Biodegradation and Thermomechanical Behavior of 3D-Printed PLA Scaffolds Under Static and Stirring Biomimetic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
- The description of the experimental setups for degradation is given below, as follows:
- (1)
- Setup for the static degradation experiment: A total of 60 mL SBF was added to a Berzelius beaker. Subsequently, 21 scaffolds were immersed in the fluid. The beaker was then placed in an incubator and maintained at a temperature of 37 °C, standard for the human body. Aluminum foil was used to prevent the liquid from evaporating. The aluminium foil was perforated with tiny holes to promote oxygenation and eliminate anaerobic contamination.
- (2)
- Setup for the dynamic degradation experiment: The dynamic experiment involved active stirring. As in the case of the static experiment, 60 mL SBF was placed in a Berzelius beaker. A total of 21 samples were immersed in the solution together with two rotating magnets. The beaker was then placed on a magneto-thermo stirrer. The temperature of the solution was maintained constant at 37 °C and regularly checked with a thermometer. Aluminum foil was used to prevent the liquid from evaporating. To allow oxygenation, small holes were made in the aluminum foil. The liquid with the scaffolds was in constant motion at 500 RPM.
2.2.1. Weight Loss Evaluation
2.2.2. DSC Measurements
2.2.3. DMA Measurements
2.2.4. Morphological Analysis
3. Results
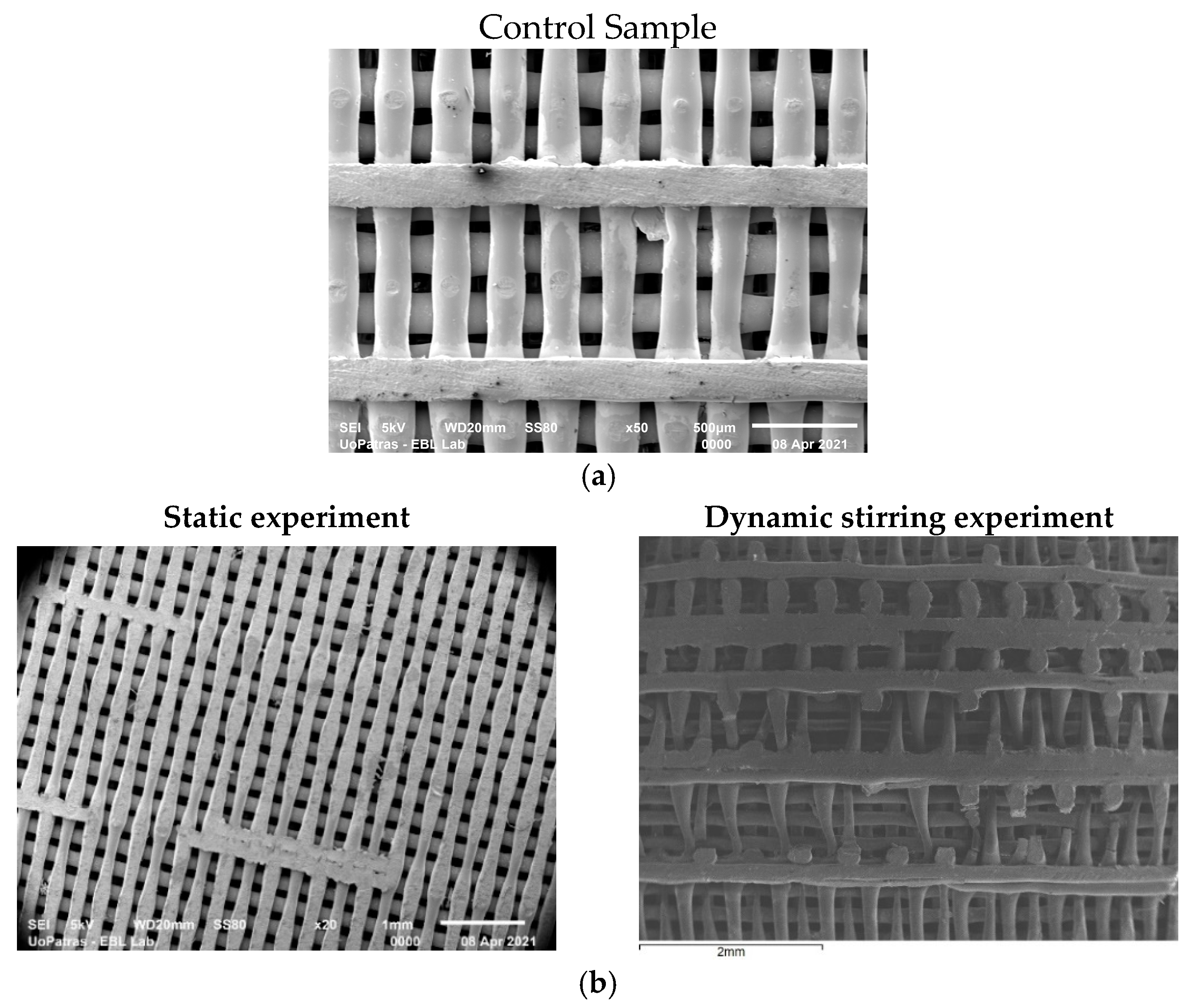
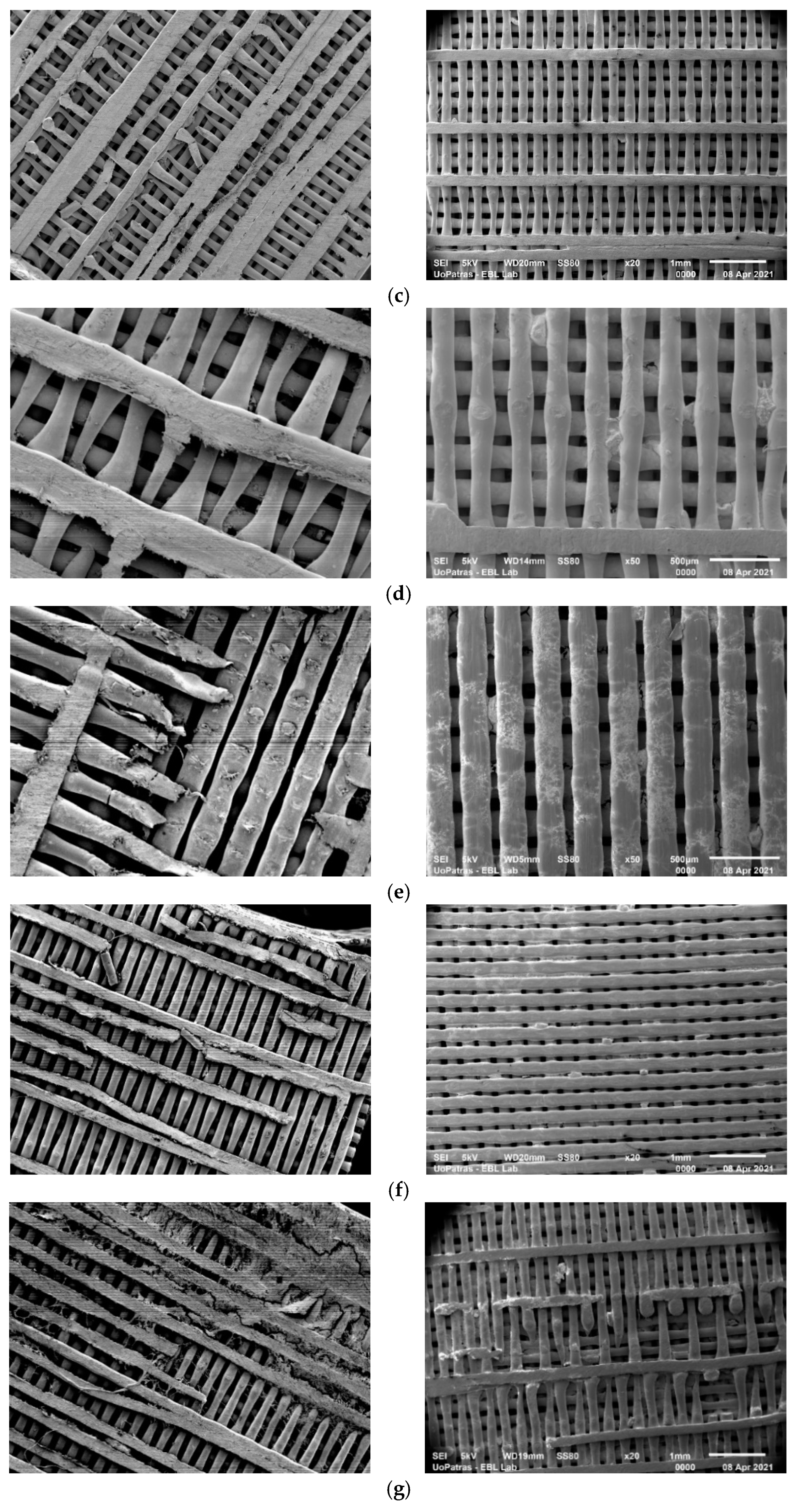
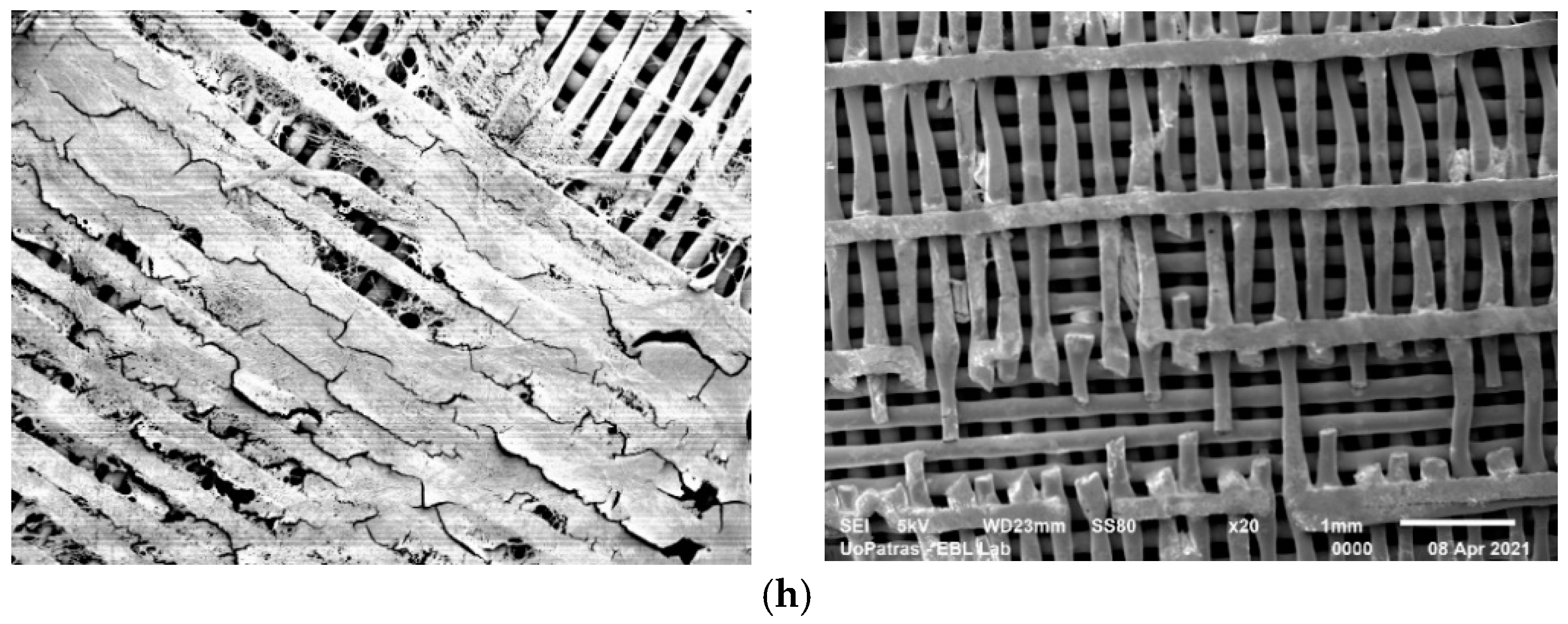
3.1. SEM Analysis
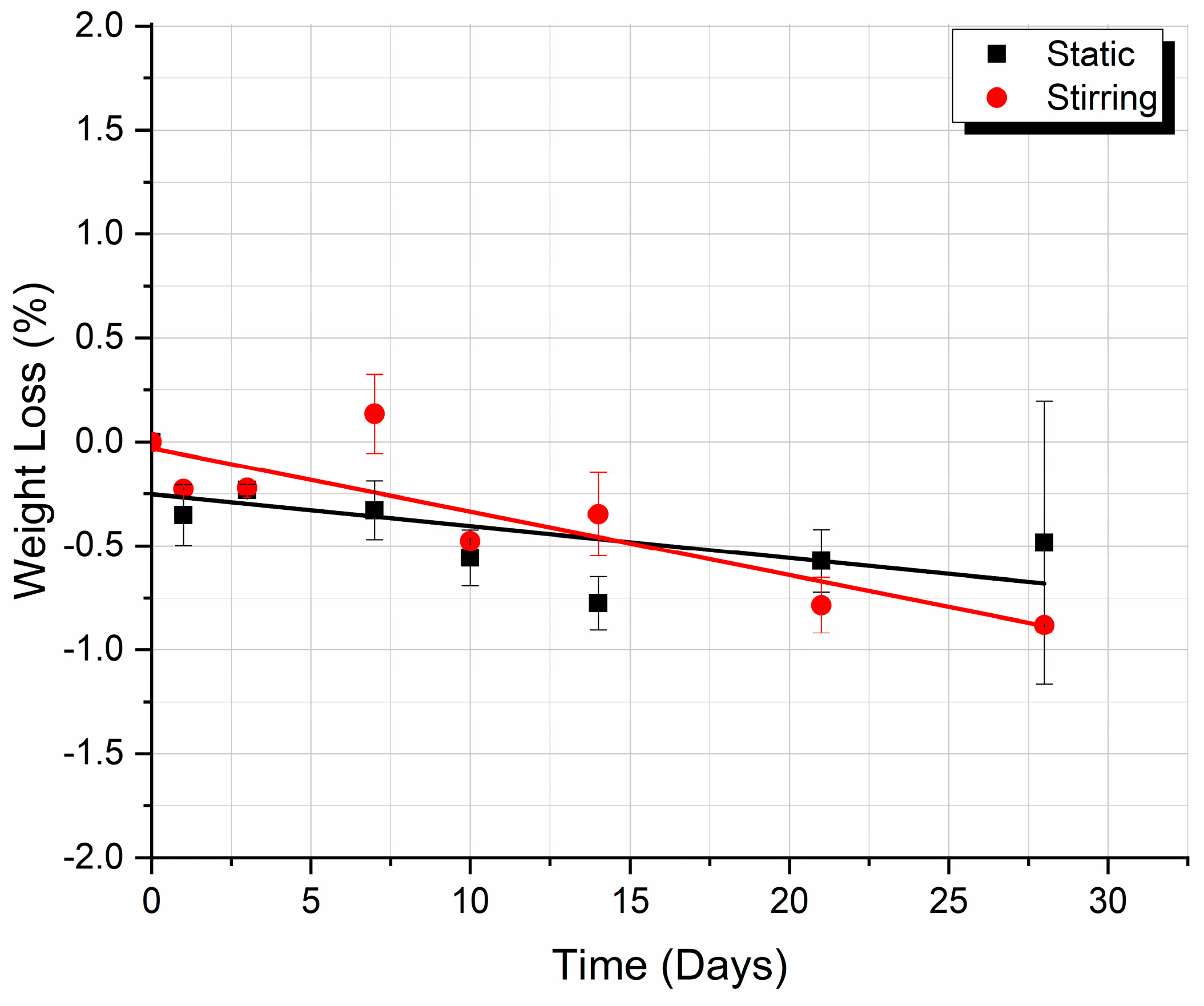
3.2. Weight Loss
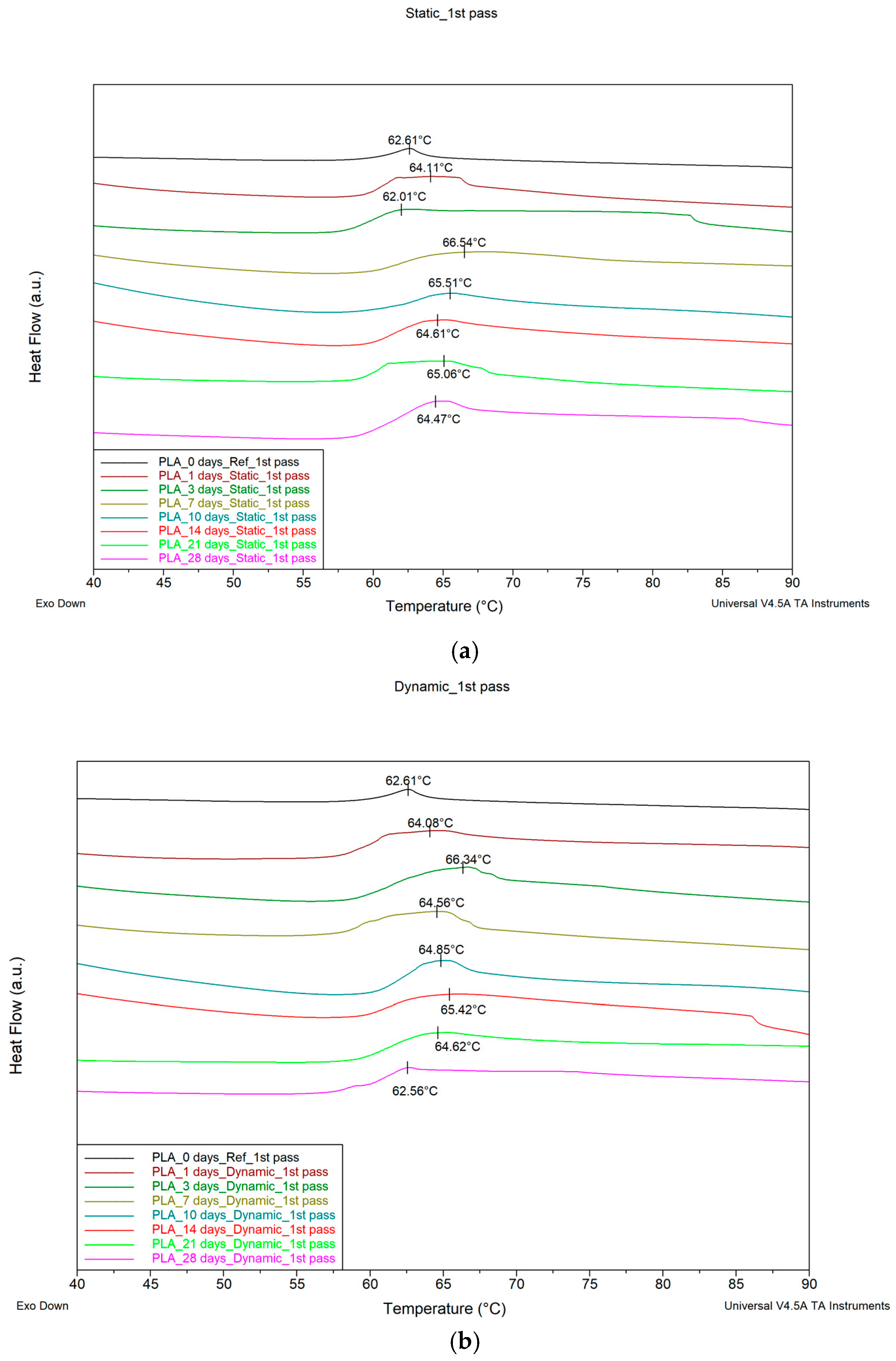
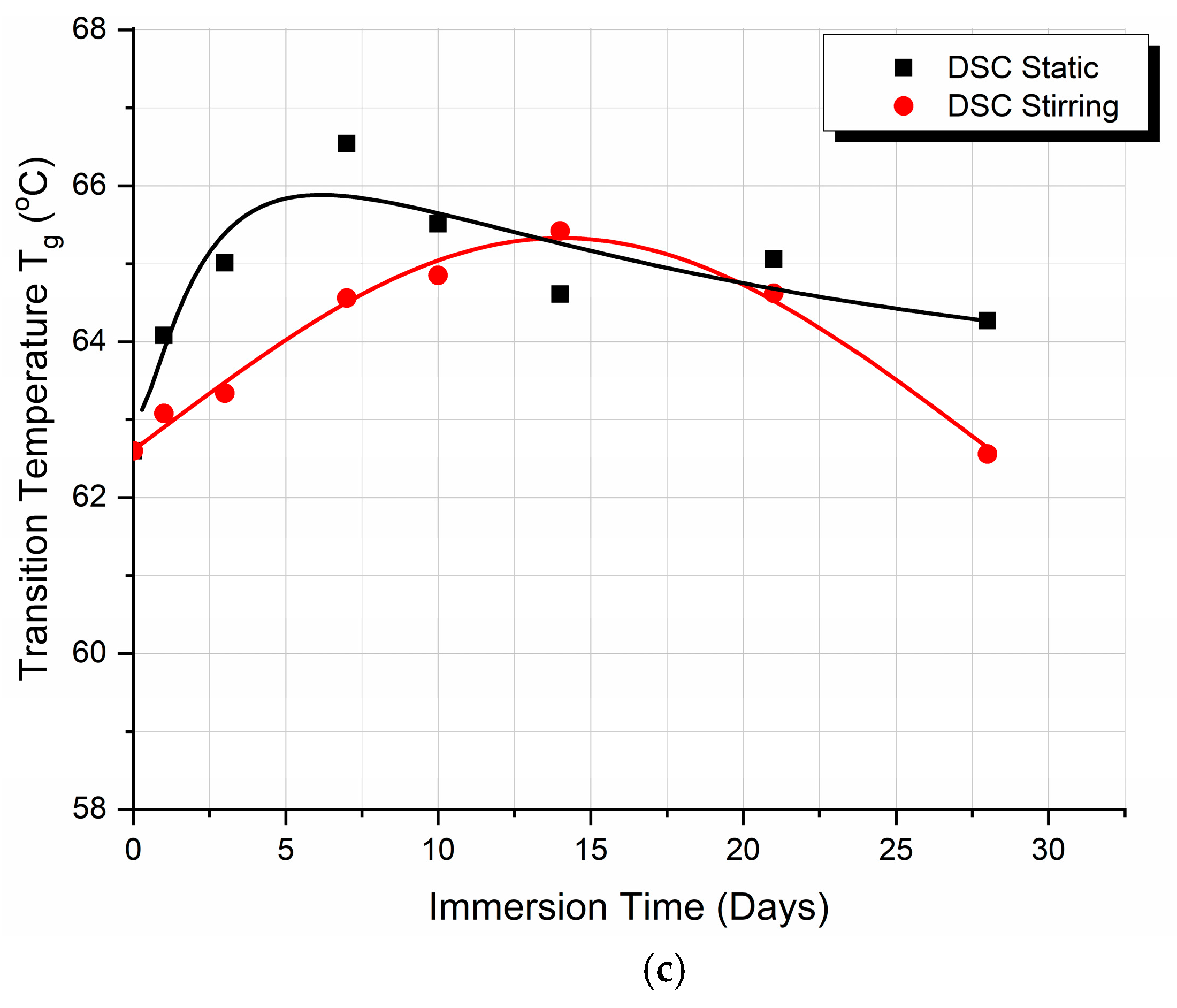
