3D Spongin Scaffolds as Templates for Electro-Assisted Deposition of Selected Iron Oxides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
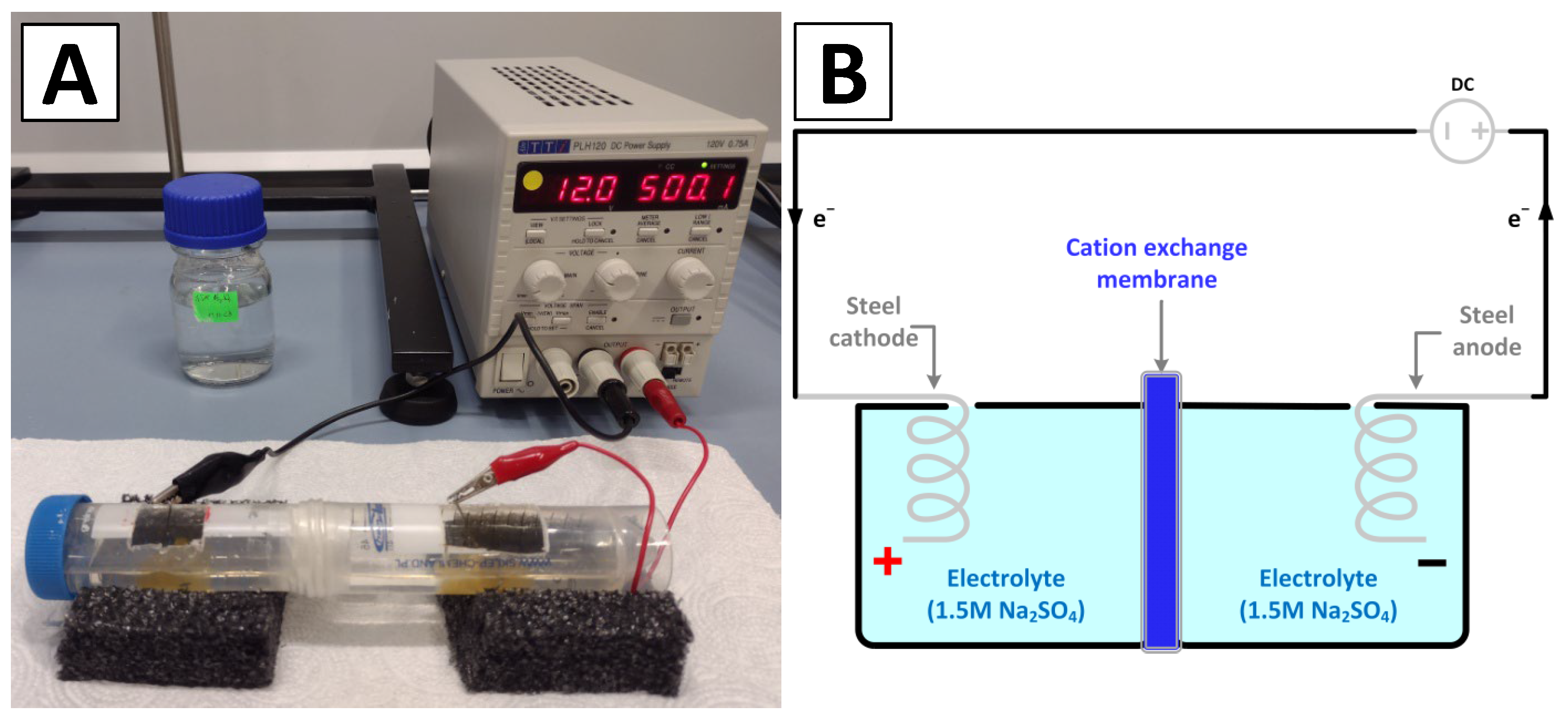
2.2. Electrolyzer Setup
2.3. Deposition of Iron Oxide
2.4. Characterization Techniques
2.4.1. Digital Microscopy
2.4.2. Scanning Electron Microscopy (SEM) with Energy-Dispersive X-ray Analysis (EDX)
2.4.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.4. X-ray Diffraction (XRD)
3. Results
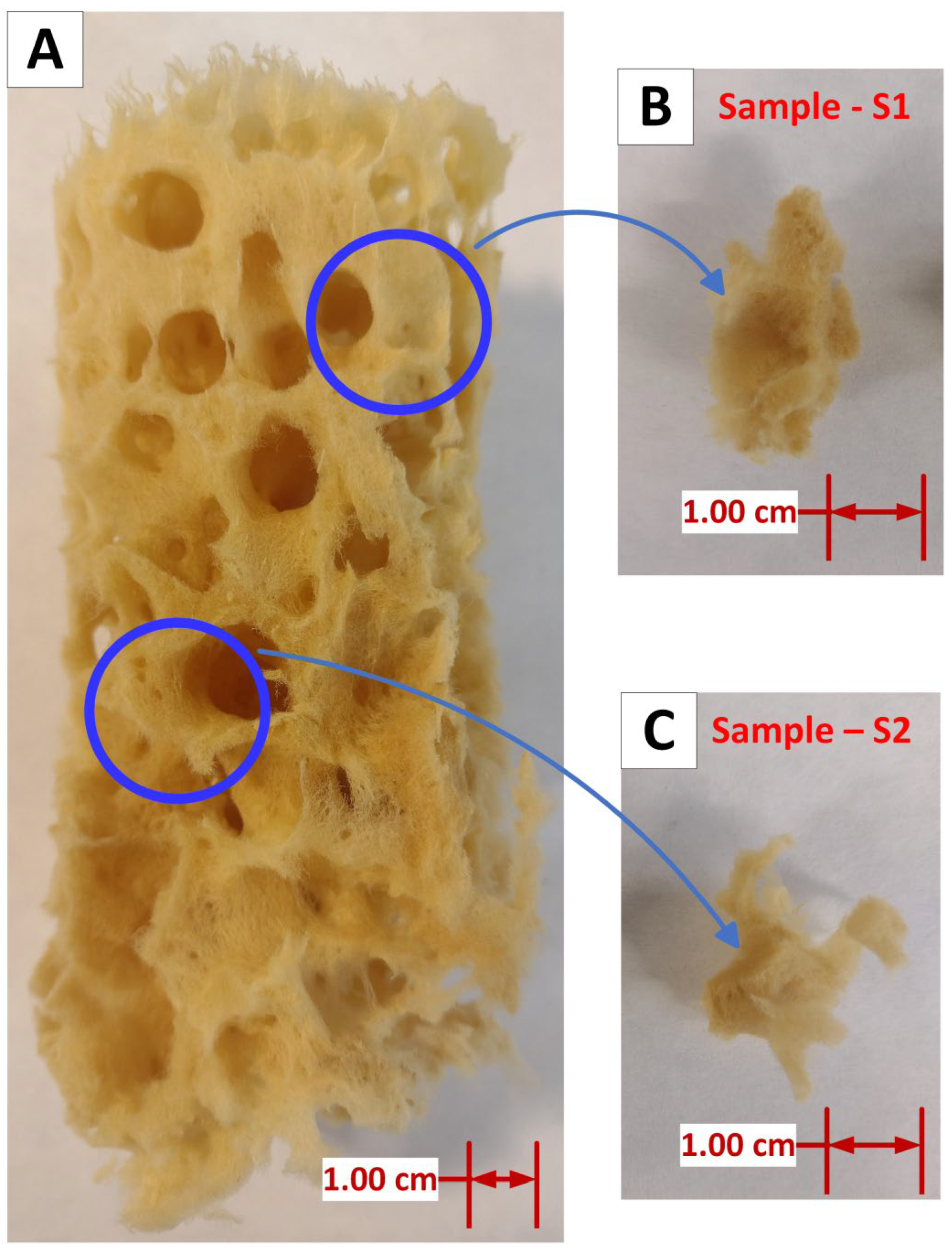
3.1. Morphology and Microstructure of Processed Spongin Scaffolds
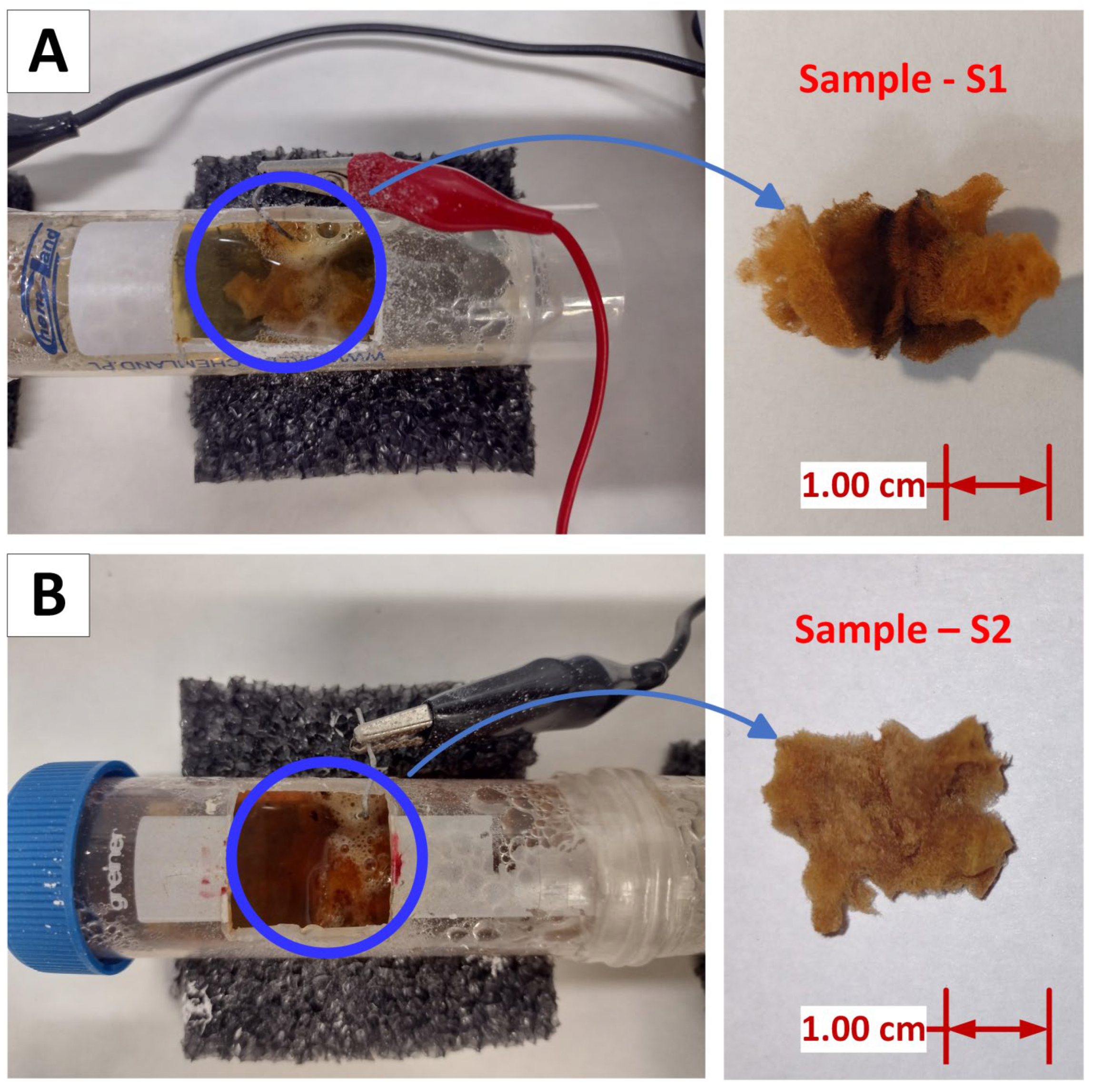
- The original spongin sample displays a yellowish, reticulated microfibrous network. In contrast, samples S1 (Figure 5B) and S2 (Figure 5D) exhibit a uniform rusty hue, which is consistent with the deposition of iron oxide closely associated with the organic matrix of the spongin fibers. The iron oxide deposition is visible as a cohesive surface layer, indicating a significant interaction between the oxide and the spongin components. Additionally, upon closer inspection of sample S1 (Figure 5C), a fiber with a fine, pale-yellow deposit is visible, suggesting a distinct oxide incorporation phase that survived even after sonication at room temperature for 1 h. The rust-colored deposit is most noticeable in sample S2, where the fibers are tightly compacted and show a texture that suggests greater oxide accumulation.
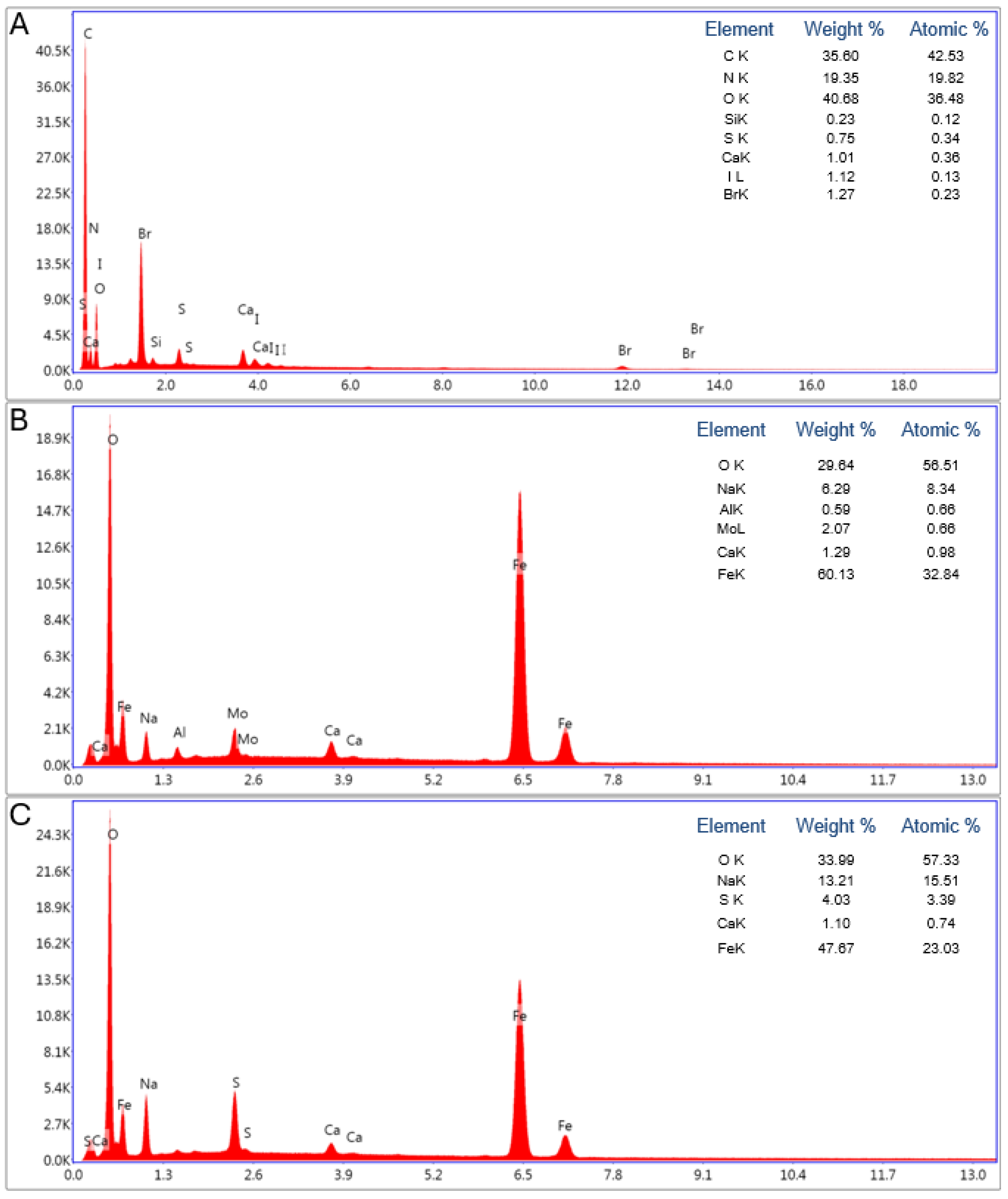
3.2. Chemical Composition of Samples
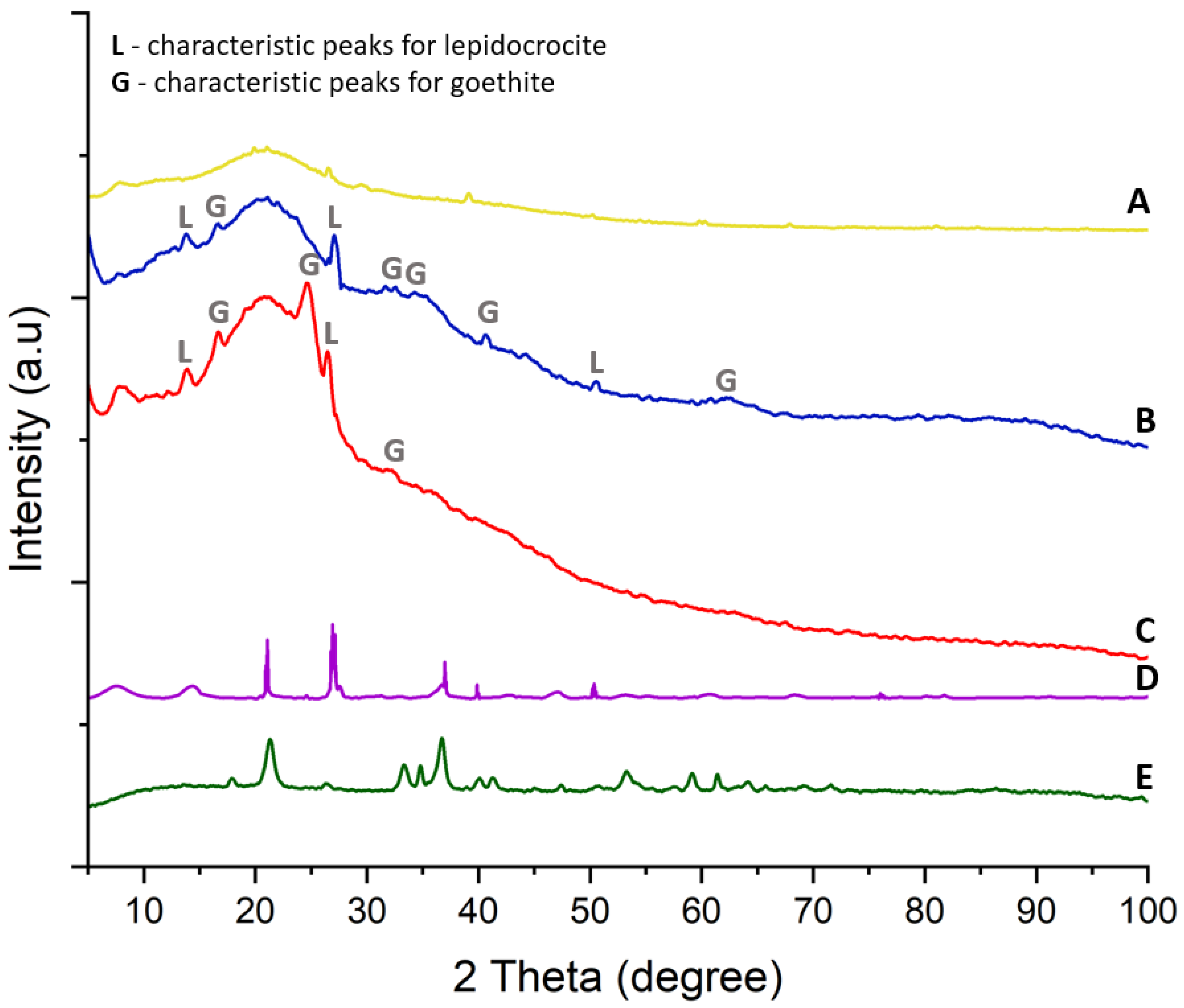
3.3. Characterization of the Crystalline Structure of the Iron Oxide–Hydroxide–Spongin Composites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tsurkan, D.; Wysokowski, M.; Petrenko, I.; Voronkina, A.; Khrunyk, Y.; Fursov, A.; Ehrlich, H. Modern Scaffolding Strategies Based on Naturally Pre-Fabricated 3D Biomaterials of Poriferan Origin. Appl. Phys. A 2020, 126, 382. [Google Scholar] [CrossRef]
