Innervation of an Ultrasound-Mediated PVDF-TrFE Scaffold for Skin-Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
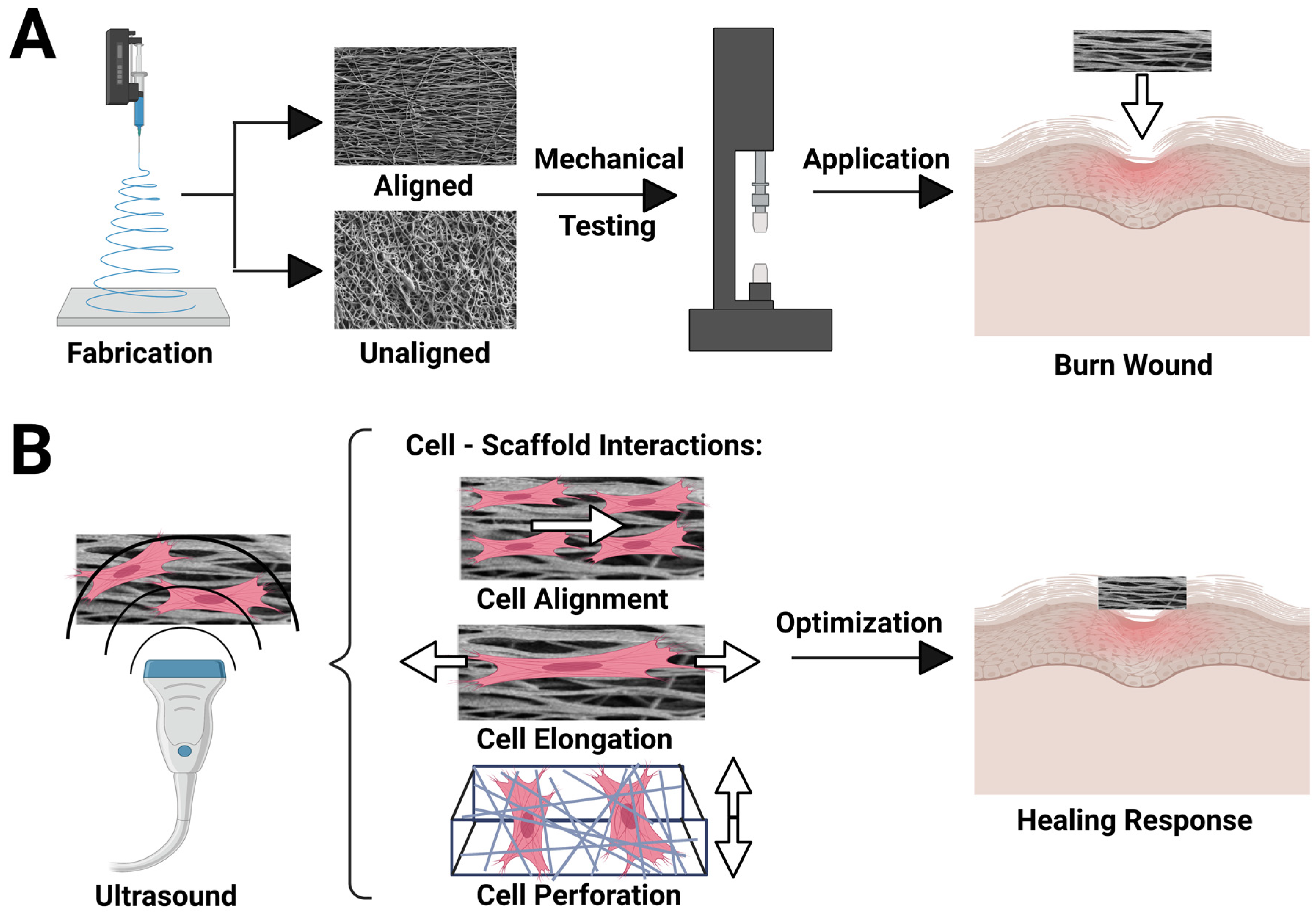
2.1. Fabrication of PVDF-TrFE Scaffolds
2.2. Porosity Measurement of PVDF-TrFE Fibers
2.3. Mechanical Testing of PVDF-TrFE Fibers
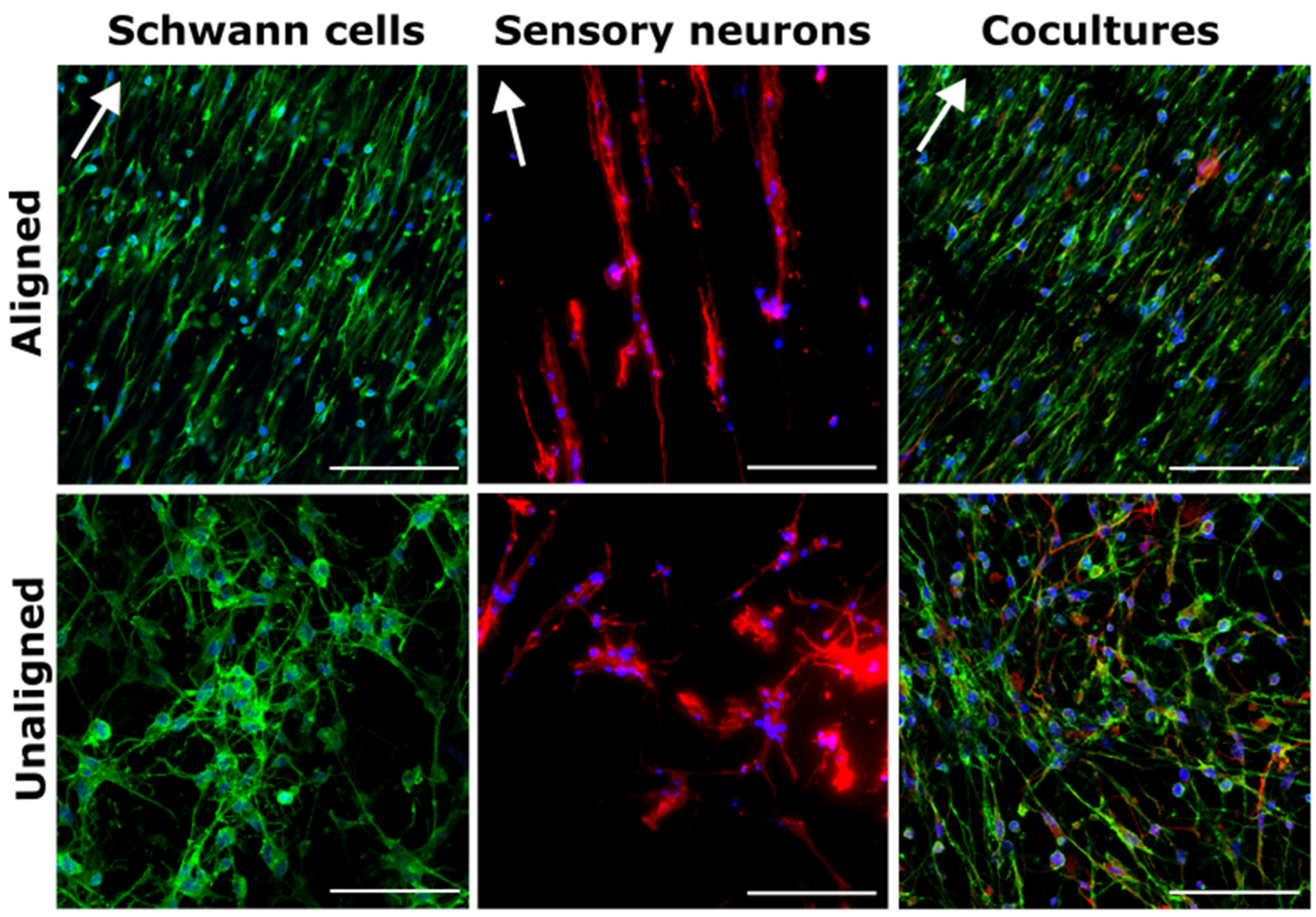
2.4. Cell Culture
2.5. hK Viability Assay
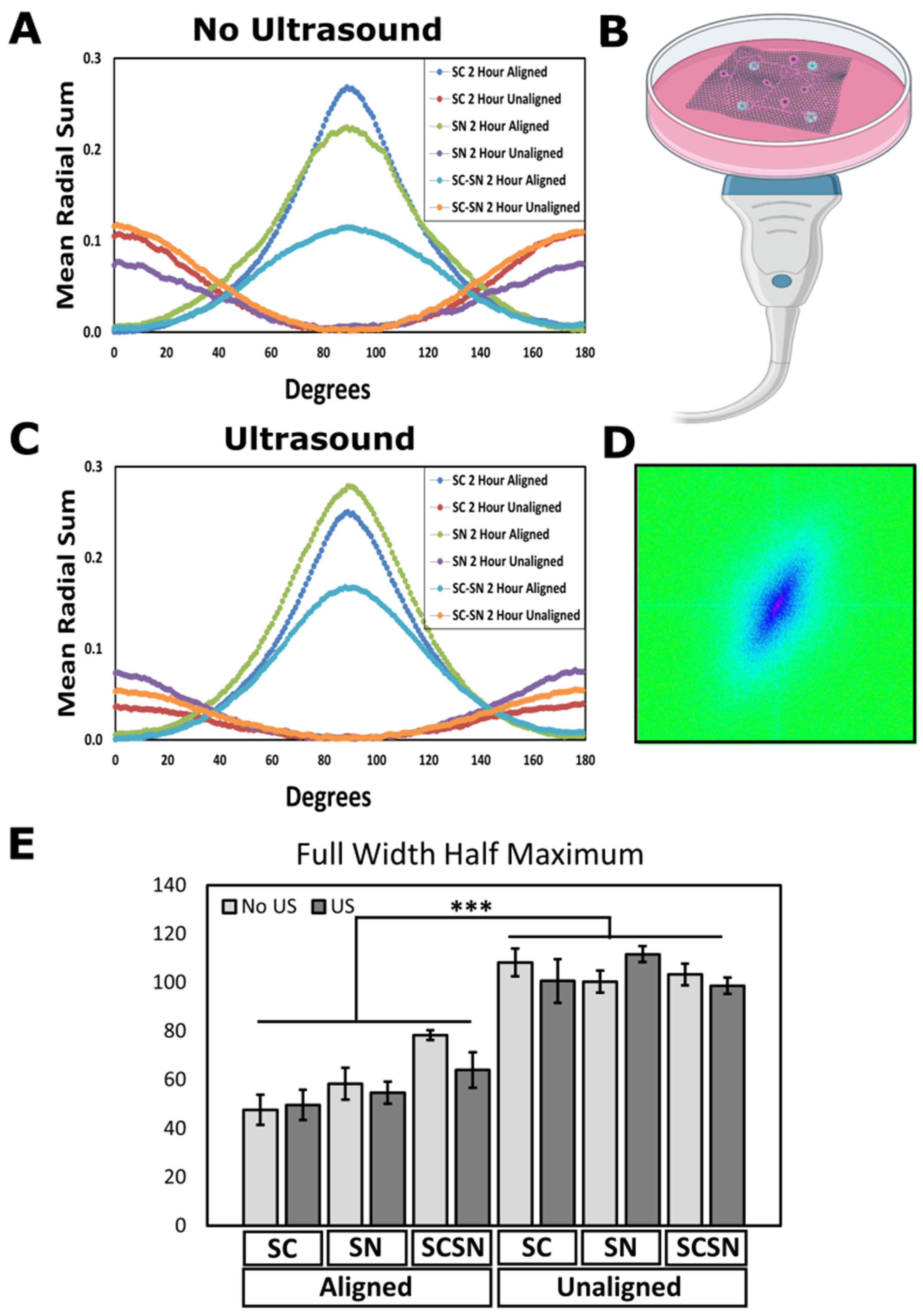
2.6. Ultrasound Stimulation
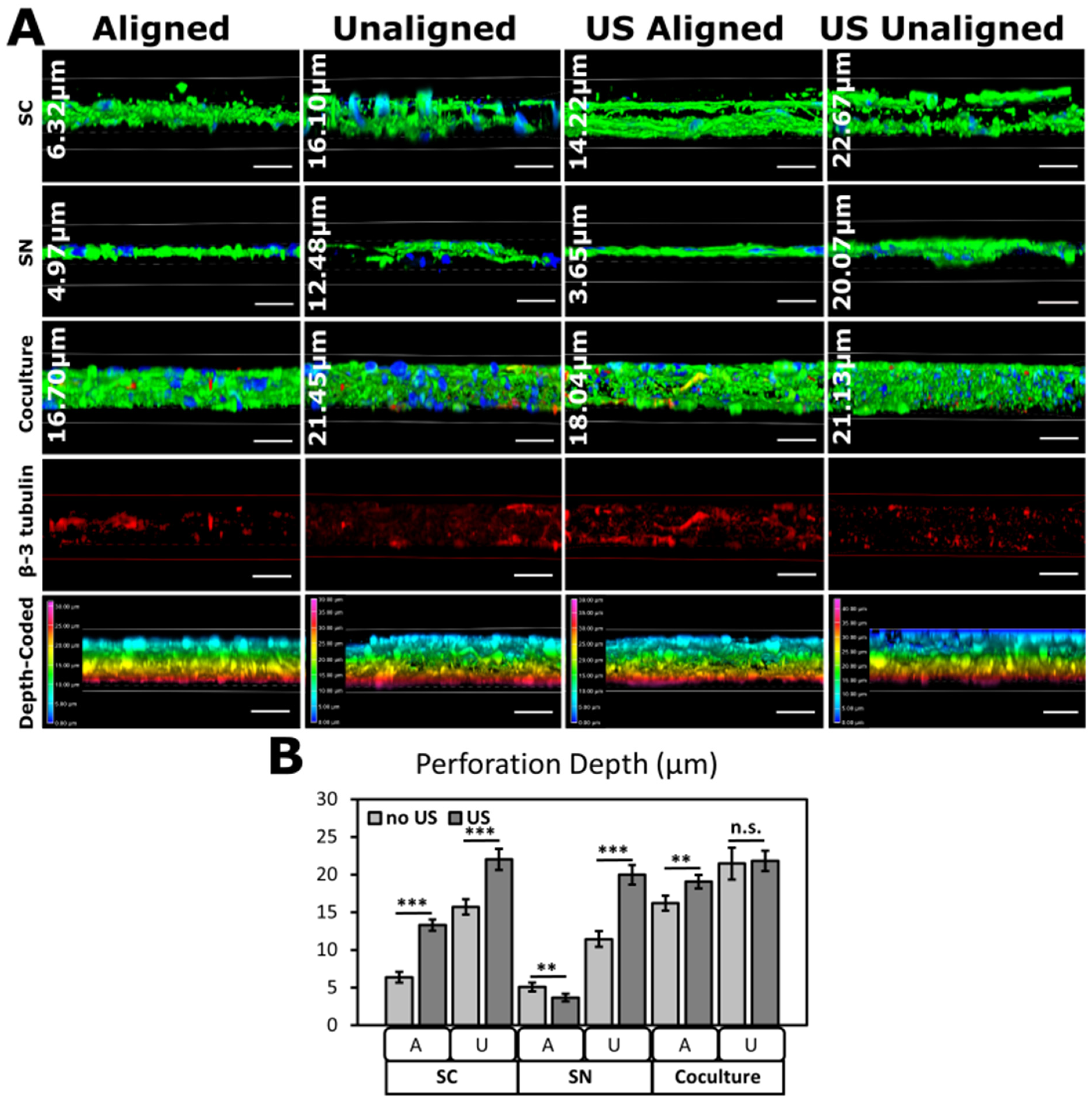
2.7. Immunofluorescence Staining and Confocal Microscopy
2.8. Statistical Analysis
3. Results and Discussion
3.1. PVDF-TrFE Scaffold Physical Characterization
3.2. Cell Alignment Quantification on Aligned and Unaligned PVDF-TrFE
3.3. Verification of hK Viability on PVDF-TrFE
3.4. Ultrasonic Stimulation of Cells on PVDF-TrFE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menorca, R.M.G.; Fussell, T.S.; Elfar, J.C. Nerve Physiology: Mechanisms of Injury and Recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Hall, S. The Response to Injury in the Peripheral Nervous System. J. Bone Jt. Surg. Br. 2005, 87, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Helm, P.A.; Johnson, R.E.; McIntosh Carlton, A. Peripheral Neurological Problems in the Acute Burn Patient. Burns 1977, 2, 123–125. [Google Scholar] [CrossRef]
- Gabriel, V.; Kowalske, K.J.; Holavanahalli, R.K. Assessment of Recovery from Burn-Related Neuropathy by Electrodiagnostic Testing. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2009, 30, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Burns. Available online: https://www.cdc.gov/masstrauma/factsheets/public/burns.pdf (accessed on 10 May 2023).