3.3. Differential Scanning Calorimetry (DSC)
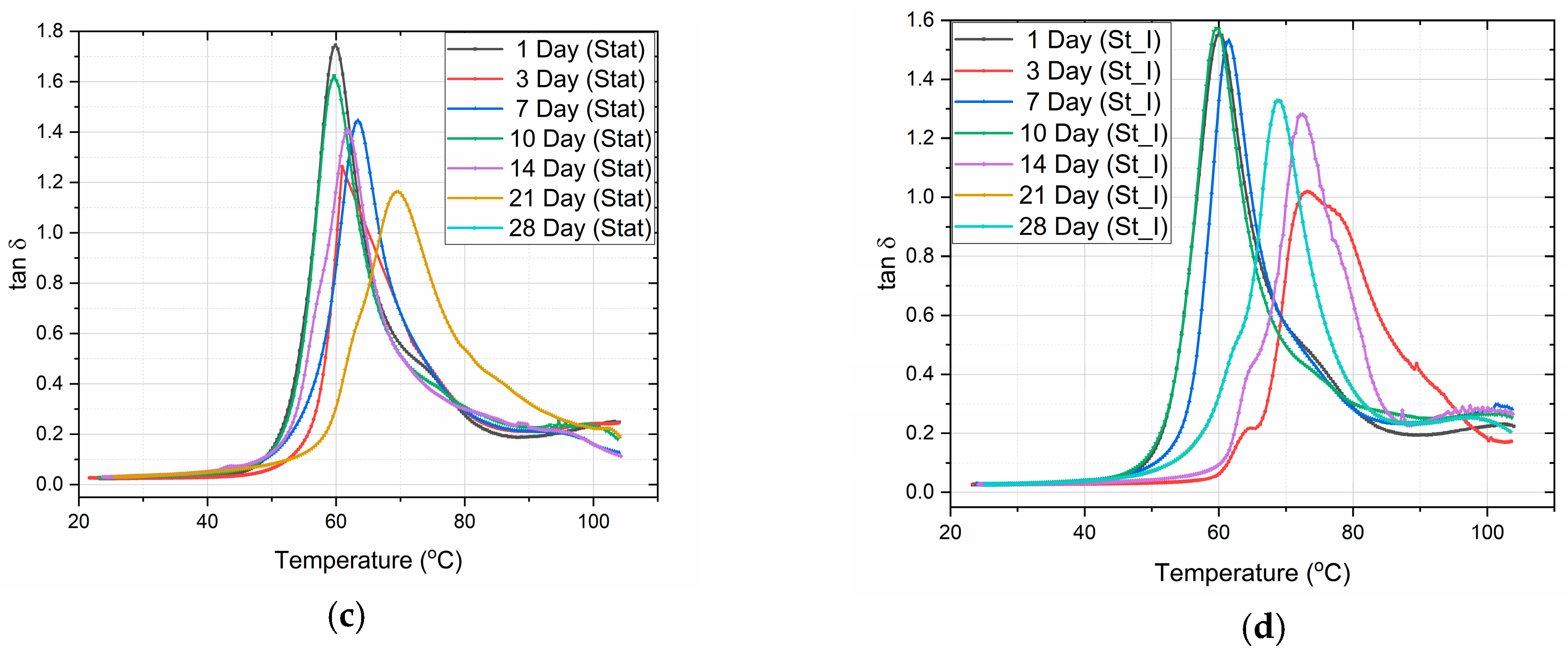
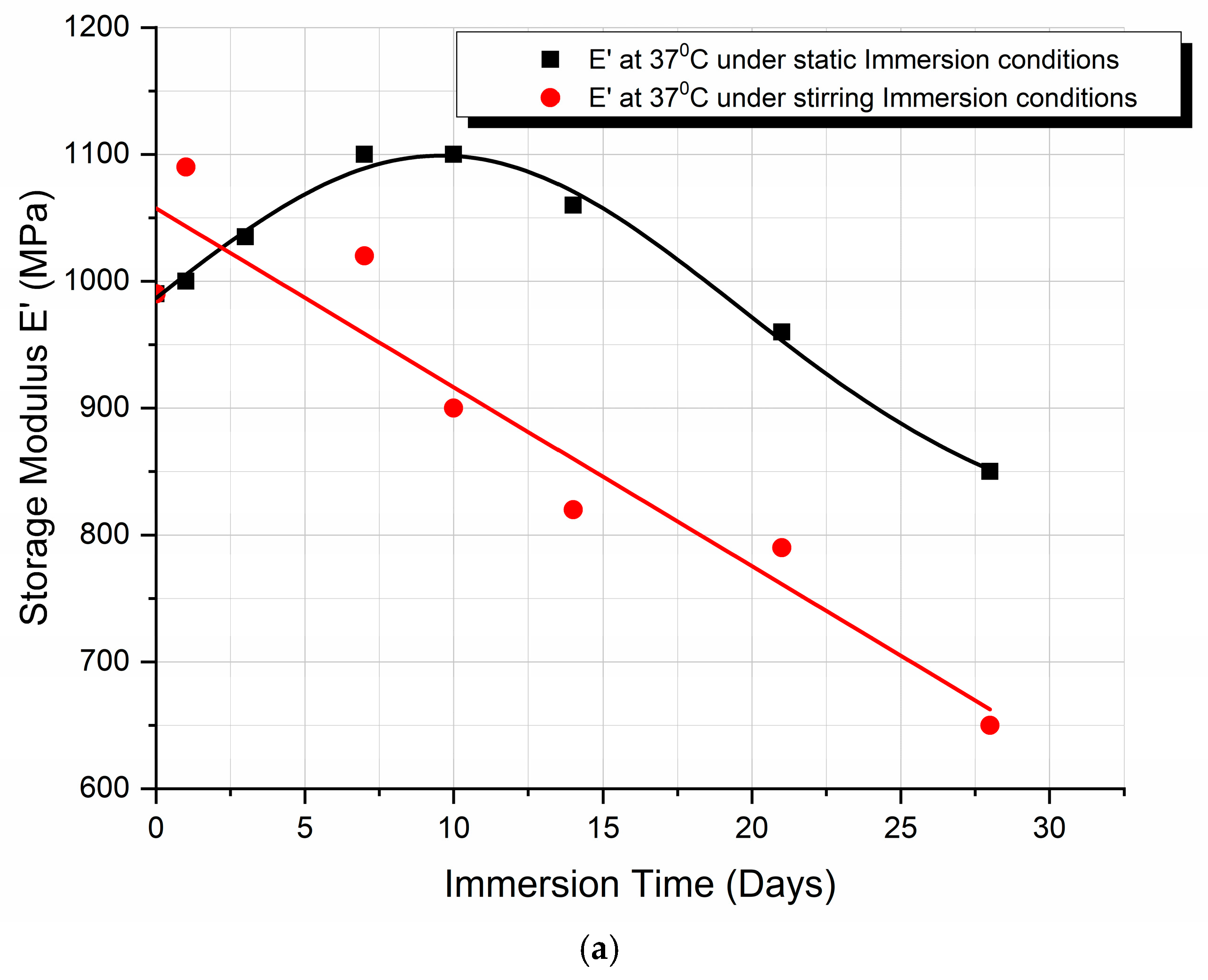
3.4. Dynamic Mechanical Analysis (DMA)
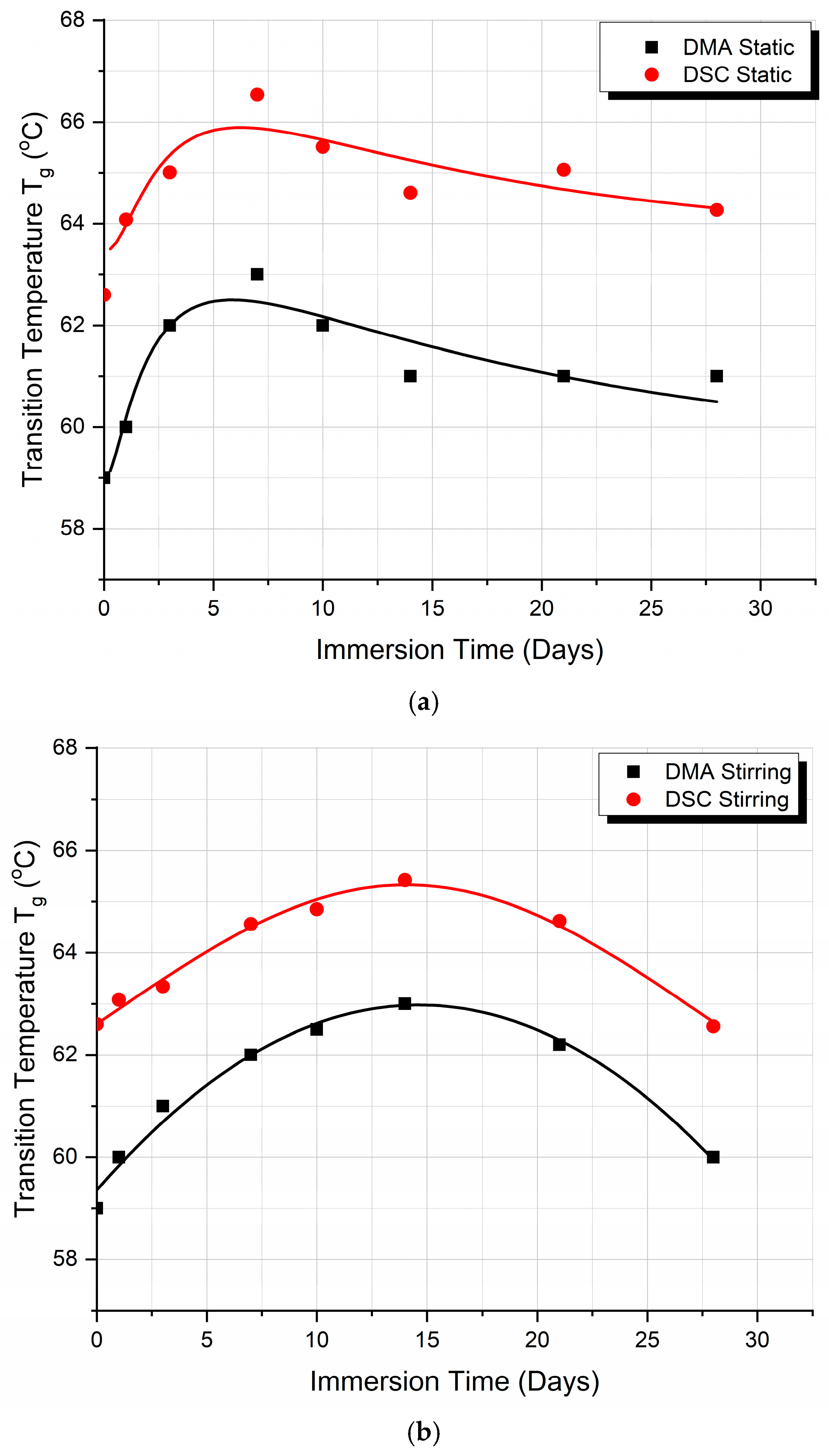
3.5. DMA and DSC Comparison
4. Discussion
4.1. Structural Changes Observed in SEM Micrographs
4.2. Weight Loss Analysis
4.3. Differential Scanning Calorimetry (DSC) Discussion
- According to the theory, three competitive phenomena occur in thermoplastics, which affect their glass transition temperature Tg, as follows:
- The first phenomenon is that the absorption of moisture by the polymer enables plasticization. The macromolecules of the polymer chains acquire greater mobility. Therefore, the material needs less energy to change from the rigid state (glassy state) to the elastomeric state (rubbery state). Consequently, the Tg value decreases.
