Remote Wearable Neuroimaging Devices for Health Monitoring and Neurophenotyping: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Identifying the Research Question
- What health conditions are studied remotely with wearable neuroimaging devices?
- Which wearable neuroimaging devices are prevalent in remote health studies?
- How does remotely collected neuroimaging data quality compare to that provided by collecting neuroimaging data using traditional/in-person clinical-setting devices?
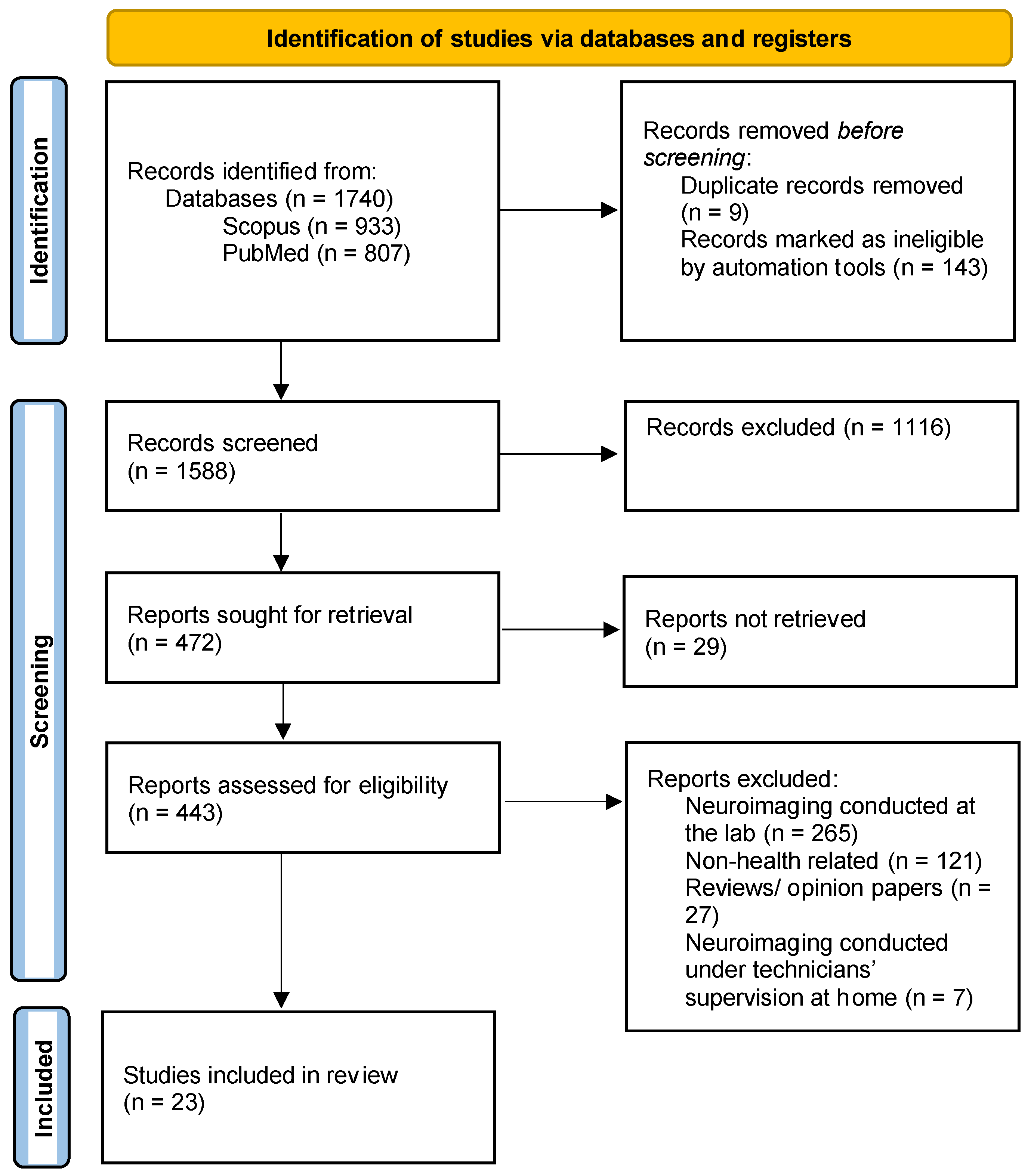
2.2. Identify Relevant Studies
2.3. Selection of Eligible Studies
- Related to a healthcare application;
- Full text was available;
- Concerned non-invasive neuroimaging using EEG, fNIRS, or PPG;
- Self-applied, unattended neuroimaging conducted outside clinics and research centers.
