Clinical Applications of Micro/Nanobubble Technology in Neurological Diseases
Abstract
1. Introduction
2. Principles
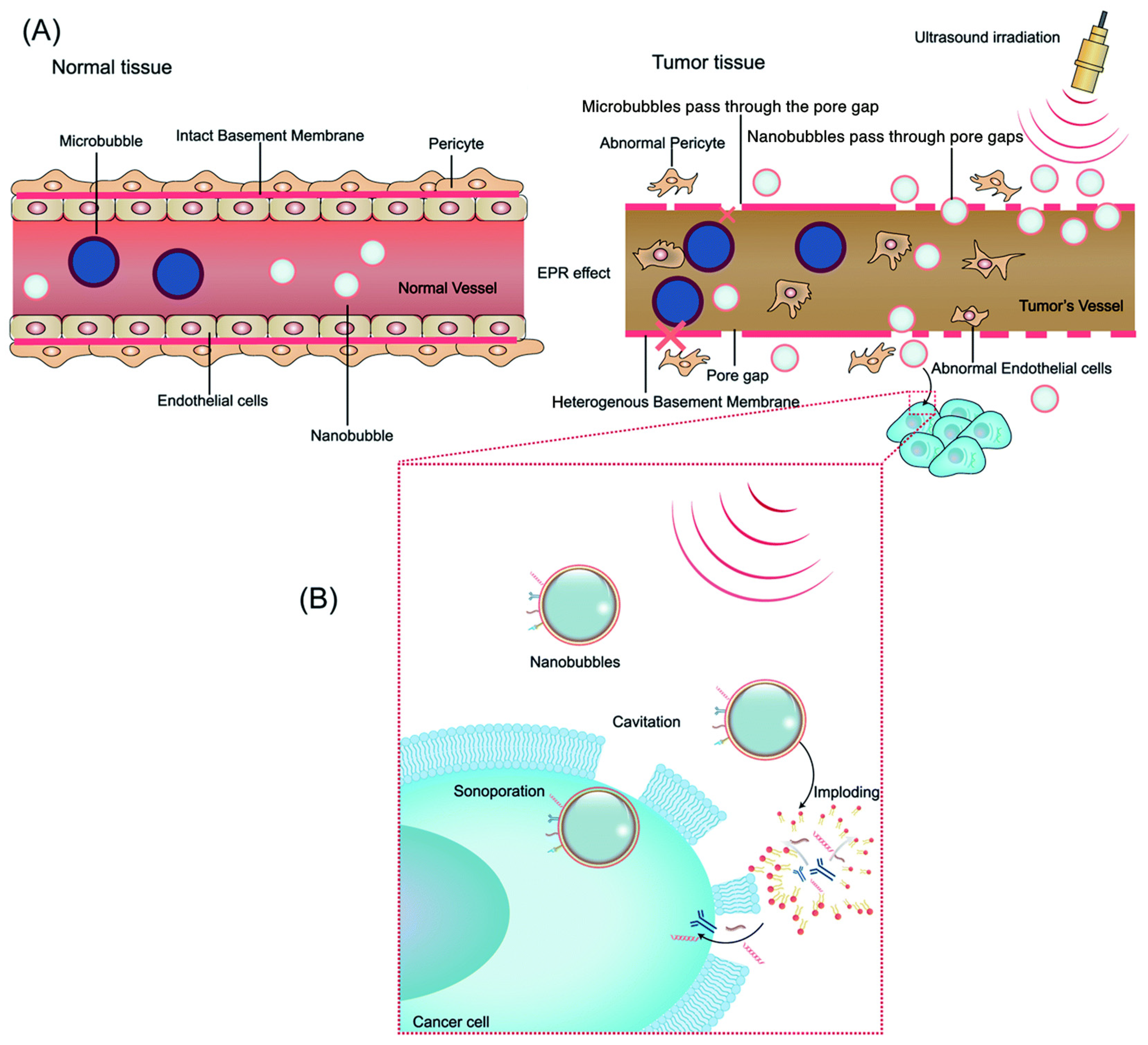
2.1. Micro/Nanobubble Technology
2.2. Fabrication and Functionalization
| Preparation Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Mechanical Agitation |
|
| [23,30,56,57] |
| Acoustic Cavitation |
|
| [23,38,58,59] |
| Hydrodynamic Cavitation |
|
| [30,34,60] |
| Membrane Dispersion |
|
| [61,62,63] |
| Electrolysis |
|
| [30,43] |
| Compression–decompression |
|
| [18,30,64] |
| Microfluidics |
|
| [30,48,65,66,67] |
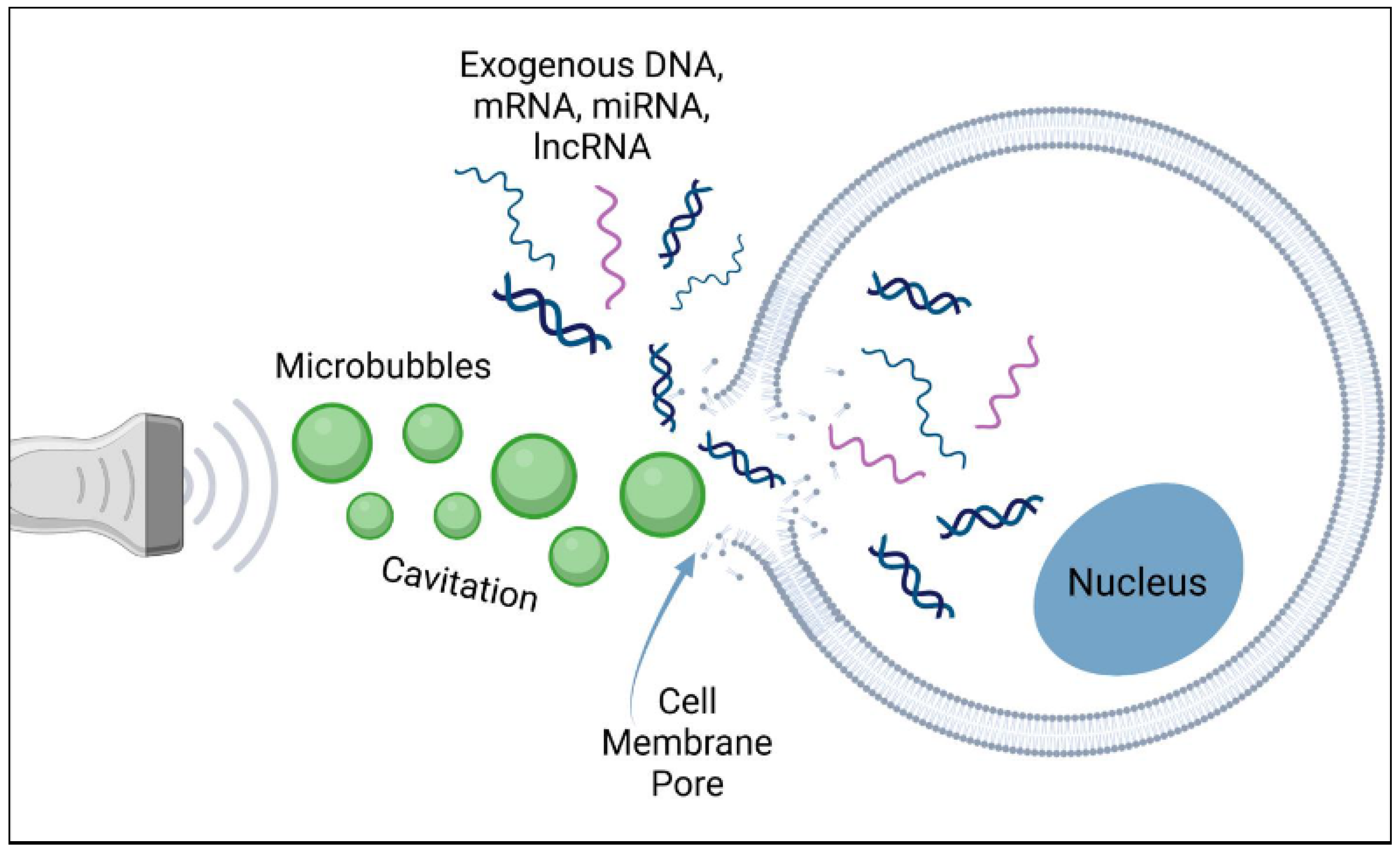
2.3. Principles of Ultrasound-Mediated Drug Delivery
2.3.1. Nanobubble Applications in Imaging
2.3.2. Nanobubble Applications in Drug Delivery
3. Current Clinical Applications
3.1. Stroke
3.1.1. Ischemic Stroke
3.1.2. Clinical Studies
3.2. Oncology
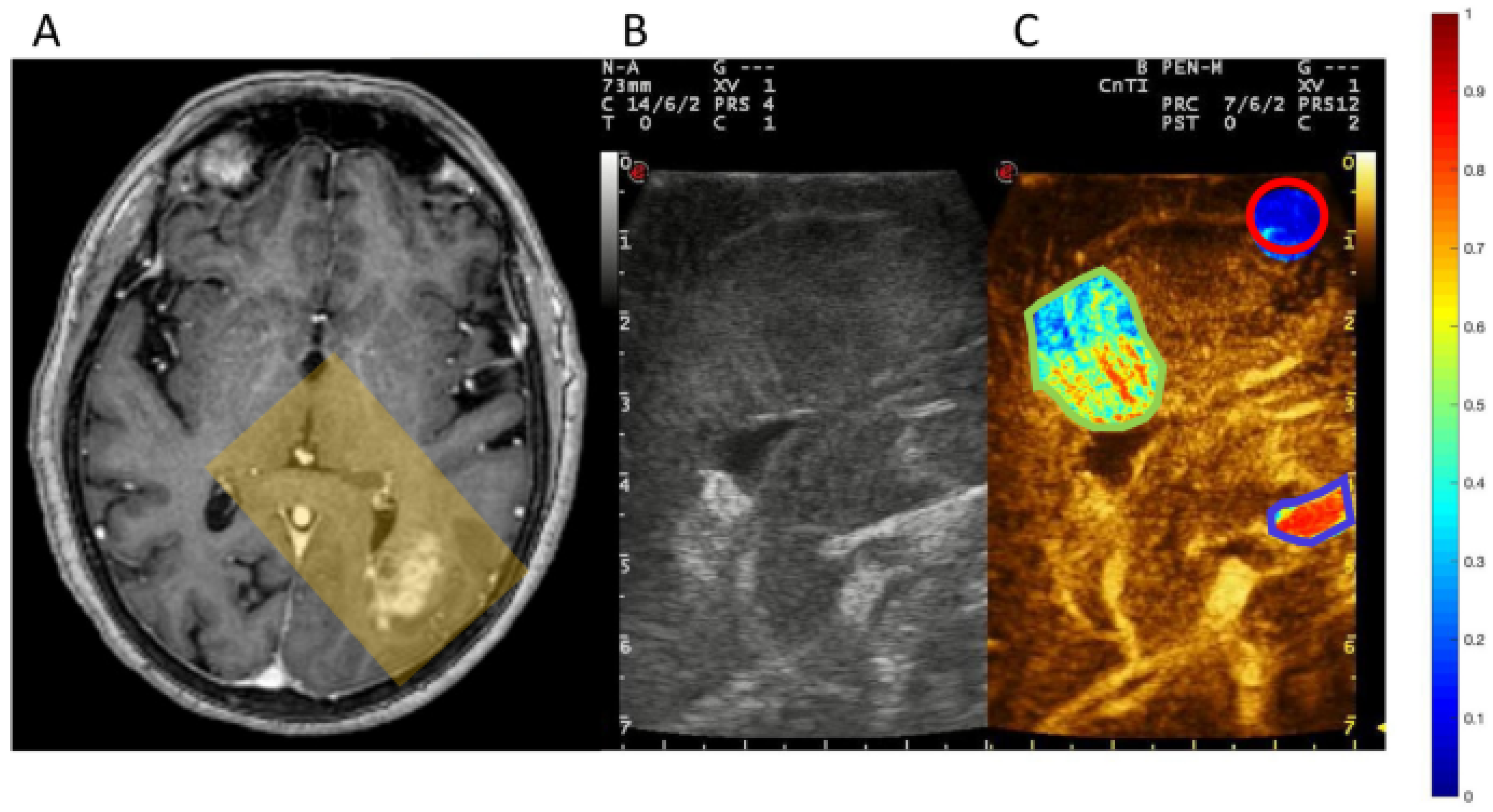
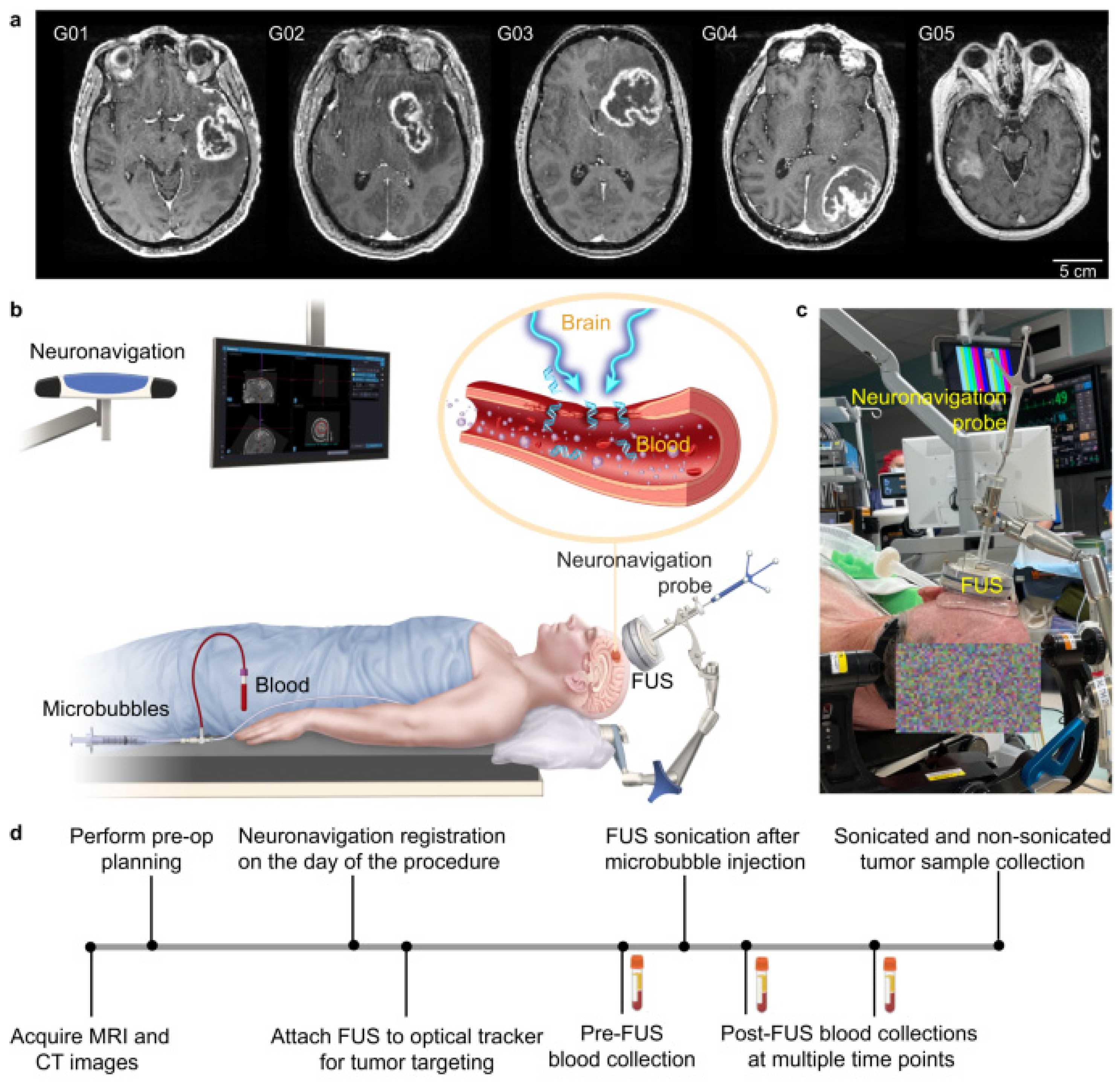
3.2.1. Brain Tumors
3.2.2. Clinical Trials
3.3. Degenerative Diseases
3.3.1. Alzheimer’s Disease
3.3.2. Clinical Studies
3.3.3. Parkinson’s Disease
3.3.4. Clinical Studies
3.4. Other Neurological Diseases
3.4.1. Myasthenia Gravis
3.4.2. Clinical Studies
3.4.3. Amyotrophic Lateral Sclerosis (ALS)
3.4.4. Clinical Studies
4. Clinical Trials in Progress
5. Challenges and Limitations
6. Technological and Clinical Advancements
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, L.; Yang, L.; Jin, J.; Yang, F.; Liu, D.; Hu, K.; Wang, Q.; Yue, Y.; Gu, N. Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics 2020, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, M.; Aronson, L.; Pattilachan, T.; Sautto, F.; Daines, B.; Thommes, D.; Shar, A.; Razavi, M. Bubble-based drug delivery systems: Next-generation diagnosis to therapy. J. Funct. Biomater. 2023, 14, 373. [Google Scholar] [CrossRef] [PubMed]
- Helfield, B.; Zou, Y.; Matsuura, N. Acoustically-stimulated nanobubbles: Opportunities in medical ultrasound imaging and therapy. Front. Phys. 2021, 9, 654374. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery. Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 2336. [Google Scholar] [CrossRef]
- Bouakaz, A.; Escoffre, J.M. From concept to early clinical trials: 30 years of microbubble-based ultrasound-mediated drug delivery research. Adv. Drug Deliv. Rev. 2024, 206, 115199. [Google Scholar] [CrossRef]
- Kogan, P.; Gessner, R.C.; Dayton, P.A. Microbubbles in imaging: Applications beyond ultrasound. Bubble Sci. Eng. Technol. 2010, 2, 3–8. [Google Scholar] [CrossRef][Green Version]
- Lindner, J.R. Microbubbles in medical imaging: Current applications and future directions. Nat. Rev. Drug Discov. 2004, 3, 527–533. [Google Scholar] [CrossRef]
- Sirsi, S.; Borden, M. Microbubble compositions, properties and biomedical applications. Bubble Sci. Eng. Technol. 2009, 1, 3–17. [Google Scholar] [CrossRef]
- Tan, B.H.; An, H.; Ohl, C.-D. Stability of surface and bulk nanobubbles. Curr. Opin. Colloid Interface Sci. 2021, 53, 101428. [Google Scholar] [CrossRef]