- The second phenomenon concerns the change in the density of the polymer. The biological fluid entering the polymer occupies the empty space that exists between the macromolecules of the polymer. The structure of the polymer becomes more compact, while the mobility of the macromolecules is more limited. Consequently, more energy is required to transition from the rigid state to the elastomeric state. In this case, the Tg value increases.
- The third phenomenon has to do with the chemical interaction of water present in the biological fluid with the polymer. Water creates hydrogen bonds with the macromolecules of the polymer. Consequently, the mobility of macromolecules is limited. Therefore, more energy is required to transition from the rigid state to the elastomeric state. When this happens, the Tg value increases.
- To summarize and correlate events, we found the following:
- At the point of the intersection of the curves (day 17), it may be deduced that, in static immersion, the layer has formed, the liquid can no longer penetrate the polymer—or rather the penetration rate becomes very small—and, from then on, the Tg value reaches a plateau and remains almost stable.
- In stirred immersion, the layer detaches, and the liquid penetrates the polymer matrix. The factor of plasticization dominates other phenomena; in addition, since the voids become saturated and the hydrogen bonds are weak, the Tg value is reduced.
4.4. Dynamic Mechanical Analysis (DMA) Discussion
4.5. Discussion on the DMA and DSC Comparison
5. Conclusions
- The analysis of the SEM images performed on the scaffolds after different immersion times showed that the scaffolds in the static experiment visibly and considerably degrade after 28 days of immersion, while the scaffolds in the dynamic experiment are subjected to layer line breakage.
- The speed of weight reduction in stirred immersion is greater than the corresponding one in static immersion.
- In both cases, Tg initially rises because the filling of the polymer voids with fluid happens more quickly than the other processes.
- Finally, as illustrated in Figure 5b, the storage modulus showed a 10% improvement in the case of static immersion, followed by a constant linear degradation that peaked at −15% after 28 days of immersion. Conversely, a linear degradation of the storage modulus to −40% is noted for the specimens held under stirring conditions. Both types of E′ variation fit within the mechanisms described.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varma, M.V.; Kandasubramanian, B.; Ibrahim, S.M. 3D printed scaffolds for biomedical applications. Mater. Chem. Phys. 2020, 255, 123642. [Google Scholar] [CrossRef]
- Zhan, L.; Wang, L.; Deng, J.; Zheng, Y.; Ke, Q.; Yang, X.; Zhang, X.; Jia, W.; Huang, C. Enhanced cellular infiltration of tissue-engineered scaffolds fabricated by PLLA nanogrooved microfibers. Nano Res. 2023, 16, 1614–1625. [Google Scholar] [CrossRef]
- Haycock, J.W. 3D Cell Culture: A review of current approaches and techniques. In 3D Cell Culture Methods in Molecular Biology; Haycock, J.W., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 695, pp. 1–15. [Google Scholar]
- Londono, R.; Badylak, S.F. Biologic scaffolds for regenerative medicine: Mechanisms of in vivo remodeling. Ann. Biomed. Eng. 2015, 43, 577–592. [Google Scholar] [CrossRef]
- Dąbrowska-Gralak, M.; Sadło, J.; Głuszewski, W.; Łyczko, K.; Przybytniak, G.; Lewandowska, H. The combined effect of humidity and electron beam irradiation on collagen type I—Implications for collagen-based devices. Mater. Today Commun. 2022, 31, 103255. [Google Scholar] [CrossRef]
- Yiwen, X. Tribological Properties of Micro/Nano-Textured Surfaces Under Physiological Conditions. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 23 November 2022. [Google Scholar]
- Rouabhia, M.; Park, H.; Meng, S.; Derbali, H.; Zhang, Z. Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS ONE 2013, 19, e71660. [Google Scholar] [CrossRef]
- Fatemi, M.; Manduca, A.; Greenleaf, J.F. Imaging elastic properties of biological tissues by low-frequency harmonic vibration. Proc. IEEE 2003, 91, 1503–1519. [Google Scholar] [CrossRef]
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef]
- Hukins, D.W.L.; Mahomed, A.; Kukureka, S.N. Accelerated aging for testing polymeric biomaterials and medical devices. Med. Eng. Phys. 2008, 30, 1270–1274. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Z.; Song, Y.; Liu, S.; Qi, Y.; Wang, X.; Wang, Q.; Cui, C. Mechanical properties and in vitro biodegradation of newly developed porous Zn scaffolds for biomedical applications. Mater. Des. 2016, 108, 136–144. [Google Scholar] [CrossRef]
- Carfì Pavia, F.; La Carrubba, V.; Brucato, V. Tuning of biodegradation rate of PLLA scaffolds via blending with PLA. Int. J. Mater. Form. 2009, 2, 713. [Google Scholar] [CrossRef]
- LeBlon, C.E.; Pai, R.; Fodor, C.R.; Golding, A.S.; Coulter, J.P.; Jedlicka, S.S. In vitro comparative biodegradation analysis of salt-leached porous polymer scaffolds. J. Appl. Polym. Sci. 2013, 128, 2701–2712. [Google Scholar] [CrossRef]
- Agrawal, C.M.; McKinney, J.S.; Lanctot, D.; Athanasiou, K.A. Effects of fluid flow on the in vitro degradation kinetics of biodegradable scaffolds for tissue engineering. Biomaterials 2000, 21, 2443–2452. [Google Scholar] [CrossRef]
- Feier, A.M.; Portan, D.; Manu, D.R.; Kostopoulos, V.; Kotrotsos, A.; Strnad, G.; Dobreanu, M.; Salcudean, A.; Bataga, T. Primary MSCs for personalized medicine: Ethical challenges, isolation and biocompatibility evaluation of 3d electrospun and printed scaffolds. Biomedicines 2022, 10, 1563. [Google Scholar] [CrossRef]
- Mano, J.F.; Koniarova, D.; Reis, R.L. Thermal properties of thermoplastic starch/synthetic polymer blends with potential biomedical applicability. J. Mater. Sci. Mater. Med. 2003, 14, 127–135. [Google Scholar] [CrossRef]
- AnkerMake. Explore the 3D Printing Frontier—Ankermake Europe. Available online: https://www.ankermake.com/eu-en/blogs/maintenance-guides/how-to-tell-if-filament-is-wet (accessed on 29 November 2024).