- Entekhabi, E.; Nazarpak, M.H.; Shafieian, M.; Mohammadi, H.; Firouzi, M.; Hassannejad, Z. Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun polycaprolactone nanofibers for peripheral nerve regeneration. J. Biomed. Mater. Res. A 2020, 109, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, L.; Zhang, L. A Case Study in Natural Fibre Material (Luffa Sponge) Development Using E2-Material-Driven Design. Sustainability 2024, 16, 3490. [Google Scholar] [CrossRef]
- Saeed, A.; Iqbal, M. Loofa (Luffa cylindrica) Sponge: Review of Development of the Biomatrix as a Tool for Biotechnological Applications. Biotechnol. Prog. 2013, 29, 573–600. [Google Scholar] [CrossRef] [PubMed]
- Gurjar, A.K.; Kulkarni, S.M.; Joladarashi, S.; Doddamani, S. Investigation of Mechanical Properties of Luffa Fibre Reinforced Natural Rubber Composites: Implications of Process Parameters. J. Mater. Res. Technol. 2024, 29, 4232–4244. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Abdulkareem, S.A.; Adeyanju, C.A.; Abdulkareem, M.T.; Odimayomi, K.P.; Iwuozor, K.O.; Amoloye, M.A.; Belgore, R.O. Production and Properties of the Fibrillated Plastic Composite from Recycled Polystyrene and Luffa cylindrica. Polym. Bull. 2023, 80, 9569–9588. [Google Scholar] [CrossRef]
- Farooq, S.; Ahmad, M.I.; Zheng, S.; Ali, U.; Li, Y.; Shixiu, C.; Zhang, H. A Review on Marine Collagen: Sources, Extraction Methods, Colloids Properties, and Food Applications. Collagen Leather 2024, 6, 11. [Google Scholar] [CrossRef]
- Lagopati, N.; Pippa, N.; Gatou, M.-A.; Papadopoulou-Fermeli, N.; Gorgoulis, V.G.; Gazouli, M.; Pavlatou, E.A. Marine-Originated Materials and Their Potential Use in Biomedicine. Appl. Sci. 2023, 13, 9172. [Google Scholar] [CrossRef]
- Jesionowski, T.; Norman, M.; Zółtowska-Aksamitowska, S.; Petrenko, I.; Joseph, Y.; Ehrlich, H. Marine Spongin: Naturally Prefabricated 3D Scaffold-Based Biomaterial. Mar. Drugs 2018, 16, 88. [Google Scholar] [CrossRef]
- Alves, A.L.; Marques, C.F.; Almeida, M.; Pérez-Martín, R.I.; Reis, R.L.; Silva, T.H. Isolation and Characterization of Marine-Derived Collagens and Gelatins in the Perspective of Biomedical Application. In Handbook of the Extracellular Matrix; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–28. [Google Scholar]
- Ehrlich, H.; Wysokowski, M.; Zółtowska-Aksamitowska, S.; Petrenko, I.; Jesionowski, T. Collagens of Poriferan Origin. Mar. Drugs 2018, 16, 79. [Google Scholar] [CrossRef]