- Alheshibri, M.; Qian, J.; Jehannin, M.; Craig, V.S. A history of nanobubbles. Langmuir 2016, 32, 11086–11100. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Chen, S.; Su, S.; Huang, Y.; Liu, B.; Sun, H. A review and perspective on micro and nanobubbles: What They Are and Why They Matter. Miner. Eng. 2022, 189, 107906. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Chen, C.-W.; Tseng, Y.-S.; Kuo, C.-H.; Wu, C.-H.; Di Dong, C. Advances in micro-and nano bubbles technology for application in biochemical processes. Environ. Technol. Innov. 2021, 23, 101729. [Google Scholar] [CrossRef]
- Batchelor, D.V.; Armistead, F.J.; Ingram, N.; Peyman, S.A.; McLaughlan, J.R.; Coletta, P.L.; Evans, S.D. The influence of nanobubble size and stability on ultrasound enhanced drug delivery. Langmuir 2022, 38, 13943–13954. [Google Scholar] [CrossRef]
- Jin, J.; Yang, L.; Chen, F.; Gu, N. Drug delivery system based on nanobubbles. Interdiscip. Mater. 2022, 1, 471–494. [Google Scholar] [CrossRef]
- Batchelor, D.V.; Abou-Saleh, R.H.; Coletta, P.L.; McLaughlan, J.R.; Peyman, S.A.; Evans, S.D. Nested nanobubbles for ultrasound-triggered drug release. ACS Appl. Mater. Interfaces 2020, 12, 29085–29093. [Google Scholar] [CrossRef]
- Hansen, H.H.; Cha, H.; Ouyang, L.; Zhang, J.; Jin, B.; Stratton, H.; Nguyen, N.-T.; An, H. Nanobubble technologies: Applications in therapy from molecular to cellular level. Biotechnol. Adv. 2023, 63, 108091. [Google Scholar] [CrossRef]
- Oh, S.H.; Han, J.G.; Kim, J.-M. Long-term stability of hydrogen nanobubble fuel. Fuel 2015, 158, 399–404. [Google Scholar] [CrossRef]
- Lyu, T.; Wu, Y.; Zhang, Y.; Fan, W.; Wu, S.; Mortimer, R.J.; Pan, G. Nanobubble aeration enhanced wastewater treatment and bioenergy generation in constructed wetlands coupled with microbial fuel cells. Sci. Total Environ. 2023, 895, 165131. [Google Scholar] [CrossRef]
- Park, B.; Yoon, S.; Choi, Y.; Jang, J.; Park, S.; Choi, J. Stability of engineered micro or nanobubbles for biomedical applications. Pharmaceutics 2020, 12, 1089. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Suzuki, R.; Mori, T.; Takahashi, H.; Natsugari, H.; Omata, D.; Unga, J.; Uruga, H.; Sugii, M.; Kawakami, S. Development of fluorous lipid-based nanobubbles for efficiently containing perfluoropropane. Int. J. Pharm. 2015, 487, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Hwang, J.; Lee, K.; Choi, Y.; Kim, K.; Koo, H.-J.; Hong, J.W.; Choi, J. Oxygen-carrying micro/nanobubbles: Composition, synthesis techniques and potential prospects in photo-triggered theranostics. Molecules 2018, 23, 2210. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Takizawa, T.; Negishi, Y.; Utoguchi, N.; Maruyama, K. Effective gene delivery with novel liposomal bubbles and ultrasonic destruction technology. Int. J. Pharm. 2008, 354, 49–55. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Xu, D.; Zhu, L.; Hu, M.; Liu, Q.; Lan, W.; Jiang, J.; Wang, L. G250 antigen-targeting drug-loaded nanobubbles combined with ultrasound targeted nanobubble destruction: A potential novel treatment for renal cell carcinoma. Int. J. Nanomed. 2020, 15, 81–95. [Google Scholar] [CrossRef]