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solís, V.; Martínez-Guerra, E.; Piñón-Balderrama, C.I.; Compean Martínez, I.; Saavedra-Leos, M.Z. Application of differential scanning calorimetry (DSC) and modulated differential scanning calorimetry (MDSC) in food and drug industries. Polymers 2020, 12, 5. [Google Scholar] [CrossRef]
- Sun, W.Q. DSC Analysis of thermophysical properties for biomaterials and formulations. Methods Mol. Biol. 2021, 2180, 285–302. [Google Scholar]
- Mano, J.F.; Reis, R.L.; Cunha, A.M. Effects of moisture and degradation time over the mechanical dynamical performance of starch-based biomaterials. J. Appl. Polym. Sci. 2000, 78, 2345–2357. [Google Scholar] [CrossRef]
- Jones, D.S. Dynamic mechanical analysis of polymeric systems of pharmaceutical and biomedical significance. Int. J. Pharm. 1999, 179, 167–178. [Google Scholar] [CrossRef]
- Portan, D.V.; Kroustalli, A.A.; Deligianni, D.D.; Papanicolaou, G.C. On the biocompatibility between TiO2 nanotubes layer and human osteoblasts. J. Biomed. Mater. Res. 2012, 100, 2546–2553. [Google Scholar] [CrossRef]
- Portan, D.V.; Deligianni, D.D.; Deligianni, K.; Tyllianakis, M.; Papanicolaou, G.C. Modeling of the interaction between osteoblasts and biocompatible substrates as a function of adhesion strength. J. Biomed. Mater. Res. 2018, 106, 621–628. [Google Scholar] [CrossRef]
- Portan, D.V.; Ntoulias, C.; Mantzouranis, G.; Fortis, A.P.; Deligianni, D.D.; Polyzos, D.; Kostopoulos, V. Gradient 3D printed PLA scaffolds on biomedical titanium: Mechanical evaluation and biocompatibility. Polymers 2021, 13, 682. [Google Scholar] [CrossRef]
- Odelius, K.; Höglund, A.; Kumar, S.; Hakkarainen, M.; Ghosh, A.K.; Bhatnagar, N.; Albertsson, A.C. Porosity and pore size regulate the degradation product profile of polylactide. Biomacromolecules 2011, 12, 1250–1258. [Google Scholar] [CrossRef]
- Lam, C.X.F.; Savalani, M.M.; Teoh, S.H.; Hutmacher, D.W. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: Accelerated versus simulated physiological conditions. Biomed. Mater. 2008, 3, 034108. [Google Scholar] [CrossRef]
- Bengi, Y.; Pazarceviren, A.E.; Tezcaner, A.; Evis, Z. Historical development of simulated body fluids used in biomedical applications: A review. Microchem. J. 2020, 155, 104713. [Google Scholar]
- Domingos, M.; Chiellini, F.; Cometa, S.; De Giglio, E.; Grillo-Fernandes, E.; Bártolo, P.; Chiellini, E. Evaluation of in vitro degradation of PCL scaffolds fabricated via BioExtrusion. Part 1: Influence of the degradation environment. Virtual Phys. Prototyp. 2010, 5, 65–73. [Google Scholar]
- Iftikhar, A.; Qaiser, Z.; Sarfraz, W.; Ejaz, U.; Aqeel, M.; Rizvi, Z.F.; Khalid, N. Understanding the leaching of plastic additives and subsequent risks to ecosystems. Water Emerg. Contam. Nanoplast. 2024, 3, 5. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Yang, H.; He, W.; Li, B.; Zhu, X.; Liu, S.; Jia, S.; Li, R.; Tang, K.H.D. Leaching of chemicals from microplastics: A review of chemical types, leaching mechanisms and influencing factors. Sci. Total Environ. 2024, 906, 167666. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portan, D.V.; Bampounis, G.; Koliadima, A.; Patsidis, A.C.; Kontaxis, L.C.; Papanicolaou, G.C. Biodegradation and Thermomechanical Behavior of 3D-Printed PLA Scaffolds Under Static and Stirring Biomimetic Conditions. Biomimetics 2024, 9, 743. https://doi.org/10.3390/biomimetics9120743
Portan DV, Bampounis G, Koliadima A, Patsidis AC, Kontaxis LC, Papanicolaou GC. Biodegradation and Thermomechanical Behavior of 3D-Printed PLA Scaffolds Under Static and Stirring Biomimetic Conditions. Biomimetics. 2024; 9(12):743. https://doi.org/10.3390/biomimetics9120743
Chicago/Turabian StylePortan, Diana V., Georgios Bampounis, Athanasia Koliadima, Anastasios C. Patsidis, Lykourgos C. Kontaxis, and George C. Papanicolaou. 2024. "Biodegradation and Thermomechanical Behavior of 3D-Printed PLA Scaffolds Under Static and Stirring Biomimetic Conditions" Biomimetics 9, no. 12: 743. https://doi.org/10.3390/biomimetics9120743
APA StylePortan, D. V., Bampounis, G., Koliadima, A., Patsidis, A. C., Kontaxis, L. C., & Papanicolaou, G. C. (2024). Biodegradation and Thermomechanical Behavior of 3D-Printed PLA Scaffolds Under Static and Stirring Biomimetic Conditions. Biomimetics, 9(12), 743. https://doi.org/10.3390/biomimetics9120743