- Kubiak, A.; Voronkina, A.; Pajewska-Szmyt, M.; Kotula, M.; Leśniewski, B.; Ereskovsky, A.; Heimler, K.; Rogoll, A.; Vogt, C.; Rahimi, P.; et al. Creation of a 3D Goethite–Spongin Composite Using an Extreme Biomimetics Approach. Biomimetics 2023, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Szatkowski, T.; Wysokowski, M.; Lota, G.; Pęziak, D.; Bazhenov, V.V.; Nowaczyk, G.; Walter, J.; Molodtsov, S.L.; Stöcker, H.; Himcinschi, C.; et al. Novel Nanostructured Hematite-Spongin Composite Developed Using an Extreme Biomimetic Approach. RSC Adv. 2015, 5, 79031–79040. [Google Scholar] [CrossRef]
- Tsurkan, D.; Simon, P.; Schimpf, C.; Motylenko, M.; Rafaja, D.; Roth, F.; Inosov, D.S.; Makarova, A.A.; Stepniak, I.; Petrenko, I.; et al. Extreme Biomimetics: Designing of the First Nanostructured 3D Spongin–Atacamite Composite and Its Application. Adv. Mater. 2021, 33, 53. [Google Scholar] [CrossRef] [PubMed]
- Szatkowski, T.; Kopczyński, K.; Motylenko, M.; Borrmann, H.; Mania, B.; Graś, M.; Lota, G.; Bazhenov, V.V.; Rafaja, D.; Roth, F.; et al. Extreme Biomimetics: A Carbonized 3D Spongin Scaffold as a Novel Support for Nanostructured Manganese Oxide(IV) and Its Electrochemical Applications. Nano Res. 2018, 11, 4199–4214. [Google Scholar] [CrossRef]
- Szatkowski, T.; Jesionowski, T. Hydrothermal Synthesis of Spongin-Based Materials. In Extreme Biomimetics; Ehrlich, H., Ed.; Springer: Cham, Switzerland, 2017; pp. 241–274. [Google Scholar]
- Meenatchi, T.; Subadevi, R.; Kumar, P.; Raghu, S.; Liu, W.-R.; Sivakumar, M. An Impact of Sea Sponge-Derived Hard Carbon with the Symbiosis of Sodium Ion Battery and Biomedical Applications. J. Taiwan Inst. Chem. Eng. 2024, 154, 105083. [Google Scholar] [CrossRef]
- Petrenko, I.; Summers, A.P.; Simon, P.; Zółtowska-Aksamitowska, S.; Motylenko, M.; Schimpf, C.; Rafaja, D.; Roth, F.; Kummer, K.; Brendler, E.; et al. Extreme Biomimetics: Preservation of Molecular Detail in Centimeter-Scale Samples of Biological Meshes Laid down by Sponges. Sci. Adv. 2019, 5, eaax2805. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Kotula, M.; Leśniewski, B.; Pajewska-Szmyt, M. Iron-Sponges Interrelations: From Biocorrosion to Nanostructured Biocomposites. Lett. Appl. NanoBioSci. 2022, 12, 64. [Google Scholar]