- Čolić, M.; Kraljević Pavelić, S.; Peršurić, Ž.; Agaj, A.; Bulog, A.; Pavelić, K. Enhancing the bioavailability and activity of natural antioxidants with nanobubbles and nanoparticles. Redox Rep. 2024, 29, 2333619. [Google Scholar] [CrossRef]
- Cavalli, R.; Soster, M.; Argenziano, M. Nanobubbles: A promising efficient tool for therapeutic delivery. Ther. Deliv. 2016, 7, 117–138. [Google Scholar] [CrossRef]
- Pasupathy, R.; Pandian, P.; Selvamuthukumar, S. Nanobubbles: A novel targeted drug delivery system. Braz. J. Pharm. Sci. 2022, 58, e19608. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, S.; Zhang, L.; Hu, J. Generation and stability of bulk nanobubbles: A review and perspective. Curr. Opin. Colloid Interface Sci. 2021, 53, 101439. [Google Scholar] [CrossRef]
- Paknahad, A.A.; Kerr, L.; Wong, D.A.; Kolios, M.C.; Tsai, S.S. Biomedical nanobubbles and opportunities for microfluidics. RSC Adv. 2021, 11, 32750–32774. [Google Scholar] [CrossRef]
- Foudas, A.W.; Kosheleva, R.I.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C.; Kyzas, G.Z. Fundamentals and applications of nanobubbles: A review. Chem. Eng. Res. Des. 2023, 189, 64–86. [Google Scholar] [CrossRef]
- Favvas, E.P.; Kyzas, G.Z.; Efthimiadou, E.K.; Mitropoulos, A.C. Bulk nanobubbles, generation methods and potential applications. Curr. Opin. Colloid Interface Sci. 2021, 54, 101455. [Google Scholar] [CrossRef]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Marcelino, K.R.; Wongkiew, S.; Sun, L.; Guo, W.; Khanal, S.K.; Lu, H. Untapped potential: Applying microbubble and nanobubble technology in water and wastewater treatment and ecological restoration. ACS EST Eng. 2022, 2, 1558–1573. [Google Scholar] [CrossRef]
- Brennen, C.E. Cavitation and Bubble Dynamics; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Seo, H.-B.; Lee, S.-Y. High-concentration nanobubble generation by megasonic cavitation and atomization. Colloid Interface Sci. Commun. 2023, 52, 100687. [Google Scholar] [CrossRef]
- Xu, Q.; Nakajima, M.; Ichikawa, S.; Nakamura, N.; Shiina, T. A comparative study of microbubble generation by mechanical agitation and sonication. Innov. Food Sci. Emerg. Technol. 2008, 9, 489–494. [Google Scholar] [CrossRef]
- Nirmalkar, N.; Pacek, A.; Barigou, M. On the existence and stability of bulk nanobubbles. Langmuir 2018, 34, 10964–10973. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Shafaei, S.Z.; Hassanzadeh, A.; Azizi, A.; Gharabaghi, M.; Ahmadi, R.; Shahbazi, B. Study of effective parameters on generating submicron (nano)-bubbles using the hydrodynamic cavitation. Physicochem. Probl. Miner. Process. 2020, 56, 884–904. [Google Scholar] [CrossRef]