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. A concise review on tissue engineered artificial skin grafts for chronic wound treatment: Can we reconstruct functional skin tissue in vitro? Cells 2020, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.; Wu, P.; Zhang, W.; Zheng, Y.; Li, Q.; Jin, R.; Chen, H.; You, C.; Guo, S.; Han, C.; et al. Regeneration of Skin Appendages and Nerves: Current Status and Further Challenges. J. Transl. Med. 2020, 18, 53. [Google Scholar] [CrossRef]
- Pirotte, N.; Leynen, N.; Artois, T.; Smeets, K. Do You Have the Nerves to Regenerate? The Importance of Neural Signalling in the Regeneration Process. Dev. Biol. 2016, 409, 4–15. [Google Scholar] [CrossRef]
- Das, S.; Gordián-Vélez, W.J.; Ledebur, H.C.; Mourkioti, F.; Rompolas, P.; Chen, H.I.; Serruya, M.D.; Cullen, D.K. Innervation: The Missing Link for Biofabricated Tissues and Organs. npj Regen. Med. 2020, 5, 11. [Google Scholar] [CrossRef]
- Lehmann, H.C.; Höke, A. Schwann Cells as a Therapeutic Target for Peripheral Neuropathies. CNS Neurol. Disord. Drug Targets 2010, 9, 801–806. [Google Scholar] [CrossRef]
- Chen, Z.-L.; Yu, W.-M.; Strickland, S. Peripheral Regeneration. Annu. Rev. Neurosci. 2007, 30, 209–233. [Google Scholar] [CrossRef]
- Höke, A. Mechanisms of Disease: What Factors Limit the Success of Peripheral Nerve Regeneration in Humans? Nat. Clin. Pract. Neurol. 2006, 2, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.L.; Brophy, P.J. Mechanisms of Axon Ensheathment and Myelin Growth. Nat. Rev. Neurosci. 2005, 6, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, O.A.; Gordon, T. Effects of Short- and Long-Term Schwann Cell Denervation on Peripheral Nerve Regeneration, Myelination, and Size. Glia 2000, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Y.; Gordon, T. The Cellular and Molecular Basis of Peripheral Nerve Regeneration. Mol. Neurobiol. 1997, 14, 67–116. [Google Scholar] [CrossRef] [PubMed]
- Bunge, R.P.; Bunge, M.B.; Eldridge, C.F. Linkage between Axonal Ensheathment and Basal Lamina Production by Schwann Cells. Annu. Rev. Neurosci. 1986, 9, 305–328. [Google Scholar] [CrossRef]
- Bunge, M.B.; Clark, M.B.; Dean, A.C.; Eldridge, C.F.; Bunge, R.P. Schwann Cell Function Depends upon Axonal Signals and Basal Lamina Components. Ann. N. Y. Acad. Sci. 1990, 580, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R. Torsional Elasticity of Human Skin In Vivo. Pflug. Arch. 1973, 342, 255–260. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Arinzeh, T.L. The Influence of Piezoelectric Scaffolds on Neural Differentiation of Human Neural Stem/Progenitor Cells. Tissue Eng. Part A 2012, 18, 2063–2072. [Google Scholar] [CrossRef]
- Orkwis, J.A.; Wolf, A.K.; Shahid, S.M.; Smith, C.; Esfandiari, L.; Harris, G.M. Development of a Piezoelectric PVDF-TrFE Fibrous Scaffold to Guide Cell Adhesion, Proliferation, and Alignment. Macromol. Biosci. 2020, 20, e2000197. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Collins, G.; Arinzeh, T.L. Neurite Extension of Primary Neurons on Electrospun Piezoelectric Scaffolds. Acta Biomater. 2011, 7, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Gryshkov, O.; Al Halabi, F.; Kuhn, A.I.; Leal-Marin, S.; Freund, L.J.; Förthmann, M.; Meier, N.; Barker, S.-A.; Haastert-Talini, K.; Glasmacher, B. PVDF and P(VDF-TrFE) Electrospun Scaffolds for Nerve Graft Engineering: A Comparative Study on Piezoelectric and Structural Properties, and In Vitro Biocompatibility. Int. J. Mol. Sci. 2021, 22, 11373. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Livingston Arinzeh, T. Electrospun Nanofibrous Materials for Neural Tissue Engineering. Polymers 2011, 3, 413–426. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Wu, S.; Arinzeh, T.L.; Bunge, M.B. Enhanced Noradrenergic Axon Regeneration into Schwann Cell-Filled PVDF-TrFE Conduits after Complete Spinal Cord Transection. Biotechnol. Bioeng. 2017, 114, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Ikai, H.; Tamura, T.; Watanabe, T.; Itou, M.; Sugaya, A.; Iwabuchi, S.; Mikuni-Takagaki, Y.; Deguchi, S. Low-Intensity Pulsed Ultrasound Accelerates Periodontal Wound Healing after Flap Surgery. J. Periodontal Res. 2008, 43, 212–216. [Google Scholar] [CrossRef]
- Nakao, J.; Fujii, Y.; Kusuyama, J.; Bandow, K.; Kakimoto, K.; Ohnishi, T.; Matsuguchi, T. Low-Intensity Pulsed Ultrasound (LIPUS) Inhibits LPS-Induced Inflammatory Responses of Osteoblasts through TLR4-MyD88 Dissociation. Bone 2014, 58, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T.; Mochida, J.; Miyazaki, T.; Watanabe, T.; Iwabuchi, S.; Ando, K.; Hotta, T.; Sakai, D. Low-Intensity Pulsed Ultrasound Stimulates Cell Proliferation and Proteoglycan Production in Rabbit Intervertebral Disc Cells Cultured in Alginate. Biomaterials 2006, 27, 354–361. [Google Scholar] [CrossRef]