- Vedaprakash, L.; Senthilkumar, P.; Inbakandan, D.; Venkatesan, R. Marine Biofouling and Corrosion on Long-Term Behavior of Marine Structures. In A Treatise on Corrosion Science, Engineering and Technology; Kamachi Mudali, U., Subba Rao, T., Ningshen, S., Pillai, R.G., George, R.P., Sridhar, T.M., Eds.; Indian Institute of Metals Series; Springer: Singapore, 2022; pp. 447–466. [Google Scholar]
- Refait, P.; Memet, J.-B.; Bon, C.; Sabot, R.; Génin, J.-M.R. Formation of the Fe(II)–Fe(III) Hydroxysulphate Green Rust during Marine Corrosion of Steel. Corros. Sci. 2003, 45, 833–845. [Google Scholar] [CrossRef]
- Kubiak, A.; Pajewska-Szmyt, M.; Kotula, M.; Leśniewski, B.; Voronkina, A.; Rahimi, P.; Falahi, S.; Heimler, K.; Rogoll, A.; Vogt, C.; et al. Spongin as a Unique 3D Template for the Development of Functional Iron-Based Composites Using Biomimetic Approach In Vitro. Mar. Drugs 2023, 21, 460. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, H.; Hao, Z.; Yu, M.; Chen, X.; Chen, J. Electrodeposition of (Hydro)Oxides for an Oxygen Evolution Electrode. Chem. Sci. 2020, 11, 10614–10625. [Google Scholar] [CrossRef]
- Kale, M.B.; Borse, R.A.; Gomaa Abdelkader Mohamed, A.; Wang, Y. Electrocatalysts by Electrodeposition: Recent Advances, Synthesis Methods, and Applications in Energy Conversion. Adv. Funct. Mater. 2021, 31, 2101313. [Google Scholar] [CrossRef]
- Schubert, T.; Zein El Abedin, S.; Abbott, A.P.; Mckenzie, K.J.; Ryder, K.S.; Endres, F. Electrodeposition of Metals. In Electrodeposition from Ionic Liquids; Wiley: Hoboken, NJ, USA, 2008; pp. 83–123. [Google Scholar]
- Lee, S.A.; Yang, J.W.; Choi, S.; Jang, H.W. Nanoscale Electrodeposition: Dimension Control and 3D Conformality. Exploration 2021, 1, 20210012. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-S.; Ou, Y.-H.; Lin, Y.-P. Electrodeposition of Iron Oxide Nanorods on Carbon Nanofiber Scaffolds as an Anode Material for Lithium-Ion Batteries. Electrochim. Acta 2010, 55, 3240–3244. [Google Scholar] [CrossRef]