- Li, T.; Cui, Z.; Sun, J.; Jiang, C.; Li, G. Generation of bulk nanobubbles by self-developed venturi-type circulation hydrodynamic cavitation device. Langmuir 2021, 37, 12952–12960. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T. Preparation method and application of nanobubbles: A review. Coatings 2023, 13, 1510. [Google Scholar] [CrossRef]
- Ulatowski, K.; Sobieszuk, P.; Mróz, A.; Ciach, T. Stability of nanobubbles generated in water using porous membrane system. Chem. Eng. Process. Process Intensif. 2019, 136, 62–71. [Google Scholar] [CrossRef]
- Jadhav, A.J.; Barigou, M. Electrochemically induced bulk nanobubbles. Ind. Eng. Chem. Res. 2021, 60, 17999–18006. [Google Scholar] [CrossRef]
- Ulatowski, K.; Jeżak, R.; Sobieszuk, P. Impact of process parameters on the diameter of nanobubbles generated by electrolysis on platinum-coated titanium electrodes using Box–Behnken experimental design. Energies 2021, 14, 2542. [Google Scholar] [CrossRef]
- Kikuchi, K.; Ioka, A.; Oku, T.; Tanaka, Y.; Saihara, Y.; Ogumi, Z. Concentration determination of oxygen nanobubbles in electrolyzed water. J. Colloid Interface Sci. 2009, 329, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Y.; Huang, Q.; Wang, X.; Zhou, L.; Wang, X.; Wen, B.; Guan, N.; Hu, J.; Zhou, X. The generation and stability of bulk nanobubbles by compression-decompression method: The role of dissolved gas. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130488. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Wereley, S.T.; Shaegh, S.A.M. Fundamentals and Applications of Microfluidics; Artech House: New Yor, NY, USA, 2019. [Google Scholar]
- Labarre, L.A.; Saint-Jalmes, A.; Vigolo, D. Microfluidics investigation of the effect of bulk nanobubbles on surfactant-stabilised foams. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130169. [Google Scholar] [CrossRef]
- Tabeling, P. Introduction to Microfluidics; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- Ren, Z.; Xu, P.; Han, H.; Ohl, C.-D.; Zuo, Z.; Liu, S. Removal of surface-attached micro-and nanobubbles by ultrasonic cavitation in microfluidics. Ultrason. Sonochemistry 2024, 109, 107011. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, T.; Yaparatne, S.; Graf, J.; Garcia-Segura, S.; Apul, O. Electrostatic forces and higher order curvature terms of Young–Laplace equation on nanobubble stability in water. NPJ Clean Water 2022, 5, 18. [Google Scholar] [CrossRef]
- Lee, M.; Lee, E.Y.; Lee, D.; Park, B.J. Stabilization and fabrication of microbubbles: Applications for medical purposes and functional materials. Soft Matter 2015, 11, 2067–2079. [Google Scholar] [CrossRef]
- Su, C.; Ren, X.; Nie, F.; Li, T.; Lv, W.; Li, H.; Zhang, Y. Current advances in ultrasound-combined nanobubbles for cancer-targeted therapy: A review of the current status and future perspectives. RSC Adv. 2021, 11, 12915–12928. [Google Scholar] [CrossRef]
- Toljan, K.; Ashok, A.; Labhasetwar, V.; Hussain, M.S. Nanotechnology in stroke: New trails with smaller scales. Biomedicines 2023, 11, 780. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, D.V.; Armistead, F.J.; Ingram, N.; Peyman, S.A.; Mclaughlan, J.R.; Coletta, P.L.; Evans, S.D. Nanobubbles for therapeutic delivery: Production, stability and current prospects. Curr. Opin. Colloid Interface Sci. 2021, 54, 101456. [Google Scholar] [CrossRef]
