mediated transdermal delivery of melittin for rheumatoid arthritis treatment. J. Control. Release 2021, 336, 537–548. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, J.; Li, W.; Yang, L.; Dong, H.; Zhang, X. Programmable Polymeric Microneedles for Combined Chemotherapy and Antioxidative Treatment of Rheumatoid Arthritis. ACS Appl. Mater. Interfaces 2021, 13, 55559–55568. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chen, Y.; Gu, X.; Li, Y.; Zhao, H.; Shao, W.; Ma, T.; Wu, C.; Wang, Q. Co-delivery of drugs by adhesive transdermal patches equipped with dissolving microneedles for the treatment of rheumatoid arthritis. J. Control. Release 2024, 365, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Long, L.; Zhang, F.; Hu, X.; Zhang, J.; Hu, C.; Wang, Y.; Xu, J. Microneedle-mediated vascular endothelial growth factor delivery promotes angiogenesis and functional recovery after stroke. J. Control. Release 2021, 338, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, B.; Guo, M.; Peng, W.; Wang, D.; Guo, Q.; Wang, S.; Ming, D.; Zheng, B. Microneedle patch based on molecular motor as a spatio-temporal controllable dosing strategy of L-DOPA for Parkinson’s disease. Chem. Eng. J. 2022, 427, 131555. [Google Scholar] [CrossRef]
- Li, B.; Lu, G.; Liu, W.; Liao, L.; Ban, J.; Lu, Z. Formulation and Evaluation of PLGA Nanoparticulate-Based Microneedle System for Potential Treatment of Neurological Diseases. Int. J. Nanomed. 2023, 18, 3745–3760. [Google Scholar] [CrossRef]
- McGuckin, M.B.; Hutton, A.R.J.; Davis, E.R.; Sabri, A.H.B.; Ripolin, A.; Himawan, A.; Naser, Y.A.; Ghanma, R.; Greer, B.; McCarthy, H.O.; et al. Transdermal Delivery of Pramipexole Using Microneedle Technology for the Potential Treatment of Parkinson’s Disease. Mol. Pharm. 2024, 21, 2512–2533. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Y.; Shi, S.; Liang, M.; Yang, D.; Sui, N.; Yu, W.W.; Wang, L.; Zhu, Z. Machine Learning Guided Discovery of Superoxide Dismutase Nanozymes for Androgenetic Alopecia. Nano Lett. 2022, 22, 8592–8600. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, C.; Tan, R.; Zhang, J.; Fang, Q.; Jin, R.; Mi, X.; Sun, D.; Xue, Y.; Wang, Y.; et al. Artificial Intelligence-Assisted Bioinformatics, Microneedle, and Diabetic Wound Healing: A “New Deal” of an Old Drug. ACS Appl. Mater. Interfaces 2022, 14, 37396–37409. [Google Scholar] [CrossRef]
- Yuan, Y.; Han, Y.; Yap, C.W.; Kochhar, J.S.; Li, H.; Xiang, X.; Kang, L. Prediction of drug permeation through microneedled skin by machine learning. Bioeng. Transl. Med. 2023, 8, e10512. [Google Scholar] [CrossRef]
- Biswas, A.A.; Dhondale, M.R.; Singh, M.; Agrawal, A.K.; Muthudoss, P.; Mishra, B.; Kumar, D. Development and comparison of machine learning models for in-vitro drug permeation prediction from microneedle patch. Eur. J. Pharm. Biopharm. 2024, 199, 114311. [Google Scholar] [CrossRef]
- Lee, S.H.; Thunemann, M.; Lee, K.; Cleary, D.R.; Tonsfeldt, K.J.; Oh, H.; Azzazy, F.; Tchoe, Y.; Bourhis, A.M.; Hossain, L.; et al. Scalable Thousand Channel Penetrating Microneedle Arrays on Flex for Multimodal and Large Area Coverage BrainMachine Interfaces. Adv. Funct. Mater. 2022, 32, 2112045. [Google Scholar] [CrossRef]
- Friedel, M.; Thompson, I.A.P.; Kasting, G.; Polsky, R.; Cunningham, D.; Soh, H.T.; Heikenfeld, J. Opportunities and challenges in the diagnostic utility of dermal interstitial fluid. Nat. Biomed. Eng. 2023, 7, 1541–1555. [Google Scholar] [CrossRef]