2.4. Data Charting
- The health conditions tracked using remote neuroimaging;
- Number of participants who participated in remote neuroimaging monitoring;
- Remote neuroimaging procedures and findings;
- The types of devices used to collect neuroimaging data remotely;
- Notes on the usability of the devices, including barriers and facilitators of use;
- Did the study report a comparison between mobile neuroimaging data and neuroimaging data collected at a clinic and/or research center? If so, how did the quality of mobile neuroimaging data compare to that of clinical neuroimaging data?
2.5. Collating, Summarizing, and Reporting the Results
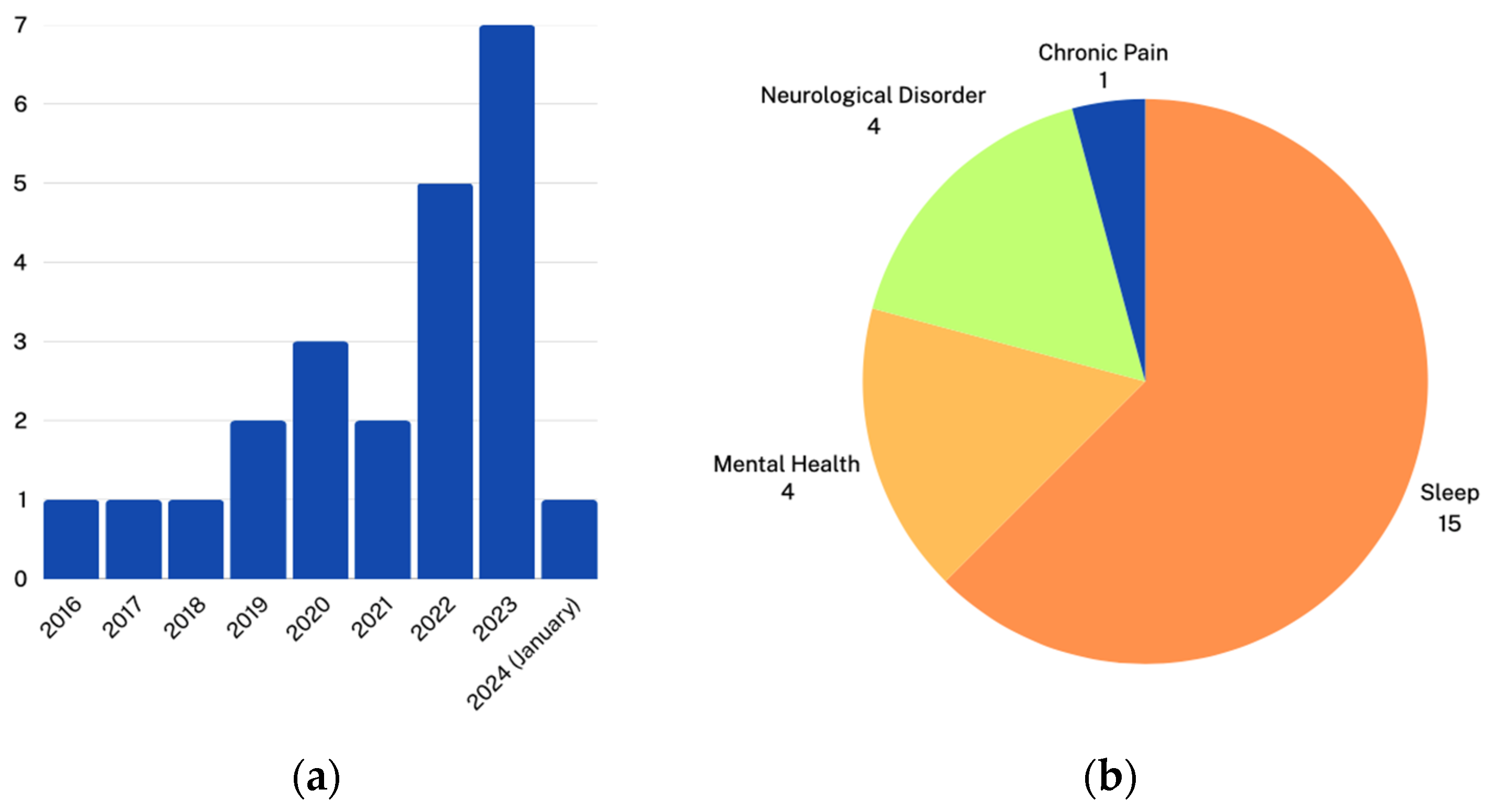
3. Results
3.1. Remote Monitoring Using Mobile EEG Devices
3.1.1. Neurological Disorders
3.1.2. Mental Health
3.1.3. Sleep Monitoring and Disorders
3.2. Remote Neurofeedback Interventions
3.3. Mobile Device Charastristicts
3.4. Quality of Mobile EEGs Compared to Clinical EEGs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onnela, J.P.; Rauch, S. Harnessing Smartphone-Based Digital Phenotyping to Enhance Behavioral and Mental Health. Neuropsychopharmacol 2016, 41, 1691–1696. [Google Scholar] [CrossRef]
- Mohr, D.C.; Zhang, M.; Schueller, S.M. Personal Sensing: Understanding Mental Health Using Ubiquitous Sensors and Machine Learning. Annu. Rev. Clin. Psychol. 2017, 13, 23–47. [Google Scholar] [CrossRef]
- Torous, J.; Onnela, J.P.; Keshavan, M. New dimensions and new tools to realize the potential of RDoC: Digital phenotyping via smartphones and connected devices. Transl. Psychiatry 2017, 7, e1053. [Google Scholar] [CrossRef]
- Insel, T.R. Digital Phenotyping: Technology for a New Science of Behavior. JAMA 2017, 318, 1215–1216. [Google Scholar] [CrossRef] [PubMed]
- Huckvale, K.; Venkatesh, S.; Christensen, H. Toward Clinical Digital Phenotyping: A Timely Opportunity to Consider Purpose, Quality, and Safety. NPJ Digit. Med. 2019, 2, 88. [Google Scholar] [CrossRef] [PubMed]
- Radonjić, N.V.; Hess, J.L.; Rovira, P.; Andreassen, O.; Buitelaar, J.K.; Ching, C.R.K.; Franke, B.; Hoogman, M.; Jahanshad, N.; McDonald, C.; et al. Structural Brain Imaging Studies Offer Clues about the Effects of the Shared Genetic Etiology among Neuropsychiatric Disorders. Mol. Psychiatry 2021, 26, 2101–2110. [Google Scholar] [CrossRef]