- Abdalkader, R.; Kawakami, S.; Unga, J.; Higuchi, Y.; Suzuki, R.; Maruyama, K.; Yamashita, F.; Hashida, M. The development of mechanically formed stable nanobubbles intended for sonoporation-mediated gene transfection. Drug Deliv. 2017, 24, 320–327. [Google Scholar] [CrossRef]
- Melich, R.; Valour, J.-P.; Urbaniak, S.; Padilla, F.; Charcosset, C. Preparation and characterization of perfluorocarbon microbubbles using Shirasu Porous Glass (SPG) membranes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 233–243. [Google Scholar] [CrossRef]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochemistry 2011, 18, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Brotchie, A.; Grieser, F.; Ashokkumar, M. Effect of power and frequency on bubble-size distributions in acoustic cavitation. Phys. Rev. Lett. 2009, 102, 084302. [Google Scholar] [CrossRef]
- Zheng, H.; Zheng, Y.; Zhu, J. Recent developments in hydrodynamic cavitation reactors: Cavitation mechanism, reactor design, and applications. Engineering 2022, 19, 180–198. [Google Scholar] [CrossRef]
- Ahmed, A.K.A.; Sun, C.; Hua, L.; Zhang, Z.; Zhang, Y.; Zhang, W.; Marhaba, T. Generation of nanobubbles by ceramic membrane filters: The dependence of bubble size and zeta potential on surface coating, pore size and injected gas pressure. Chemosphere 2018, 203, 327–335. [Google Scholar] [CrossRef]
- Dhungana, P.; Bhandari, B. Development of a continuous membrane nanobubble generation method applicable in liquid food processing. Int. J. Food Sci. Technol. 2021, 56, 4268–4277. [Google Scholar] [CrossRef]
- Joseph, S.; Bunjes, H. Evaluation of Shirasu Porous Glass (SPG) membrane emulsification for the preparation of colloidal lipid drug carrier dispersions. Eur. J. Pharm. Biopharm. 2014, 87, 178–186. [Google Scholar] [CrossRef]
- Ke, S.; Xiao, W.; Quan, N.; Dong, Y.; Zhang, L.; Hu, J. Formation and stability of bulk nanobubbles in different solutions. Langmuir 2019, 35, 5250–5256. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.; Abdelbaset, R.; Mahmoud, A.A.; Elkasabgy, N.A. Merits and advances of microfluidics in the pharmaceutical field: Design technologies and future prospects. Drug Deliv. 2022, 29, 1549–1570. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, A.A.; Zalloum, I.O.; Karshafian, R.; Kolios, M.C.; Tsai, S.S. High throughput microfluidic nanobubble generation by microporous membrane integration and controlled bubble shrinkage. J. Colloid Interface Sci. 2024, 653, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Salari, A.; Wang, Y.; He, X.; Kerr, L.; Darbandi, A.; de Leon, A.C.; Exner, A.A.; Kolios, M.C.; Yuen, D. Microfluidic generation of monodisperse nanobubbles by selective gas dissolution. Small 2021, 17, 2100345. [Google Scholar] [CrossRef]