- Oharazawa, A.; Maimaituxun, G.; Watanabe, K.; Nishiyasu, T.; Fujii, N. Metabolome analyses of skin dialysate: Insights into skin interstitial fluid biomarkers. J. Dermatol. Sci. 2024, 114, 141–147. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Chang, H.; Zheng, M.; Yu, X.; Than, A.; Seeni, R.Z.; Kang, R.; Tian, J.; Khanh, D.P.; Liu, L.; Chen, P.; et al. A Swellable Microneedle Patch to Rapidly Extract Skin Interstitial Fluid for Timely Metabolic Analysis. Adv. Mater. 2017, 29, 1702243. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, Z.; Chang, H.; Wang, L.; Chew, S.W.T.; Lio, D.C.S.; Cui, M.; Liu, L.; Tee, B.C.K.; Xu, C. Osmosis-Powered Hydrogel Microneedles for Microliters of Skin Interstitial Fluid Extraction within Minutes. Adv. Healthc. Mater. 2020, 9, 1901683. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, J.; He, W.; Iftikhar, T.; Zhang, C.; Su, L.; Zhang, X. Enhanced Interstitial Fluid Extraction and Rapid Analysis via Vacuum Tube-Integrated Microneedle Array Device. Adv. Sci. 2024, 11, 2308716. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zada, S.; Yang, L.; Dong, H. Microneedle-Based Device for Biological Analysis. Front. Bioeng. Biotechnol. 2022, 10, 851134. [Google Scholar] [CrossRef] [PubMed]
- Himawan, A.; Vora, L.K.; Permana, A.D.; Sudir, S.; Nurdin, A.R.; Nislawati, R.; Hasyim, R.; Scott, C.J.; Donnelly, R.F. Where Microneedle Meets Biomarkers: Futuristic Application for Diagnosing and Monitoring Localized External Organ Diseases. Adv. Healthc. Mater. 2023, 12, e2202066. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.; Wang, Y.; Zhao, X.; Shi, S.; Wang, X.; Zhou, N.; Shen, D.; Kang, Q.; Chen, L. Skin Interstitial Fluid-Based SERS Tags Labeled Microneedles for Tracking of Peritonitis Progression and Treatment Effect. ACS Sens. 2023, 8, 372–380. [Google Scholar] [CrossRef]
- Wang, Z.; Luan, J.; Seth, A.; Liu, L.; You, M.; Gupta, P.; Rathi, P.; Wang, Y.; Cao, S.; Jiang, Q.; et al. Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid. Nat. Biomed. Eng. 2021, 5, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Y.; Liu, T.; Wu, C.; Li, J.; Cheng, J.; Wei, W.; Yang, F.; Zhou, L.; Zhang, Y.; et al. Microneedle Array Encapsulated with Programmed DNA Hydrogels for Rapidly Sampling and Sensitively Sensing of Specific MicroRNA in Dermal Interstitial Fluid. ACS Nano 2022, 16, 18366–18375. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.T.; Yang, Z.; Sutarlie, L.; Thangaveloo, M.; Yu, Y.; Salleh, N.; Chin, J.S.; Xiong, Z.; Becker, D.L.; Loh, X.J.; et al. Battery-free and AI-enabled multiplexed sensor patches for wound monitoring. Sci. Adv. 2023, 9, eadg6670. [Google Scholar] [CrossRef]
- Shirzaei Sani, E.; Xu, C.; Wang, C.; Song, Y.; Min, J.; Tu, J.; Solomon, S.A.; Li, J.; Banks, J.L.; Armstrong, D.G.; et al. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Sci. Adv. 2023, 9, eadf7388. [Google Scholar] [CrossRef]
- Johnson, A.C.; Buchanan, E.P.; Khechoyan, D.Y. Wound infection: A review of qualitative and quantitative assessment modalities. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhou, Z.; Zhong, G.; Xu, T.; Zhang, X. Self-Sterilizing Microneedle Sensing Patches for Machine Learning-Enabled Wound pH Visual Monitoring. Adv. Funct. Mater. 2024, 34, 2315067. [Google Scholar] [CrossRef]
- Mariani, F.; Serafini, M.; Gualandi, I.; Arcangeli, D.; Decataldo, F.; Possanzini, L.; Tessarolo, M.; Tonelli, D.; Fraboni, B.; Scavetta, E. Advanced Wound Dressing for Real-Time pH Monitoring. ACS Sens. 2021, 6, 2366–2377. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.-H.; Shen, C.-J.; Shen, C.-F.; Cheng, C.-M. Role of pH value in clinically relevant diagnosis. Diagnostics 2020, 10, 107. [Google Scholar] [CrossRef]
- Shan, J.; Zhang, X.; Kong, B.; Zhu, Y.; Gu, Z.; Ren, L.; Zhao, Y. Coordination polymer nanozymes-integrated colorimetric microneedle patches for intelligent wound infection management. Chem. Eng. J. 2022, 444, 136640. [Google Scholar] [CrossRef]
- Yang, J.; Gong, X.; Chen, S.; Zheng, Y.; Peng, L.; Liu, B.; Chen, Z.; Xie, X.; Yi, C.; Jiang, L. Development of Smartphone-Controlled and Microneedle-Based Wearable Continuous Glucose Monitoring System for Home-Care Diabetes Management. ACS Sens. 2023, 8, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Moonla, C.; Mishra, R.K.; Yu, C.; Narayan, R.; Litvan, I.; Wang, J. Wearable Electrochemical Microneedle Sensor for Continuous Monitoring of Levodopa: Toward Parkinson Management. ACS Sens. 2019, 4, 2196–2204. [Google Scholar] [CrossRef]