- Spear, L.P. Effects of Adolescent Alcohol Consumption on the Brain and Behaviour. Nat. Rev. Neurosci. 2018, 19, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, S.; Stone, A.A.; Hufford, M.R. Ecological Momentary Assessment. Annu. Rev. Clin. Psychol. 2008, 4, 1–32. [Google Scholar] [CrossRef]
- Pap, I.A.; Oniga, S. A Review of Converging Technologies in eHealth Pertaining to Artificial Intelligence. Int. J. Environ. Res. Public Health 2022, 19, 11413. [Google Scholar] [CrossRef]
- Pricillia, I.L.; Azhari, A. Electroencephalogram Detection for Insomnia Patients: A Preliminary Study. Biosaintifika: J. Biol. Biol. Educ. 2022, 14, 191–199. [Google Scholar] [CrossRef]
- Bosl, W.J. The Emerging Role of Neurodiagnostic Informatics in Integrated Neurological and Mental Health Care. Neurodiagn. J. 2018, 58, 143–153. [Google Scholar] [CrossRef]
- Shamay-Tsoory, S.G.; Mendelsohn, A. Real-Life Neuroscience: An Ecological Approach to Brain and Behavior Research. Perspect. Psychol. Sci. 2019, 14, 841–859. [Google Scholar] [CrossRef] [PubMed]
- Uchitel, J.; Vidal-Rosas, E.E.; Cooper, R.J.; Zhao, H. Wearable, Integrated EEG–fNIRS Technologies: A Review. Sensors 2021, 21, 6106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Q.-Q.; Chen, H.; Hu, X.-Q.; Li, W.-G.; Bai, Y.; Han, J.-X.; Wang, Y.; Liang, Z.-H.; Chen, D.; et al. The Applied Principles of EEG Analysis Methods in Neuroscience and Clinical Neurology. Mil. Med. Res. 2023, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, M. A Review of Electroencephalogram Signal Processing Methods for Brain-Controlled Robots. Cogn. Robot. 2021, 1, 111–124. [Google Scholar] [CrossRef]
- MuseTM EEG-Powered Meditation & Sleep Headband. Available online: https://choosemuse.com/ (accessed on 2 April 2024).
- EPOC+. Available online: https://www.emotiv.com/products/epoc (accessed on 2 April 2024).
- MindWave. Available online: https://store.neurosky.com/pages/mindwave (accessed on 2 April 2024).
- Ferrari, M.; Quaresima, V. A Brief Review on the History of Human Functional Near-Infrared Spectroscopy (fNIRS) Development and Fields of Application. NeuroImage 2012, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Mendi.Io—Better Focus, Better Performance. Available online: https://www.mendi.io/ (accessed on 3 April 2024).
- Kernel. Home. Available online: https://www.kernel.com/ (accessed on 3 April 2024).