- Yoo, J.; Jeong, D.; Son, Y.; Choi, Y.; Lee, K. Electrochemical Strategies for the Formation of Iron Oxide Nanostructures: Enhancing Photoelectrochemical Performance for Energy and Environmental Applications. Sol. RRL 2024, 8, 2300909. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, P.; Chen, J.; Dang, X.; Hu, Y.; Ai, Y.; Zheng, D.; Chen, H. One-Step Controlled Electrodeposition of Iron-Based Binary Metal Organic Nanocomposite. Appl. Surf. Sci. 2020, 504, 144504. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Mani, V.; Chen, S.-M.; Viswanath, B.; Vasantha, V.S.; Govindasamy, M. Electrodeposition of Gold Nanoparticles on a Pectin Scaffold and Its Electrocatalytic Application in the Selective Determination of Dopamine. RSC Adv. 2014, 4, 55900–55907. [Google Scholar] [CrossRef]
- Huang, J.; Daryadel, S.; Minary-Jolandan, M. Low-Cost Manufacturing of Metal–Ceramic Composites through Electrodeposition of Metal into Ceramic Scaffold. ACS Appl. Mater. Interfaces 2019, 11, 4364–4372. [Google Scholar] [CrossRef]
- Ruiz-Trejo, E.; Atkinson, A.; Brandon, N.P. Metallizing Porous Scaffolds as an Alternative Fabrication Method for Solid Oxide Fuel Cell Anodes. J. Power Sources 2015, 280, 81–89. [Google Scholar] [CrossRef]
- Petrenko, I.; Bazhenov, V.V.; Galli, R.; Wysokowski, M.; Fromont, J.; Schupp, P.J.; Stelling, A.L.; Niederschlag, E.; Stöker, H.; Kutsova, V.Z.; et al. Chitin of Poriferan Origin and the Bioelectrometallurgy of Copper/Copper Oxide. Int. J. Biol. Macromol. 2017, 104, 1626–1632. [Google Scholar] [CrossRef]
- Domingues, E.M.; Gonçalves, G.; Henriques, B.; Pereira, E.; Marques, P.A.A.P. High Affinity of 3D Spongin Scaffold towards Hg(II) in Real Waters. J. Hazard. Mater. 2021, 407, 124807. [Google Scholar] [CrossRef]
- Betancur, A.F. Quantitative Approach in Iron Oxides and Oxyhydroxides by Vibrational Analysis. Opt. Pura Apl. 2012, 45, 269–275. [Google Scholar] [CrossRef]
- Namduri, H.; Nasrazadani, S. Quantitative Analysis of Iron Oxides Using Fourier Transform Infrared Spectrophotometry. Corros. Sci. 2008, 50, 2493–2497. [Google Scholar] [CrossRef]
- Fekry, M.; Shafek, S.H.; Soliman, F.S.; Bakry, A. Impact of Poly Naphthalene Sulfonate on the Dispersion Stability of Iron Oxide Nanoparticles. Egypt. J. Pet. 2023, 32, 23–32. [Google Scholar] [CrossRef]