- Shung, K.K. Diagnostic ultrasound: Past, present, and future. J. Med. Biol. Eng. 2011, 31, 371–374. [Google Scholar] [CrossRef]
- Mason, T.J. Therapeutic ultrasound an overview. Ultrason. Sonochemistry 2011, 18, 847–852. [Google Scholar] [CrossRef]
- Tharkar, P.; Varanasi, R.; Wong, W.S.F.; Jin, C.T.; Chrzanowski, W. Nano-enhanced drug delivery and therapeutic ultrasound for cancer treatment and beyond. Front. Bioeng. Biotechnol. 2019, 7, 324. [Google Scholar] [CrossRef]
- Krut, Z.; Gazit, D.; Gazit, Z.; Pelled, G. Applications of ultrasound-mediated gene delivery in regenerative medicine. Bioengineering 2022, 9, 190. [Google Scholar] [CrossRef]
- Unnikrishnan, S.; Klibanov, A.L. Microbubbles as ultrasound contrast agents for molecular imaging: Preparation and application. Am. J. Roentgenol. 2012, 199, 292–299. [Google Scholar] [CrossRef]
- Yusefi, H.; Helfield, B. Ultrasound contrast imaging: Fundamentals and emerging technology. Front. Phys. 2022, 10, 791145. [Google Scholar] [CrossRef]
- Perera, R.H.; Hernandez, C.; Zhou, H.; Kota, P.; Burke, A.; Exner, A.A. Ultrasound imaging beyond the vasculature with new generation contrast agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechno. 2015, 7, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-K. Ultrasound microbubble contrast agents for diagnostic and therapeutic applications: Current status and future design. Chang. Gung Med. J. 2012, 35, 125–139. [Google Scholar] [CrossRef]
- Exner, A.A.; Kolios, M.C. Bursting microbubbles: How nanobubble contrast agents can enable the future of medical ultrasound molecular imaging and image-guided therapy. Curr. Opin. Colloid Interface Sci. 2021, 54, 101463. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Tiwari, A.; Verma, A.; Jain, S.K. Ultrasound-based triggered drug delivery to tumors. Drug Deliv. Transl. Res. 2018, 8, 150–164. [Google Scholar] [CrossRef]
- Pua, E.C.; Zhong, P. Ultrasound-mediated drug delivery. IEEE Eng. Med. Biol. Mag. 2009, 28, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Liu, M.; Ojha, T.; Storm, G.; Kiessling, F.; Lammers, T. Ultrasound-mediated drug delivery to the brain: Principles, progress and prospects. Drug Discov. Today: Technol. 2016, 20, 41–48. [Google Scholar] [CrossRef]
- Sitta, J.; Howard, C.M. Applications of ultrasound-mediated drug delivery and gene therapy. Int. J. Mol. Sci. 2021, 22, 11491. [Google Scholar] [CrossRef]
- Tu, J.; Yu, A.C.H. Ultrasound-mediated drug delivery: Sonoporation mechanisms, biophysics, and critical factors. BME Front. 2022, 2022, 9807347. [Google Scholar] [CrossRef]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.; Moonen, C. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef]
- Wu, R.; Yang, X.; Dong, N.; Liu, Y.; Zhang, P. Nanobubbles for tumors: Imaging and drug carriers. J. Drug Deliv. Sci. Technol. 2021, 65, 102749. [Google Scholar] [CrossRef]