- Allen, J. Photoplethysmography and Its Application in Clinical Physiological Measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, B.; Bullón-Tarrasó, E.; Pöhlchen, D.; Manafis, A.; Neumayer, H.; Besedovsky, L.; Brückl, T.; Group, B.W.; Simor, P.; Binder, F.P.; et al. The Utility of Wearable Headband Electroencephalography and Pulse Photoplethysmography to Assess Cortical and Physiological Arousal in Individuals with Stress-Related Mental Disorders. J. Sleep Res. 2023, 15, e14123. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.N.; Hani, A.J.; Cheek, J.; Thirumala, P.; Tsuchida, T.N. American Clinical Neurophysiology Society Guideline 2: Guidelines for Standard Electrode Position Nomenclature. Neurodiagn. J. 2016, 56, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zimeo Morais, G.A.; Balardin, J.B.; Sato, J.R. fNIRS Optodes’ Location Decider (fOLD): A Toolbox for Probe Arrangement Guided by Brain Regions-of-Interest. Sci. Rep. 2018, 8, 3341. [Google Scholar] [CrossRef]
- Hinrichs, H.; Scholz, M.; Baum, A.K.; Kam, J.W.Y.; Knight, R.T.; Heinze, H.-J. Comparison between a Wireless Dry Electrode EEG System with a Conventional Wired Wet Electrode EEG System for Clinical Applications. Sci. Rep. 2020, 10, 5218. [Google Scholar] [CrossRef]
- Ekandem, J.I.; Davis, T.A.; Alvarez, I.; James, M.T.; Gilbert, J.E. Evaluating the Ergonomics of BCI Devices for Research and Experimentation. Ergonomics 2012, 55, 592–598. [Google Scholar] [CrossRef]
- Radhakrishnan, B.L.; Kirubakaran, E.; Jebadurai, I.J.; Selvakumar, A.I.; Peter, J.D. Efficacy of Single-Channel EEG: A Propitious Approach for In-Home Sleep Monitoring. Front. Public Health 2022, 10, 839838. [Google Scholar] [CrossRef]
- Marzbani, H.; Marateb, H.R.; Mansourian, M. Neurofeedback: A Comprehensive Review on System Design, Methodology and Clinical Applications. Basic Clin. Neurosci. 2016, 7, 143–158. [Google Scholar] [CrossRef]
- Woo, C.-W.; Chang, L.J.; Lindquist, M.A.; Wager, T.D. Building Better Biomarkers: Brain Models in Translational Neuroimaging. Nat. Neurosci. 2017, 20, 365–377. [Google Scholar] [CrossRef]
- Ratti, E.; Waninger, S.; Berka, C.; Ruffini, G.; Verma, A. Comparison of Medical and Consumer Wireless EEG Systems for Use in Clinical Trials. Front. Hum. Neurosci. 2017, 11, 398. [Google Scholar] [CrossRef]
- von Lühmann, A.; Zheng, Y.; Ortega-Martinez, A.; Kiran, S.; Somers, D.C.; Cronin-Golomb, A.; Awad, L.N.; Ellis, T.D.; Boas, D.A.; Yücel, M.A. Towards Neuroscience of the Everyday World (NEW) Using Functional Near-Infrared Spectroscopy. Curr. Opin. Biomed. Eng. 2021, 18, 100272. [Google Scholar] [CrossRef]
- Sugden, R.J.; Pham-Kim-Nghiem-Phu, V.-L.L.; Campbell, I.; Leon, A.; Diamandis, P. Remote Collection of Electrophysiological Data with Brain Wearables: Opportunities and Challenges. Bioelectron. Med. 2023, 9, 12. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping Studies: Advancing the Methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Campanella, S.; Arikan, K.; Babiloni, C.; Balconi, M.; Bertollo, M.; Betti, V.; Bianchi, L.; Brunovsky, M.; Buttinelli, C.; Comani, S.; et al. Special Report on the Impact of the COVID-19 Pandemic on Clinical EEG and Research and Consensus Recommendations for the Safe Use of EEG. Clin. EEG Neurosci. 2021, 52, 3–28. [Google Scholar] [CrossRef]
- Faezipour, M.; Faezipour, M. Efficacy of Smart EEG Monitoring Amidst the COVID-19 Pandemic. Electronics 2021, 10, 1001. [Google Scholar] [CrossRef]
- Jørgensen, S.D.; Kidmose, P.; Mikkelsen, K.; Blech, M.; Hemmsen, M.C.; Rank, M.L.; Kjaer, T.W. Long-Term Ear-EEG Monitoring of Sleep—A Case Study during Shift Work. J. Sleep Res. 2023, 32, e13853. [Google Scholar] [CrossRef]