- Zhu, M.; Hu, X.; Tu, C.; Zhang, H.; Song, F.; Luo, Y.; Christie, P. Sorption Mechanisms of Diphenylarsinic Acid on Ferrihydrite, Goethite and Hematite Using Sequential Extraction, FTIR Measurement and XAFS Spectroscopy. Sci. Total Environ. 2019, 669, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, C.; Liu, F.; Jing, D.; Dong, Y.; Wang, L.; Ren, L. Adhesion Effect and Mechanism of Siderophore-Producing Bacteria onto Goethite and Boron-Doped Goethite. Colloid. Interface Sci. Commun. 2022, 51, 100680. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, Z.; Wang, S.; Pan, Y.; Chen, N.; Tunc, A.; Cheung, K.; Alparov, A.; Chen, W.; Deevsalar, R.; et al. Iron(II)-Activated Phase Transformation of Cd-Bearing Ferrihydrite: Implications for Cadmium Mobility and Fate under Anaerobic Conditions. Sci. Total Environ. 2022, 848, 157719. [Google Scholar] [CrossRef]
- Święch, D.; Paluszkiewicz, C.; Piergies, N.; Pięta, E.; Lelek-Borkowska, U.; Kwiatek, W. Identification of Corrosion Products on Fe and Cu Metals Using Spectroscopic Methods. Acta Phys. Pol. A 2018, 133, 286–288. [Google Scholar] [CrossRef]
- Avram, S.E.; Filip, M.R.; Barbu Tudoran, L.; Borodi, G.; Petean, I. Investigation of Ferrous Conglomerate Particles Found in Carwash Slurry and Their Environmental Implications. Stud. UBB Chem. 2023, 68, 57–70. [Google Scholar] [CrossRef]
- Ristić, M.; Opačak, I.; Musić, S. The Synthesis and Microstructure of Goethite Particles Precipitated in Highly Alkaline Media. J. Alloys Compd. 2013, 559, 49–56. [Google Scholar] [CrossRef]
- Shao, Y.; Hu, G.; Liu, Z.; Xu, X.; Zhang, M.; Ding, C.; Li, Y. Determination of Band Structure of Naturally Occurring Goethite with Al Substitution: A Case Study of Zhushan Iron Zone. Materials 2022, 15, 1465. [Google Scholar] [CrossRef]
- Yu, H.; Guo, Z.; Liu, G.; Wang, L.; Guo, H.; Jin, R.; Zhou, J. Improved Microbial Reduction of Biogenic and Abiogenic Goethite by Diesel Soot. J. Hazard. Mater. 2023, 4, 100091. [Google Scholar] [CrossRef]
- Liu, A.; Liu, J.; Pan, B.; Zhang, W. Formation of Lepidocrocite (γ-FeOOH) from Oxidation of Nanoscale Zero-Valent Iron (NZVI) in Oxygenated Water. RSC Adv. 2014, 4, 57377–57382. [Google Scholar] [CrossRef]