- Lapin, N.A.; Gill, K.; Shah, B.R.; Chopra, R. Consistent opening of the blood brain barrier using focused ultrasound with constant intravenous infusion of microbubble agent. Sci. Rep. 2020, 10, 16546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, S.; Luo, S.; Tang, P.; Wan, M.; Wu, D.; Gao, W. Ultrasound-assisted brain delivery of nanomedicines for brain tumor therapy: Advance and prospect. J. Nanobiotechnology 2022, 20, 287. [Google Scholar] [CrossRef] [PubMed]
- Venketasubramanian, N.; Yeo, L.L.; Tan, B.; Chan, B.P. Sonothrombolysis for Ischemic Stroke. J. Cardiovasc. Dev. Dis. 2024, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- El Kadi, S.; Porter, T.R.; Verouden, N.J.; van Rossum, A.C.; Kamp, O. Contrast ultrasound, Sonothrombolysis and Sonoperfusion in cardiovascular disease: Shifting to Theragnostic clinical trials. Cardiovasc. Imaging 2022, 15, 345–360. [Google Scholar]
- Zheng, L.; Shen, C.-L.; Li, J.-M.; Ma, Y.-L.; Yan, N.; Tian, X.-Q.; Zhao, Y.-Z. Assessment of the preventive effect against diabetic cardiomyopathy of FGF1-loaded nanoliposomes combined with microbubble cavitation by ultrasound. Front. Pharmacol. 2020, 10, 1535. [Google Scholar] [CrossRef]
- Nederhoed, J.H.; Tjaberinga, M.; Otten, R.H.; Evers, J.M.; Musters, R.J.; Wisselink, W.; Yeung, K.K. Therapeutic use of microbubbles and ultrasound in acute peripheral arterial thrombosis: A systematic review. Ultrasound Med. Biol. 2021, 47, 2821–2838. [Google Scholar] [CrossRef]
- Wasielewska, J.M.; White, A.R. Focused ultrasound-mediated drug delivery in humans—A path towards translation in neurodegenerative diseases. Pharm. Res. 2022, 39, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Prada, F.; Gennari, A.G.; Linville, I.M.; Mutersbaugh, M.E.; Chen, Z.; Sheybani, N.; DiMeco, F.; Padilla, F.; Hossack, J.A. Quantitative analysis of in-vivo microbubble distribution in the human brain. Sci. Rep. 2021, 11, 11797. [Google Scholar] [CrossRef]
- Yuan, J.; Xu, L.; Chien, C.-Y.; Yang, Y.; Yue, Y.; Fadera, S.; Stark, A.H.; Schwetye, K.E.; Nazeri, A.; Desai, R. First-in-human prospective trial of sonobiopsy in high-grade glioma patients using neuronavigation-guided focused ultrasound. NPJ Precis. Oncol. 2023, 7, 92. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, M.J.; Jung, H.H.; Chang, W.S.; Choi, H.S.; Rachmilevitch, I.; Zadicario, E.; Chang, J.W. One-year outcome of multiple blood–brain barrier disruptions with temozolomide for the treatment of glioblastoma. Front. Oncol. 2020, 10, 1663. [Google Scholar] [CrossRef]
- D’Haese, P.-F.; Ranjan, M.; Song, A.; Haut, M.W.; Carpenter, J.; Dieb, G.; Najib, U.; Wang, P.; Mehta, R.I.; Chazen, J.L. β-Amyloid plaque reduction in the hippocampus after focused ultrasound-induced blood–brain barrier opening in Alzheimer’s disease. Front. Hum. Neurosci. 2020, 14, 593672. [Google Scholar] [CrossRef] [PubMed]