- Chouraki, A.; Tournant, J.; Arnal, P.; Pépin, J.-L.; Bailly, S. Objective Multi-Night Sleep Monitoring at Home: Variability of Sleep Parameters between Nights and Implications for the Reliability of Sleep Assessment in Clinical Trials. Sleep 2023, 46, zsac319. [Google Scholar] [CrossRef]
- Sidelinger, L.; Zhang, M.; Frohlich, F.; Daughters, S.B. Day-to-Day Individual Alpha Frequency Variability Measured by a Mobile EEG Device Relates to Anxiety. Eur. J. Neurosci. 2023, 57, 1815–1833. [Google Scholar] [CrossRef]
- Lim, C.G.; Soh, C.P.; Lim, S.S.Y.; Fung, D.S.S.; Guan, C.; Lee, T.-S. Home-Based Brain–Computer Interface Attention Training Program for Attention Deficit Hyperactivity Disorder: A Feasibility Trial. Child Adolesc. Psychiatry Ment. Health 2023, 17, 15. [Google Scholar] [CrossRef]
- Hunkin, H.; King, D.L.; Zajac, I.T. EEG Neurofeedback During Focused Attention Meditation: Effects on State Mindfulness and Meditation Experiences. Mindfulness 2021, 12, 841–851. [Google Scholar] [CrossRef]
- Grandner, M.A.; Bromberg, Z.; Hadley, A.; Morrell, Z.; Graf, A.; Hutchison, S.; Freckleton, D. Performance of a Multisensor Smart Ring to Evaluate Sleep: In-Lab and Home-Based Evaluation of Generalized and Personalized Algorithms. Sleep 2023, 46, zsac152. [Google Scholar] [CrossRef]
- Punjabi, N.M.; Brown, T.; Aurora, R.N.; Patel, S.R.; Stosor, V.; Cho, J.H.; Helgadóttir, H.; Ágústsson, J.S.; D’Souza, G.; Margolick, J.B. Methods for home-based self-applied polysomnography: The Multicenter AIDS Cohort Study. Sleep Adv. 2022, 3, zpac011. [Google Scholar] [CrossRef]
- Kober, S.E.; Pinter, D.; Enzinger, C.; Damulina, A.; Duckstein, H.; Fuchs, S.; Neuper, C.; Wood, G. Self-Regulation of Brain Activity and Its Effect on Cognitive Function in Patients with Multiple Sclerosis—First Insights from an Interventional Study Using Neurofeedback. Clin. Neurophysiol. 2019, 130, 2124–2131. [Google Scholar] [CrossRef]
- Liang, Z.; Chapa Martell, M.A. Validity of Consumer Activity Wristbands and Wearable EEG for Measuring Overall Sleep Parameters and Sleep Structure in Free-Living Conditions. J. Health. Inf. Res. 2018, 2, 152–178. [Google Scholar] [CrossRef]
- Toedebusch, C.D.; McLeland, J.S.; Schaibley, C.M.; Banks, I.R.; Boyd, J.; Morris, J.C.; Holtzman, D.M.; Lucey, B.P. Multi-Modal Home Sleep Monitoring in Older Adults. J. Vis. Exp. 2019, 143, e58823. [Google Scholar] [CrossRef]
- Krepel, N.; Egtberts, T.; Touré-Cuq, E.; Bouny, P.; Arns, M. Evaluation of the URGOnight Tele-Neurofeedback Device: An Open-Label Feasibility Study with Follow-Up. Appl. Psychophysiol. Biofeedback 2022, 47, 43–51. [Google Scholar] [CrossRef]
- Pulver, R.L.; Kronberg, E.; Medenblik, L.M.; Kheyfets, V.O.; Ramos, A.R.; Holtzman, D.M.; Morris, J.C.; Toedebusch, C.D.; Sillau, S.H.; Bettcher, B.M.; et al. Mapping Sleep’s Oscillatory Events as a Biomarker of Alzheimer’s Disease. Alzheimers Dement. 2024, 20, 301–315. [Google Scholar] [CrossRef]
- Morssinkhof, M.W.L.; van der Werf, Y.D.; van den Heuvel, O.A.; van den Ende, D.A.; van der Tuuk, K.; den Heijer, M.; Broekman, B.F.P. Influence of Sex Hormone Use on Sleep Architecture in a Transgender Cohort. Sleep 2023, 46, zsad249. [Google Scholar] [CrossRef]
- Tabar, Y.R.; Mikkelsen, K.B.; Shenton, N.; Kappel, S.L.; Bertelsen, A.R.; Nikbakht, R.; Toft, H.O.; Henriksen, C.H.; Hemmsen, M.C.; Rank, M.L.; et al. At-Home Sleep Monitoring Using Generic Ear-EEG. Front. Neurosci. 2023, 17, 987578. [Google Scholar] [CrossRef]
- da Silva Souto, C.F.; Pätzold, W.; Paul, M.; Debener, S.; Wolf, K.I. Pre-Gelled Electrode Grid for Self-Applied EEG Sleep Monitoring at Home. Front. Neurosci. 2022, 16, 883966. [Google Scholar] [CrossRef]