- Ito, A.; Wang, T.; Nakahara, R.; Kawai, H.; Nishitani, K.; Aoyama, T.; Kuroki, H. Ultrasound Therapy with Optimal Intensity Facilitates Peripheral Nerve Regeneration in Rats through Suppression of Pro-Inflammatory and Nerve Growth Inhibitor Gene Expression. PLoS ONE 2020, 15, e0234691. [Google Scholar] [CrossRef]
- Lyu, W.; Ma, Y.; Chen, S.; Li, H.; Wang, P.; Chen, Y.; Feng, X. Flexible Ultrasonic Patch for Accelerating Chronic Wound Healing. Adv. Healthc. Mater. 2021, 10, e2100785. [Google Scholar] [CrossRef]
- Mantri, Y.; Tsujimoto, J.; Penny, W.F.; Garimella, P.S.; Anderson, C.A.; Jokerst, J.V. Point-of-Care Ultrasound as a Tool to Assess Wound Size and Tissue Regeneration after Skin Grafting. Ultrasound Med. Biol. 2021, 47, 2550–2559. [Google Scholar] [CrossRef]
- Voigt, J.; Wendelken, M.; Driver, V.; Alvarez, O.M. Low-Frequency Ultrasound (20–40 KHz) as an Adjunctive Therapy for Chronic Wound Healing: A Systematic Review of the Literature and Meta-Analysis of Eight Randomized Controlled Trials. Int. J. Low. Extrem. Wounds 2011, 10, 190–199. [Google Scholar] [CrossRef]
- Orkwis, J.A.; Wolf, A.K.; Mularczyk, Z.J.; Bryan, A.E.; Smith, C.S.; Brown, R.; Krutko, M.; McCann, A.; Collar, R.M.; Esfandiari, L.; et al. Mechanical Stimulation of a Bioactive, Functionalized PVDF-TrFE Scaffold Provides Electrical Signaling for Nerve Repair Applications. Biomater. Adv. 2022, 140, 213081. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.E.; Krutko, M.; Westphal, J.; Sheth, M.; Esfandiari, L.; Harris, G.M. Ultrasound-Activated Piezoelectric Polyvinylidene Fluoride–Trifluoroethylene Scaffolds for Tissue Engineering Applications. Mil. Med. 2023, 188 (Suppl. S6), 61–66. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Chen, J.-C. Dorsal Root Ganglia Isolation and Primary Culture to Study Neurotransmitter Release. J. Vis. Exp. JoVE 2018, 57569. [Google Scholar] [CrossRef]
- Sleigh, J.N.; Weir, G.A.; Schiavo, G. A Simple, Step-by-Step Dissection Protocol for the Rapid Isolation of Mouse Dorsal Root Ganglia. BMC Res. Notes 2016, 9, 82. [Google Scholar] [CrossRef]
- Notorgiacomo, G.; Klug, J.; Rapp, S.; Boyce, S.T.; Schutte, S.C. A Bioreactor for Studying Negative Pressure Wound Therapy on Skin Grafts. Int. Wound J. 2022, 19, 633–642. [Google Scholar] [CrossRef]
- Xu, M.; McCanna, D.J.; Sivak, J.G. Use of the Viability Reagent PrestoBlue in Comparison with AlamarBlue and MTT to Assess the Viability of Human Corneal Epithelial Cells. J. Pharmacol. Toxicol. Methods 2015, 71, 1–7. [Google Scholar] [CrossRef]
- Xu, Z.; Orkwis, J.A.; DeVine, B.M.; Harris, G.M. Extracellular Matrix Cues Modulate Schwann Cell Morphology, Proliferation, and Protein Expression. J. Tissue Eng. Regen. Med. 2020, 14, 229–242. [Google Scholar] [CrossRef]
- Ahumada, M.; Jacques, E.; Calderon, C.; Martínez-Gómez, F. Porosity in Biomaterials: A Key Factor in the Development of Applied Materials in Biomedicine. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3503–3522. [Google Scholar] [CrossRef]
- Lawrence, B.J.; Madihally, S.V. Cell Colonization in Degradable 3D Porous Matrices. Cell Adhes. Migr. 2008, 2, 9–16. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, T.; Cui, L.; Wang, Y.; Zhang, P.; Chen, X. Improved Cellular Infiltration into 3D Interconnected Microchannel Scaffolds Formed by Using Melt-Spun Sacrificial Microfibers. RSC Adv. 2016, 6, 2131–2134. [Google Scholar] [CrossRef]
- Freyman, T.M.; Yannas, I.V.; Gibson, L.B. Cellular materials as porous scaffolds for tissue engineering. Prog. Mater. Sci. 2001, 46, 273–282. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, Y.; Wang, L.; Liu, D.; Qin, C.; Shi, Y. A review of preparation methods of porous skin tissue engineering scaffolds. Mater. Today Commun. 2022, 22, 104109. [Google Scholar] [CrossRef]
- Ico, G.; Showalter, A.; Bosze, W.; Gott, S.C.; Kim, B.S.; Rao, M.P.; Myung, N.V.; Nam, J. Size-Dependent Piezoelectric and Mechanical Properties of Electrospun P(VDF-TrFE) Nanofibers for Enhanced Energy Harvesting. J. Mater. Chem. A 2016, 4, 2293–2304. [Google Scholar] [CrossRef]
- Bae, J.-H.; Chang, S.-H. A New Approach to Fabricate Poly(Vinylidene Fluoride-Trifluoroethylene) Fibers Using a Torsion-Stretching Method and Characterization of Their Piezoelectric Properties. Compos. Part B Eng. 2016, 99, 112–120. [Google Scholar] [CrossRef]