- Zafar, F.; Bano, H.; Wahab, M.F.; Corvo, F. Mild Steel Corrosion Behavior in a Coastal Megacity Relevant to China Pakistan Economic Corridor. npj Mater. Degrad. 2023, 7, 37. [Google Scholar] [CrossRef]
- Yao, S.; Jin, B.; Liu, Z.; Shao, C.; Zhao, R.; Wang, X.; Tang, R. Biomineralization: From material tactics to biological strategy. Adv. Mater. 2017, 29, 1605903. [Google Scholar] [CrossRef]
- Ramanathan, G.; Jeyakumar, G.F.S.; Sivagnanam, U.T.; Fardim, P. Biomimetic cellulose/collagen/silk fibroin as a highly interconnected 3D hybrid matrix for bone tissue engineering. Process Biochem. 2023, 129, 150–158. [Google Scholar] [CrossRef]
- Tuck, B.; Leinecker, N.; Watkin, E.; Somers, A.; Forsyth, M.; Machuca, L.L. Efficiency of a Novel Multifunctional Corrosion Inhibitor Against Biofilms Developed on Carbon Steel. Front. Bioeng. Biotechnol. 2022, 10, 803559. [Google Scholar] [CrossRef]
- Vuong, P.; McKinley, A.; Kaur, P. Understanding Biofouling and Contaminant Accretion on Submerged Marine Structures. npj Mater. Degrad. 2023, 7, 50. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.; Fan, Y.; Xu, D. Microbially Influenced Corrosion of Steel in Marine Environments: A Review from Mechanisms to Prevention. Microorganisms 2023, 11, 2299. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Zhu, M.; Wei, Y.; Sun, Y. Fe(II)-Induced Transformation from Ferrihydrite to Lepidocrocite and Goethite. J. Solid State Chem. 2007, 180, 2121–2128. [Google Scholar] [CrossRef]
- Nowacki, K.; Galiński, M.; Fursov, A.; Voronkina, A.; Meissner, H.; Petrenko, I.; Stelling, A.L.; Ehrlich, H. Electrolysis as a Universal Approach for Isolation of Diverse Chitin Scaffolds from Selected Marine Demosponges. Mar. Drugs 2022, 20, 665. [Google Scholar] [CrossRef]
- Holze, S.; Jörissen, J.; Fischer, C.; Kalvelage, H. Hydrogen Consuming Anodes for Energy Saving in Sodium Sulphate Electrolysis. Chem. Eng. Technol. 1994, 17, 382–389. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, J.; Meng, L.; Liu, L. Efficient Hydrogen Production by Saline Water Electrolysis at High Current Densities without the Interfering Chlorine Evolution. J. Mater. Chem. A Mater. 2021, 9, 22248–22253. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, K.; Zhang, T.; Zhu, X. Electrolysis Designed for Clean Production of Selective Iron Products from Coal Fly Ash Leachate. Hydrometallurgy 2021, 203, 105617. [Google Scholar] [CrossRef]