- Elbogen, E.B.; Alsobrooks, A.; Battles, S.; Molloy, K.; Dennis, P.A.; Beckham, J.C.; McLean, S.A.; Keith, J.R.; Russoniello, C. Mobile Neurofeedback for Pain Management in Veterans with TBI and PTSD. Pain Med. 2021, 22, 329–337. [Google Scholar] [CrossRef]
- Onton, J.A.; Kang, D.Y.; Coleman, T.P. Visualization of Whole-Night Sleep EEG From 2-Channel Mobile Recording Device Reveals Distinct Deep Sleep Stages with Differential Electrodermal Activity. Front. Hum. Neurosci. 2016, 10, 605. [Google Scholar] [CrossRef]
- Leach, S.; Chung, K.; Tüshaus, L.; Huber, R.; Karlen, W. A Protocol for Comparing Dry and Wet EEG Electrodes During Sleep. Front. Neurosci. 2020, 14, 586. [Google Scholar] [CrossRef]
- Musaeus, C.S.; Waldemar, G.; Andersen, B.B.; Høgh, P.; Kidmose, P.; Hemmsen, M.C.; Rank, M.L.; Kjær, T.W.; Frederiksen, K.S. Long-Term EEG Monitoring in Patients with Alzheimer’s Disease Using Ear-EEG: A Feasibility Study. J. Alzheimers Dis. 2022, 90, 1713–1723. [Google Scholar] [CrossRef]
- Debellemaniere, E.; Chambon, S.; Pinaud, C.; Thorey, V.; Dehaene, D.; Léger, D.; Chennaoui, M.; Arnal, P.J.; Galtier, M.N. Performance of an Ambulatory Dry-EEG Device for Auditory Closed-Loop Stimulation of Sleep Slow Oscillations in the Home Environment. Front. Hum. Neurosci. 2018, 12, 88. [Google Scholar] [CrossRef]
- Mentink, L.J.; Thomas, J.; Melis, R.J.F.; Olde Rikkert, M.G.M.; Overeem, S.; Claassen, J.A.H.R. Home-EEG Assessment of Possible Compensatory Mechanisms for Sleep Disruption in Highly Irregular Shift Workers—The ANCHOR Study. PLoS ONE 2020, 15, e0237622. [Google Scholar] [CrossRef]
- Levendowski, D.J.; Ferini-Strambi, L.; Gamaldo, C.; Cetel, M.; Rosenberg, R.; Westbrook, P.R. The Accuracy, Night-to-Night Variability, and Stability of Frontopolar Sleep Electroencephalography Biomarkers. J. Clin. Sleep Med. 2017, 13, 791–803. [Google Scholar] [CrossRef]
- Birch, N.; Graham, J.; Ozolins, C.; Kumarasinghe, K.; Almesfer, F. Home-Based EEG Neurofeedback Intervention for the Management of Chronic Pain. Front. Pain Res. 2022, 3, 855493. [Google Scholar] [CrossRef]
- Attar, E.T. Review of Electroencephalography Signals Approaches for Mental Stress Assessment. Neurosciences 2022, 27, 209–215. [Google Scholar] [CrossRef]
- Irani, F.; Platek, S.M.; Bunce, S.; Ruocco, A.C.; Chute, D. Functional near Infrared Spectroscopy (fNIRS): An Emerging Neuroimaging Technology with Important Applications for the Study of Brain Disorders. Clin. Neuropsychol. 2007, 21, 9–37. [Google Scholar] [CrossRef]
- Kohl, S.H.; Mehler, D.M.A.; Lührs, M.; Thibault, R.T.; Konrad, K.; Sorger, B. The Potential of Functional Near-Infrared Spectroscopy-Based Neurofeedback—A Systematic Review and Recommendations for Best Practice. Front. Neurosci. 2020, 14, 594. [Google Scholar] [CrossRef]
- Li, R.; Yang, D.; Fang, F.; Hong, K.-S.; Reiss, A.L.; Zhang, Y. Concurrent fNIRS and EEG for Brain Function Investigation: A Systematic, Methodology-Focused Review. Sensors 2022, 22, 5865. [Google Scholar] [CrossRef]
- Lee, S.-H.; Hwang, H.-H.; Kim, S.; Hwang, J.; Park, J.; Park, S. Clinical Implication of Maumgyeol Basic Service–the 2 Channel Electroencephalography and a Photoplethysmogram–Based Mental Health Evaluation Software. Clin. Psychopharmacol Neurosci 2023, 21, 583–593. [Google Scholar] [CrossRef]
- Faust, O.; Hagiwara, Y.; Hong, T.J.; Lih, O.S.; Acharya, U.R. Deep Learning for Healthcare Applications Based on Physiological Signals: A Review. Comput. Methods Programs Biomed. 2018, 161, 1–13. [Google Scholar] [CrossRef]
- Mandekar, S.; Holland, A.; Thielen, M.; Behbahani, M.; Melnykowycz, M. Advancing towards Ubiquitous EEG, Correlation of In-Ear EEG with Forehead EEG. Sensors 2022, 22, 1568. [Google Scholar] [CrossRef]