- Persano, L.; Dagdeviren, C.; Su, Y.; Zhang, Y.; Girardo, S.; Pisignano, D.; Huang, Y.; Rogers, J.A. High Performance Piezoelectric Devices Based on Aligned Arrays of Nanofibers of Poly(Vinylidenefluoride-Co-Trifluoroethylene). Nat. Commun. 2013, 4, 1633. [Google Scholar] [CrossRef] [PubMed]
- Manssor, N.A.S.; Radzi, Z.; Yahya, N.A.; Mohamad Yusof, L.; Hariri, F.; Khairuddin, N.H.; Abu Kasim, N.H.; Czernuszka, J.T. Characteristics and Young’s Modulus of Collagen Fibrils from Expanded Skin Using Anisotropic Controlled Rate Self-Inflating Tissue Expander. Skin Pharmacol. Physiol. 2016, 29, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Manschot, J.F.; Brakkee, A.J. The Measurement and Modelling of the Mechanical Properties of Human Skin In Vivo—I. The Measurement. J. Biomech. 1986, 19, 511–515. [Google Scholar] [CrossRef]
- Blair, M.J.; Jones, J.D.; Woessner, A.E.; Quinn, K.P. Skin Structure-Function Relationships and the Wound Healing Response to Intrinsic Aging. Adv. Wound Care 2020, 9, 127–143. [Google Scholar] [CrossRef]
- Adadi, N.; Yadid, M.; Gal, I.; Asulin, M.; Feiner, R.; Edri, R.; Dvir, T. Electrospun fibrous PVDF-TrFe scaffolds for cardiac tissue engineering, differentiation, and maturation. Adv. Mater. Technol. 2020, 5, 1900820. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Anniadh, A.N.; Bruyere, K.; Otténio, M.; Xie, H.; Gilchrist, M.D. Dynamic Tensile Properties of Human Skin; International Research Council on the Biomechanics of Injury: Dublin, Ireland, 2012. [Google Scholar]
- Pailler-Mattei, C.; Bec, S.; Zahouani, H. In Vivo Measurements of the Elastic Mechanical Properties of Human Skin by Indentation Tests. Med. Eng. Phys. 2008, 30, 599–606. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Lelonkiewicz, M.; Wieczorowski, M. Age-Dependent Biomechanical Properties of the Skin. Postep. Dermatol. Alergol. 2013, 30, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kumar, S.J.; Mahapatra, D.R.; Rathod, V.T. Development of P(VDF-Trfe) Films and Its Quasi-Static and Dynamic Strain Response. Int. J. Eng. Res. Technol. 2013, 2, 2598–2605. [Google Scholar] [CrossRef]
- Lam, T.-N.; Ma, C.-Y.; Hsiao, P.-H.; Ko, W.-C.; Huang, Y.-J.; Lee, S.-Y.; Jain, J.; Huang, E.-W. Tunable Mechanical and Electrical Properties of Coaxial Electrospun Composite Nanofibers of P(VDF-TrFE) and P(VDF-TrFE-CTFE). Int. J. Mol. Sci. 2021, 22, 4639. [Google Scholar] [CrossRef] [PubMed]
- Lafrance, H.; Yahia, L.; Germain, L.; Guillot, M.; Auger, F.A. Study of the Tensile Properties of Living Skin Equivalents. Biomed. Mater. Eng. 1995, 5, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Crichton, M.L.; Chen, X.; Huang, H.; Kendall, M.A.F. Elastic Modulus and Viscoelastic Properties of Full Thickness Skin Characterised at Micro Scales. Biomaterials 2013, 34, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Mak, A.F. Effective Elastic Properties for Lower Limb Soft Tissues from Manual Indentation Experiment. IEEE Trans. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 1999, 7, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Boyer, G.; Laquièze, L.; Le Bot, A.; Laquièze, S.; Zahouani, H. Dynamic Indentation on Human Skin In Vivo: Ageing Effects. Skin Res. Technol. 2009, 15, 55–67. [Google Scholar] [CrossRef]
- Boyer, G.; Zahouani, H.; Le Bot, A.; Laquieze, L. In Vivo Characterization of Viscoelastic Properties of Human Skin Using Dynamic Micro-Indentation. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 4584–4587. [Google Scholar] [CrossRef]
- Hendriks, F.M.; Brokken, D.; van Eemeren, J.T.W.M.; Oomens, C.W.J.; Baaijens, F.P.T.; Horsten, J.B.a.M. A Numerical-Experimental Method to Characterize the Non-Linear Mechanical Behaviour of Human Skin. Skin Res. Technol. 2003, 9, 274–283. [Google Scholar] [CrossRef]
- Diridollou, S.; Patat, F.; Gens, F.; Vaillant, L.; Black, D.; Lagarde, J.M.; Gall, Y.; Berson, M. In Vivo Model of the Mechanical Properties of the Human Skin under Suction. Skin Res. Technol. 2000, 6, 214–221. [Google Scholar] [CrossRef]