- Liu, Y.; Menon, R.; Putcha, A.; Huang, K.; Bonilla, L.; Vora, R.; Li, J.; Zhang, L.; Wang, Y.; Fletcher, L.; et al. Skin-Interfaced Deep-Tissue Sensing Patch via Microneedle Waveguides. Adv. Mater. Technol. 2022, 7, 2200468. [Google Scholar] [CrossRef]
- Rawson, T.M.; Gowers, S.A.N.; Freeman, D.M.E.; Wilson, R.C.; Sharma, S.; Gilchrist, M.; MacGowan, A.; Lovering, A.; Bayliss, M.; Kyriakides, M.; et al. Microneedle biosensors for real-time, minimally invasive drug monitoring of phenoxymethylpenicillin: A first-in-human evaluation in healthy volunteers. Lancet Digit. Health 2019, 1, e335–e343. [Google Scholar] [CrossRef]
- Gowers, S.A.N.; Freeman, D.M.E.; Rawson, T.M.; Rogers, M.L.; Wilson, R.C.; Holmes, A.H.; Cass, A.E.; O’Hare, D. Development of a Minimally Invasive Microneedle-Based Sensor for Continuous Monitoring of β-Lactam Antibiotic Concentrations in Vivo. ACS Sens. 2019, 4, 1072–1080. [Google Scholar] [CrossRef]
- Kadian, S.; Sahoo, S.S.; Kumari, P.; Narayan, R.J. Machine learning enabled onsite electrochemical detection of lidocaine using a microneedle array integrated screen printed electrode. Electrochim. Acta 2024, 475, 143664. [Google Scholar] [CrossRef]
- Wang, J.; Xia, L.; Liu, H.; Zhao, C.; Ming, S.; Wu, J. Colorimetric microneedle sensor using deep learning algorithm for meat freshness monitoring. Chem. Eng. J. 2024, 481, 148474. [Google Scholar] [CrossRef]
- Kadian, S.; Kumari, P.; Sahoo, S.S.; Shukla, S.; Narayan, R.J. Machine learning enabled microneedle-based colorimetric pH sensing patch for wound health monitoring and meat spoilage detection. Microchem. J. 2024, 200, 110350. [Google Scholar] [CrossRef]
- Cao, C.; Hou, C.; Wang, X.; Lv, D.; Ai, L.; Feng, Y.; Chen, P.; Wang, X.; He, M.; Yao, X. Liquid Metal-Enhanced Highly Adhesive Electrodes for Multifunctional Epidermal Bioelectronics. Adv. Funct. Mater. 2024, 2403671. [Google Scholar] [CrossRef]
- Wan, R.; Yu, J.; Quan, Z.; Ma, H.; Li, J.; Tian, F.; Wang, W.; Sun, Y.; Liu, J.; Gao, D.; et al. A reusable, healable, and biocompatible PEDOT:PSS hydrogel-based electrical bioadhesive interface for high-resolution electromyography monitoring and time–frequency analysis. Chem. Eng. J. 2024, 490, 151454. [Google Scholar] [CrossRef]
- Lv, D.; Li, X.; Huang, X.; Cao, C.; Ai, L.; Wang, X.; Ravi, S.K.; Yao, X. Microphase-Separated Elastic and Ultrastretchable Ionogel for Reliable Ionic Skin with Multimodal Sensation. Adv. Mater. 2024, 36, 2309821. [Google Scholar] [CrossRef]
- Yu, J.; Wan, R.; Tian, F.; Cao, J.; Wang, W.; Liu, Q.; Yang, H.; Liu, J.; Liu, X.; Lin, T.; et al. 3D Printing of Robust High-Performance Conducting Polymer Hydrogel-Based Electrical Bioadhesive Interface for Soft Bioelectronics. Small 2024, 20, 2308778. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Kong, S.; Lin, R.; Xie, Y.; Zheng, S.; Yin, Z.; Huang, X.; Su, L.; Zhang, X. Machine Learning Assists in the Design and Application of Microneedles. Biomimetics 2024, 9, 469. https://doi.org/10.3390/biomimetics9080469
He W, Kong S, Lin R, Xie Y, Zheng S, Yin Z, Huang X, Su L, Zhang X. Machine Learning Assists in the Design and Application of Microneedles. Biomimetics. 2024; 9(8):469. https://doi.org/10.3390/biomimetics9080469
Chicago/Turabian StyleHe, Wenqing, Suixiu Kong, Rumin Lin, Yuanting Xie, Shanshan Zheng, Ziyu Yin, Xin Huang, Lei Su, and Xueji Zhang. 2024. "Machine Learning Assists in the Design and Application of Microneedles" Biomimetics 9, no. 8: 469. https://doi.org/10.3390/biomimetics9080469
APA StyleHe, W., Kong, S., Lin, R., Xie, Y., Zheng, S., Yin, Z., Huang, X., Su, L., & Zhang, X. (2024). Machine Learning Assists in the Design and Application of Microneedles. Biomimetics, 9(8), 469. https://doi.org/10.3390/biomimetics9080469