- Chiba, T.; Kanazawa, T.; Koizumi, A.; Ide, K.; Taschereau-Dumouchel, V.; Boku, S.; Hishimoto, A.; Shirakawa, M.; Sora, I.; Lau, H.; et al. Current Status of Neurofeedback for Post-Traumatic Stress Disorder: A Systematic Review and the Possibility of Decoded Neurofeedback. Front. Hum. Neurosci. 2019, 13, 233. [Google Scholar] [CrossRef]
- Barth, B.; Mayer-Carius, K.; Strehl, U.; Wyckoff, S.N.; Haeussinger, F.B.; Fallgatter, A.J.; Ehlis, A.-C. A Randomized-Controlled Neurofeedback Trial in Adult Attention-Deficit/Hyperactivity Disorder. Sci. Rep. 2021, 11, 16873. [Google Scholar] [CrossRef]
- Islam, M.K.; Rastegarnia, A.; Yang, Z. Methods for Artifact Detection and Removal from Scalp EEG: A Review. Neurophysiol. Clin. Clin. Neurophysiol. 2016, 46, 287–305. [Google Scholar] [CrossRef]
- Gao, Z.; Cui, X.; Wan, W.; Qin, Z.; Gu, Z. Signal Quality Investigation of a New Wearable Frontal Lobe EEG Device. Sensors 2022, 22, 1898. [Google Scholar] [CrossRef]
- Radüntz, T. Signal Quality Evaluation of Emerging EEG Devices. Front. Physiol. 2018, 9, 98. [Google Scholar] [CrossRef]
| Database | Keywords |
|---|---|
| PubMed/Scopus | (“Neuroimaging Devices” OR EEG OR fNIRS OR PPG OR “brain recording” OR “brain monitoring” OR neurosensory) AND (wearable OR continuous OR remote OR wireless OR headband OR “home-based” OR “Home environment” OR “home monitoring” OR “Consumer-Grade” OR Portable OR Mobile) AND (“Healthcare Applications” OR “Mental Health” OR “Neurological Disorders” OR “Psychiatric Diseases” OR “Sleep Disorders”) |
| Article Title | Condition or Physiological Measurement | Category of the Condition | Number of Participants * | Reference |
|---|---|---|---|---|
| Long-term ear-EEG monitoring of sleep—A case study during shift work | Sleep stage classification | Sleep motoring | 1 | [38] |
| Objective multi-night sleep monitoring at home: variability of sleep parameters between nights and implications for the reliability of sleep assessment in clinical trials | Sleep stage variability | Sleep motoring | 94 | [39] |
| Day-to-day individual alpha frequency variability measured by a mobile EEG device relates to anxiety | Anxiety | Mental health | 18 | [40] |
| Home-based brain–computer interface attention training program for attention deficit hyperactivity disorder: a feasibility trial | ADHD | Mental health | 10 | [41] |
| EEG neurofeedback during focused attention meditation: Effects on state mindfulness and Meditation Experiences | Mindfulness | Mental health | 29 | [42] |
| Performance of a multisensor smart ring to evaluate sleep: in-lab and home-based evaluation of generalized and personalized algorithms | Sleep stage classification | Sleep motoring | 36 | [43] |
| Methods for home-based self-Applied polysomnography: The Multicenter AIDS Cohort Study | Sleep abnormalities in HIV patients | Sleep motoring | 960 | [44] |
| Self-regulation of brain activity and its effect on cognitive function in patients with multiple sclerosis—First insights from an interventional study using neurofeedback | Multiple Sclerosis | Neurological disorder | 14 | [45] |
| Validity of Consumer Activity Wristbands and Wearable EEG for Measuring Overall Sleep Parameters and Sleep Structure in Free-Living Conditions | Sleep stage classification | Sleep motoring | 25 | [46] |
| Multi-modal home sleep monitoring in older adults | Sleep motoring | Sleep motoring | 29 | [47] |
| Evaluation of the URGOnight Tele-neurofeedback Device: An Open-label Feasibility Study with Follow-up. | Sleep quality | Sleep disorders | 37 | [48] |
| Mapping sleep’s oscillatory events as a biomarker of Alzheimer’s disease. | Alzheimer’s Disease | Neurological disorder | 205 | [49] |