- Rezai, A.R.; Ranjan, M.; D’Haese, P.-F.; Haut, M.W.; Carpenter, J.; Najib, U.; Mehta, R.I.; Chazen, J.L.; Zibly, Z.; Yates, J.R. Noninvasive hippocampal blood− brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc. Natl. Acad. Sci. USA 2020, 117, 9180–9182. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Pardo, J.A.; Gasca-Salas, C.; Fernández-Rodríguez, B.; Rodríguez-Rojas, R.; Del Alamo, M.; Obeso, I.; Hernández-Fernández, F.; Trompeta, C.; Martínez-Fernández, R.; Matarazzo, M. Striatal blood–brain barrier opening in Parkinson’s disease dementia: A pilot exploratory study. Mov. Disord. 2022, 37, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Du, T.; Yang, C.-L.; Li, T.; Li, X.-L.; Liu, W.; Zhang, P.; Dong, J.; Si, W.-Y.; Duan, R.-S. Extracellular vesicles encapsulated with caspase-1 inhibitor ameliorate experimental autoimmune myasthenia gravis through targeting macrophages. J. Control. Release 2023, 364, 458–472. [Google Scholar] [CrossRef]
- Gomes, G.S.; Frank, L.A.; Contri, R.V.; Longhi, M.S.; Pohlmann, A.R.; Guterres, S.S. Nanotechnology-based alternatives for the topical delivery of immunosuppressive agents in psoriasis. Int. J. Pharm. 2023, 631, 122535. [Google Scholar] [CrossRef]
- Abrahao, A.; Meng, Y.; Llinas, M.; Huang, Y.; Hamani, C.; Mainprize, T.; Aubert, I.; Heyn, C.; Black, S.E.; Hynynen, K. First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun. 2019, 10, 4373. [Google Scholar] [CrossRef]
- Mehta, R.I.; Carpenter, J.S.; Mehta, R.I.; Haut, M.W.; Wang, P.; Ranjan, M.; Najib, U.; D’Haese, P.-F.; Rezai, A.R. Ultrasound-mediated blood–brain barrier opening uncovers an intracerebral perivenous fluid network in persons with Alzheimer’s disease. Fluids Barriers CNS 2023, 20, 46. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Gould, A.; Amidei, C.; Ward, R.; Schmidt, K.A.; Zhang, D.Y.; Gomez, C.; Bebawy, J.F.; Liu, B.P.; Bouchoux, G. Repeated blood–brain barrier opening with an implantable ultrasound device for delivery of albumin-bound paclitaxel in patients with recurrent glioblastoma: A phase 1 trial. Lancet Oncol. 2023, 24, 509–522. [Google Scholar] [CrossRef]
- Karakatsani, M.E.; Ji, R.; Murillo, M.F.; Kugelman, T.; Kwon, N.; Lao, Y.-H.; Liu, K.; Pouliopoulos, A.N.; Honig, L.S.; Duff, K.E. Focused ultrasound mitigates pathology and improves spatial memory in Alzheimer’s mice and patients. Theranostics 2023, 13, 4102. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, P.B.; Latt, S.; Ravi, K.; Razavi, M. Clinical Applications of Micro/Nanobubble Technology in Neurological Diseases. Biomimetics 2024, 9, 645. https://doi.org/10.3390/biomimetics9100645
Patel PB, Latt S, Ravi K, Razavi M. Clinical Applications of Micro/Nanobubble Technology in Neurological Diseases. Biomimetics. 2024; 9(10):645. https://doi.org/10.3390/biomimetics9100645
Chicago/Turabian StylePatel, Parth B., Sun Latt, Karan Ravi, and Mehdi Razavi. 2024. "Clinical Applications of Micro/Nanobubble Technology in Neurological Diseases" Biomimetics 9, no. 10: 645. https://doi.org/10.3390/biomimetics9100645
APA StylePatel, P. B., Latt, S., Ravi, K., & Razavi, M. (2024). Clinical Applications of Micro/Nanobubble Technology in Neurological Diseases. Biomimetics, 9(10), 645. https://doi.org/10.3390/biomimetics9100645