- Liang, X.; Boppart, S.A. Biomechanical Properties of In Vivo Human Skin from Dynamic Optical Coherence Elastography. IEEE Trans. Biomed. Eng. 2010, 57, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Agache, P.G.; Monneur, C.; Leveque, J.L.; De Rigal, J. Mechanical Properties and Young’s Modulus of Human Skin In Vivo. Arch. Dermatol. Res. 1980, 269, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Ankersen, J.; Birkbeck, A.E.; Thomson, R.D.; Vanezis, P. Puncture Resistance and Tensile Strength of Skin Simulants. Proc. Inst. Mech. Eng. H 1999, 213, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Jacquemoud, C.; Bruyere-Garnier, K.; Coret, M. Methodology to Determine Failure Characteristics of Planar Soft Tissues Using a Dynamic Tensile Test. J. Biomech. 2007, 40, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Ottenio, M.; Tran, D.; Ní Annaidh, A.; Gilchrist, M.D.; Bruyère, K. Strain Rate and Anisotropy Effects on the Tensile Failure Characteristics of Human Skin. J. Mech. Behav. Biomed. Mater. 2015, 41, 241–250. [Google Scholar] [CrossRef]
- Kirby, M.A.; Tang, P.; Liou, H.-C.; Kuriakose, M.; Pitre, J.J.; Pham, T.N.; Ettinger, R.E.; Wang, R.K.; O’Donnell, M.; Pelivanov, I. Probing Elastic Anisotropy of Human Skin In Vivo with Light Using Non-Contact Acoustic Micro-Tapping OCE and Polarization Sensitive OCT. Sci. Rep. 2022, 12, 3963. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Tsai, W.-C.; Chang, S.-H. Collagen-PVA Aligned Nanofiber on Collagen Sponge as Bi-Layered Scaffold for Surface Cartilage Repair. J. Biomater. Sci. Polym. Ed. 2017, 28, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Moncayo-Donoso, M.; Rico-Llanos, G.A.; Garzón-Alvarado, D.A.; Becerra, J.; Visser, R.; Fontanilla, M.R. The Effect of Pore Directionality of Collagen Scaffolds on Cell Differentiation and In Vivo Osteogenesis. Polymers 2021, 13, 3187. [Google Scholar] [CrossRef]
- Valenzuela-Rojo, R.D.; López-Cervantes, J.; Sánchez-Machado, D.I.; Escárcega-Galaz, A.A.; del Rosario Martínez-Macias, M. Antibacterial, Mechanical and Physical Properties of Collagen—Chitosan Sponges from Aquatic Source. Sustain. Chem. Pharm. 2020, 15, 100218. [Google Scholar] [CrossRef]
- Ghodbane, S.A.; Dunn, M.G. Physical and Mechanical Properties of Cross-Linked Type I Collagen Scaffolds Derived from Bovine, Porcine, and Ovine Tendons. J. Biomed. Mater. Res. A 2016, 104, 2685–2692. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yoshizawa, Y.; Yanagiguchi, K.; Ikeda, T.; Yamada, S.; Hayashi, Y. The Characterization of Fish (Tilapia) Collagen Sponge as a Biomaterial. Int. J. Polym. Sci. 2015, 2015, 957385. [Google Scholar] [CrossRef]
- Seo, Y.-K.; Youn, H.-H.; Park, C.-S.; Song, K.-Y.; Park, J.-K. Reinforced Bioartificial Dermis Constructed with Collagen Threads. Biotechnol. Bioprocess Eng. 2008, 13, 745–751. [Google Scholar] [CrossRef]
- Patel, J.M.; Jackson, R.C.; Schneider, G.L.; Ghodbane, S.A.; Dunn, M.G. Carbodiimide Cross-Linking Counteracts the Detrimental Effects of Gamma Irradiation on the Physical Properties of Collagen-Hyaluronan Sponges. J. Mater. Sci. Mater. Med. 2018, 29, 75. [Google Scholar] [CrossRef] [PubMed]
- Cady, E.; Orkwis, J.A.; Weaver, R.; Conlin, L.; Madigan, N.N.; Harris, G.M. Micropatterning Decellularized ECM as a Bioactive Surface to Guide Cell Alignment, Proliferation, and Migration. Bioengineering 2020, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, C.; Zhang, L.; Huang, Z.; Zhao, P.; Chen, R.; Pang, M.; Chen, Z.; He, L.; Luo, C.; et al. Interaction of IPSC-Derived Neural Stem Cells on Poly(L-Lactic Acid) Nanofibrous Scaffolds for Possible Use in Neural Tissue Engineering. Int. J. Mol. Med. 2018, 41, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of Nano/Micro Scale Poly(L-Lactic Acid) Aligned Fibers and Their Potential in Neural Tissue Engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.; Deumens, R.; Beckmann, C.; Olde Damink, L.; Schügner, F.; Heschel, I.; Sellhaus, B.; Weis, J.; Jahnen-Dechent, W.; Brook, G.A.; et al. In Vitro Cell Alignment Obtained with a Schwann Cell Enriched Microstructured Nerve Guide with Longitudinal Guidance Channels. Biomaterials 2009, 30, 169–179. [Google Scholar] [CrossRef]
- Jin, G.-Z.; Kim, M.; Shin, U.S.; Kim, H.-W. Neurite Outgrowth of Dorsal Root Ganglia Neurons Is Enhanced on Aligned Nanofibrous Biopolymer Scaffold with Carbon Nanotube Coating. Neurosci. Lett. 2011, 501, 10–14. [Google Scholar] [CrossRef]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. The Effect of the Alignment of Electrospun Fibrous Scaffolds on Schwann Cell Maturation. Biomaterials 2008, 29, 653–661. [Google Scholar] [CrossRef]
- Harris, G.M.; Madigan, N.N.; Lancaster, K.Z.; Enquist, L.W.; Windebank, A.J.; Schwartz, J.; Schwarzbauer, J.E. Nerve Guidance by a Decellularized Fibroblast Extracellular Matrix. Matrix Biol. J. Int. Soc. Matrix Biol. 2017, 60–61, 176–189. [Google Scholar] [CrossRef]