- Gunawan, G.; Haris, A.; Prasetya, N.B.A.; Pratista, E.; Amrullah, A. Ferrate(VI) Synthesis Using Fe(OH) from Waste Iron Electrolysis and Its Application for the Removal of Metal Ions and Anions in Water. Indones. J. Chem. 2021, 21, 1397. [Google Scholar] [CrossRef]




| Pure Spongin | Sample S1 | Sample S2 | Vibrational Assignment |
|---|---|---|---|
| 3410 | 3408 | 3410 | –OH stretching |
| 3300 | 3300 | 3303 | –NH stretching |
| - | 3143 | 3140 | –OH stretching |
| 2931 | 2929 | 2933 | –CH2, –CH3 stretching |
| 1633 | 1633 | 1633 | C=O stretching |
| 1536 | 1536 | 1537 | –NH deformational |
| 1244 | 1245 | 1244 | C–N stretching |
| - | 1150 | - | –OH deformational |
| 1030 | - | - | C–O stretching |
| - | 1021 | - | Fe–OH |
| - | 890 | 890 | –OH bending |
| - | 794 | 794 | –OH bending |
| - | 742 | - | –OH deformational |
| - | 635 | 632 | Fe–O stretching |
| - | 570 | - | Fe–O stretching |
| 472 | 460 | 462 | N–H stretching |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacki, K.; Kubiak, A.; Nowicki, M.; Tsurkan, D.; Ehrlich, H.; Jesionowski, T. 3D Spongin Scaffolds as Templates for Electro-Assisted Deposition of Selected Iron Oxides. Biomimetics 2024, 9, 387. https://doi.org/10.3390/biomimetics9070387
Nowacki K, Kubiak A, Nowicki M, Tsurkan D, Ehrlich H, Jesionowski T. 3D Spongin Scaffolds as Templates for Electro-Assisted Deposition of Selected Iron Oxides. Biomimetics. 2024; 9(7):387. https://doi.org/10.3390/biomimetics9070387
Chicago/Turabian StyleNowacki, Krzysztof, Anita Kubiak, Marek Nowicki, Dmitry Tsurkan, Hermann Ehrlich, and Teofil Jesionowski. 2024. "3D Spongin Scaffolds as Templates for Electro-Assisted Deposition of Selected Iron Oxides" Biomimetics 9, no. 7: 387. https://doi.org/10.3390/biomimetics9070387
APA StyleNowacki, K., Kubiak, A., Nowicki, M., Tsurkan, D., Ehrlich, H., & Jesionowski, T. (2024). 3D Spongin Scaffolds as Templates for Electro-Assisted Deposition of Selected Iron Oxides. Biomimetics, 9(7), 387. https://doi.org/10.3390/biomimetics9070387