| Influence of sex hormone use on sleep architecture in a transgender cohort. | Sleep quality | Sleep disorders | 73 | [50] |
| At-home sleep monitoring using generic ear-EEG | Sleep stage classification | Sleep motoring | 10 | [51] |
| Pre-gelled Electrode Grid for Self-Applied EEG Sleep Monitoring at Home | Sleep stage classification | Sleep motoring | 12 | [52] |
| Mobile Neurofeedback for Pain Management in Veterans with TBI and PTSD | Depression, anger, sleep disturbance, suicidal ideation, and chronic pain | Mental health | 36 | [53] |
| Visualization of whole-night sleep EEG from 2-channel mobile recording device reveals distinct deep sleep stages with differential electrodermal activity | Sleep stage classification | Sleep motoring | 51 | [54] |
| A Protocol for Comparing Dry and Wet EEG Electrodes During Sleep. | Sleep stage classification | Sleep motoring | 4 | [55] |
| Long-Term EEG Monitoring in Patients with Alzheimer’s Disease Using Ear-EEG: A Feasibility Study | Alzheimer’s Disease | Neurological disorder | 10 | [56] |
| Performance of an Ambulatory Dry-EEG Device for Auditory Closed-Loop Stimulation of Sleep Slow Oscillations in the Home Environment | Sleep quality | Sleep disorders | 90 | [57] |
| Home-EEG assessment of possible compensatory mechanisms for sleep disruption in highly irregular shift workers—The ANCHOR study | Sleep quality | Sleep disorders | 10 | [58] |
| The Accuracy, Night-to-Night Variability, and Stability of Frontopolar Sleep Electroencephalography Biomarkers | Sleep monitoring | Sleep motoring | 63 | [59] |
| Home-Based EEG Neurofeedback Intervention for the Management of Chronic Pain | Chronic Pain, depression, anxiety, and quality of life score | Pain management | 16 | [60] |
| Device Type | Number of Studies |
|---|---|
| EEG headset | 12 |
| Ear-EEG | 3 |
| Medical-grade device (EEG) | 3 |
| Single-channel EEG | 2 |
| EEG mask | 1 |
| 2-channel EEG | 1 |
| Data Quality Test | Data Quality Assessment | Gold-Standard Data | Reference |
|---|---|---|---|
| Bayesian t-test on spectral correlations | High correlation for TP9 and TP10 channels. Low correlation for AF7 and AF8 channels. | HD-EEG data collected at the clinic | [40] |
| Agreement in sleep stage classification | High (Cohen’s Kappa = 0.72) | Scalpe EEG collected at home | [38] |
| Linear mixed model on sleep stage classification | No statically significant difference was found between types of equipment. | PSG data collected at home | [51] |
| Agreement in sleep stage classification | Moderate to high (Cohen’s Kappa = 0.58 to 0.83) | PSG data collected at the clinic | [52] |
| Agreement in sleep stage classification | High agreement between expert-reviewed PSG and automatically detected sleep stages from EEG data (higher than 80% for all sleep stages except N1, with a mean Cohen’s Kappa = 0.67) | PSG data collected at the clinic | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emish, M.; Young, S.D. Remote Wearable Neuroimaging Devices for Health Monitoring and Neurophenotyping: A Scoping Review. Biomimetics 2024, 9, 237. https://doi.org/10.3390/biomimetics9040237
Emish M, Young SD. Remote Wearable Neuroimaging Devices for Health Monitoring and Neurophenotyping: A Scoping Review. Biomimetics. 2024; 9(4):237. https://doi.org/10.3390/biomimetics9040237
Chicago/Turabian StyleEmish, Mohamed, and Sean D. Young. 2024. "Remote Wearable Neuroimaging Devices for Health Monitoring and Neurophenotyping: A Scoping Review" Biomimetics 9, no. 4: 237. https://doi.org/10.3390/biomimetics9040237
APA StyleEmish, M., & Young, S. D. (2024). Remote Wearable Neuroimaging Devices for Health Monitoring and Neurophenotyping: A Scoping Review. Biomimetics, 9(4), 237. https://doi.org/10.3390/biomimetics9040237