- Wu, S.; Chen, M.-S.; Maurel, P.; Lee, Y.-S.; Bunge, M.B.; Arinzeh, T.L. Aligned Fibrous PVDF-TrFE Scaffolds with Schwann Cells Support Neurite Extension and Myelination In Vitro. J. Neural Eng. 2018, 15, 056010. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Liu, Z.; Hu, M.; Wang, C.; Zhang, X.; Shi, B.; Fan, Y.; Cui, Y.; Li, Z.; Ren, K. Piezoelectric Nanofibrous Scaffolds as In Vivo Energy Harvesters for Modifying Fibroblast Alignment and Proliferation in Wound Healing. Nano Energy 2018, 43, 63–71. [Google Scholar] [CrossRef]
- Agarwal, R.; Liu, G.; Tam, N.W.; Gratzer, P.F.; Frampton, J.P. Precision Cell Delivery in Biphasic Polymer Systems Enhances Growth of Keratinocytes in Culture and Promotes Their Attachment on Acellular Dermal Matrices. J. Tissue Eng. Regen. Med. 2019, 13, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Gómez, N.; Freije, A.; Gandarillas, A. Keratinocyte Differentiation by Flow Cytometry. Methods Mol. Biol. 2020, 2109, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Foss, M.; Besenbacher, F. Influence of Nanoscale Surface Topography on Protein Adsorption and Cellular Response. Nano Today 2010, 5, 66–78. [Google Scholar] [CrossRef]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties. Colloids Interfaces 2019, 3, 48. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Davidsen, M.B.; Teixeira, J.F.L.; Dehli, J.; Karlsson, C.; Kraft, D.; Souza, P.P.C.; Foss, M. Post-Treatments of Polydopamine Coatings Influence Cellular Response. Colloids Surf. B Biointerfaces 2021, 207, 111972. [Google Scholar] [CrossRef]
- Gorbahn, M.; Klein, M.O.; Lehnert, M.; Ziebart, T.; Brüllmann, D.; Köper, I.; Wagner, W.; Al-Nawas, B.; Veith, M. Promotion of Osteogenic Cell Response Using Quasicovalent Immobilized Fibronectin on Titanium Surfaces: Introduction of a Novel Biomimetic Layer System. J. Oral Maxillofac. Surg. 2012, 70, 1827–1834. [Google Scholar] [CrossRef]
- Teixeira, L.N.; Crippa, G.E.; Trabuco, A.C.; Gimenes, R.; Zaghete, M.A.; Palioto, D.B.; de Oliveira, P.T.; Rosa, A.L.; Beloti, M.M. In Vitro Biocompatibility of Poly(Vinylidene Fluoride-Trifluoroethylene)/Barium Titanate Composite Using Cultures of Human Periodontal Ligament Fibroblasts and Keratinocytes. Acta Biomater. 2010, 6, 979–989. [Google Scholar] [CrossRef]
- Ranjbarvan, P.; Golchin, A.; Azari, A.; Niknam, Z. The bilayer skin substitute based on human adipose-derived mesenchymal stem cells and neonate keratinocytes on the 3D nanofibrous PCL-platelet gel scaffold. Polym. Bull. 2022, 79, 4013–4030. [Google Scholar] [CrossRef]
- Ventre, D.; Puzan, M.; Ashbolt, E.; Koppes, A. Enhanced Total Neurite Outgrowth and Secondary Branching in Dorsal Root Ganglion Neurons Elicited by Low Intensity Pulsed Ultrasound. J. Neural Eng. 2018, 15, 046013. [Google Scholar] [CrossRef] [PubMed]
- Ventre, D.M.; Cluff, A.; Gagnon, C.; Diaz Vera, D.; Koppes, R.A.; Koppes, A.N. The Effects of Low Intensity Focused Ultrasonic Stimulation on Dorsal Root Ganglion Neurons and Schwann Cells In Vitro. J. Neurosci. Res. 2021, 99, 374–391. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Xu, T.-M.; Wei, Y.-B.; Gao, X.-X.; Sun, J.-C.; Wang, Y.; Kong, Q.-J.; Shi, J.-G. Low-Intensity Pulsed Ultrasound Treatment Accelerates Angiogenesis by Activating YAP/TAZ in Human Umbilical Vein Endothelial Cells. Ultrasound Med. Biol. 2018, 44, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-X.; Liu, Y.-J.; Chen, X.; Zhang, X.; Xu, J.; Yang, K.; Wang, D.; Lin, S.; Ye, J. Low-Intensity Pulsed Ultrasound Protects Retinal Ganglion Cell from Optic Nerve Injury Induced Apoptosis via Yes Associated Protein. Front. Cell. Neurosci. 2018, 12, 160. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westphal, J.A.; Bryan, A.E.; Krutko, M.; Esfandiari, L.; Schutte, S.C.; Harris, G.M. Innervation of an Ultrasound-Mediated PVDF-TrFE Scaffold for Skin-Tissue Engineering. Biomimetics 2024, 9, 2. https://doi.org/10.3390/biomimetics9010002
Westphal JA, Bryan AE, Krutko M, Esfandiari L, Schutte SC, Harris GM. Innervation of an Ultrasound-Mediated PVDF-TrFE Scaffold for Skin-Tissue Engineering. Biomimetics. 2024; 9(1):2. https://doi.org/10.3390/biomimetics9010002
Chicago/Turabian StyleWestphal, Jennifer A., Andrew E. Bryan, Maksym Krutko, Leyla Esfandiari, Stacey C. Schutte, and Greg M. Harris. 2024. "Innervation of an Ultrasound-Mediated PVDF-TrFE Scaffold for Skin-Tissue Engineering" Biomimetics 9, no. 1: 2. https://doi.org/10.3390/biomimetics9010002
APA StyleWestphal, J. A., Bryan, A. E., Krutko, M., Esfandiari, L., Schutte, S. C., & Harris, G. M. (2024). Innervation of an Ultrasound-Mediated PVDF-TrFE Scaffold for Skin-Tissue Engineering. Biomimetics, 9(1), 2. https://doi.org/10.3390/biomimetics9010002






