Efficient and Selective Oxygenation of Cycloalkanes and Alkyl Aromatics with Oxygen through Synergistic Catalysis of Bimetallic Active Centers in Two-Dimensional Metal-Organic Frameworks Based on Metalloporphyrins
Abstract
1. Introduction
2. Experimental Section
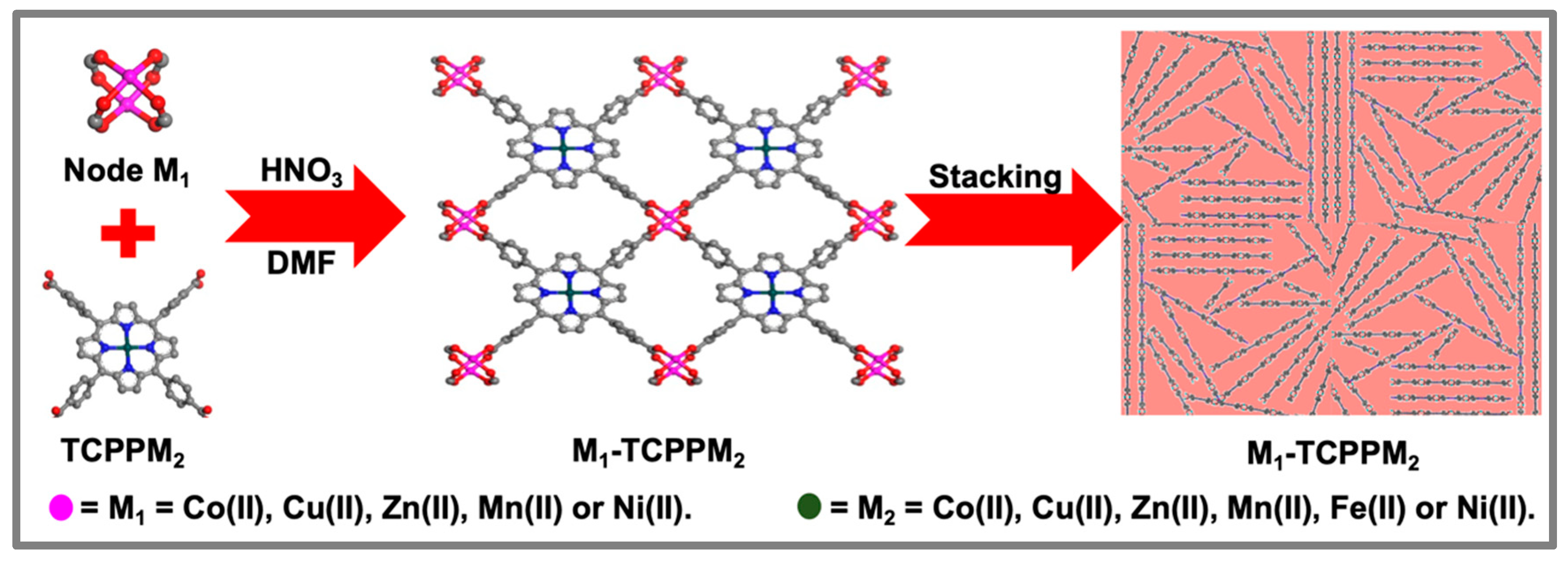
2.1. Syntheses of Two-Dimensional MOF M1-TCPPM2
2.2. Catalytic C–H Bonds Oxygenation with Oxygen
2.3. Apparent Kinetic Research
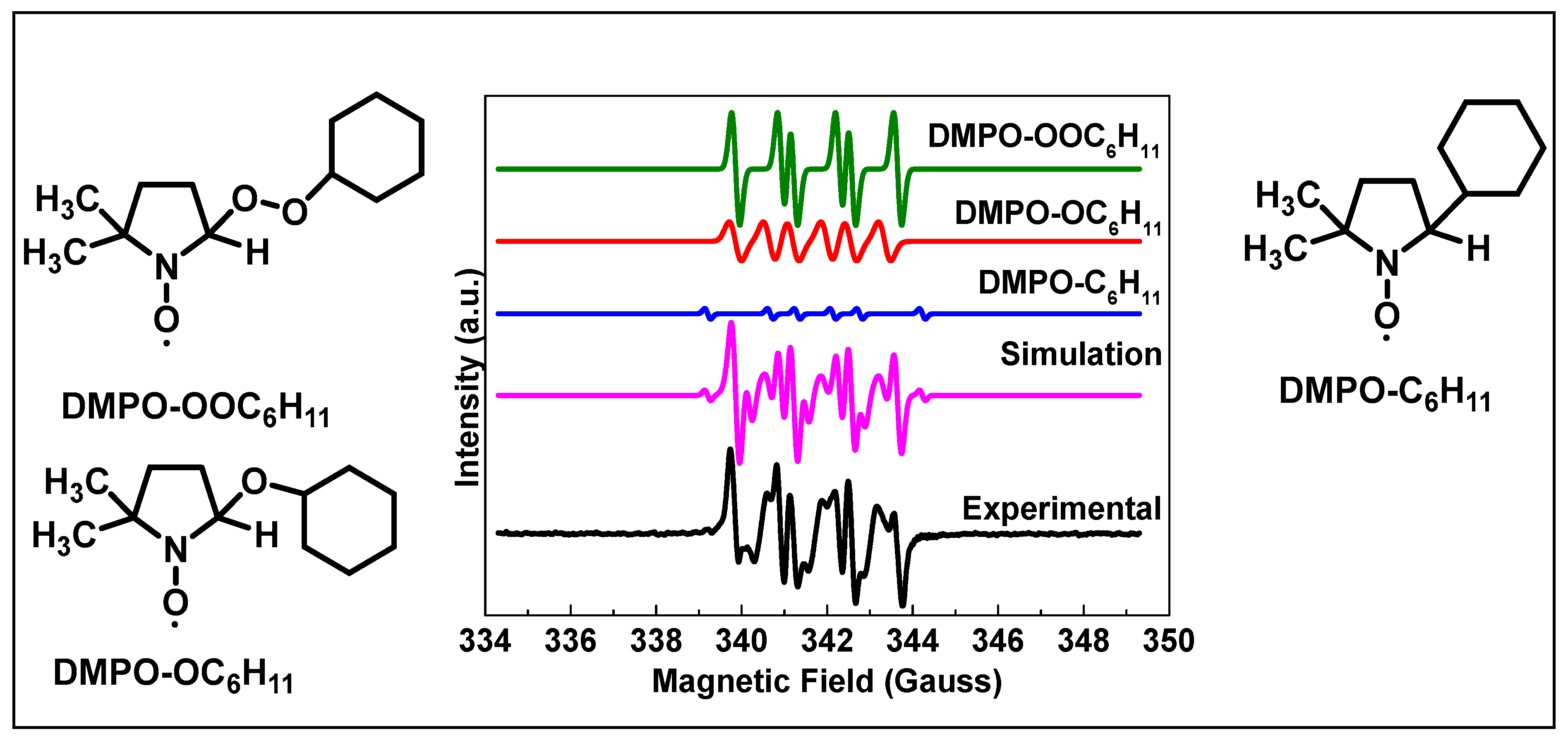
2.4. Electron Paramagnetic Resonance (EPR) Analyses
3. Results and Discussion
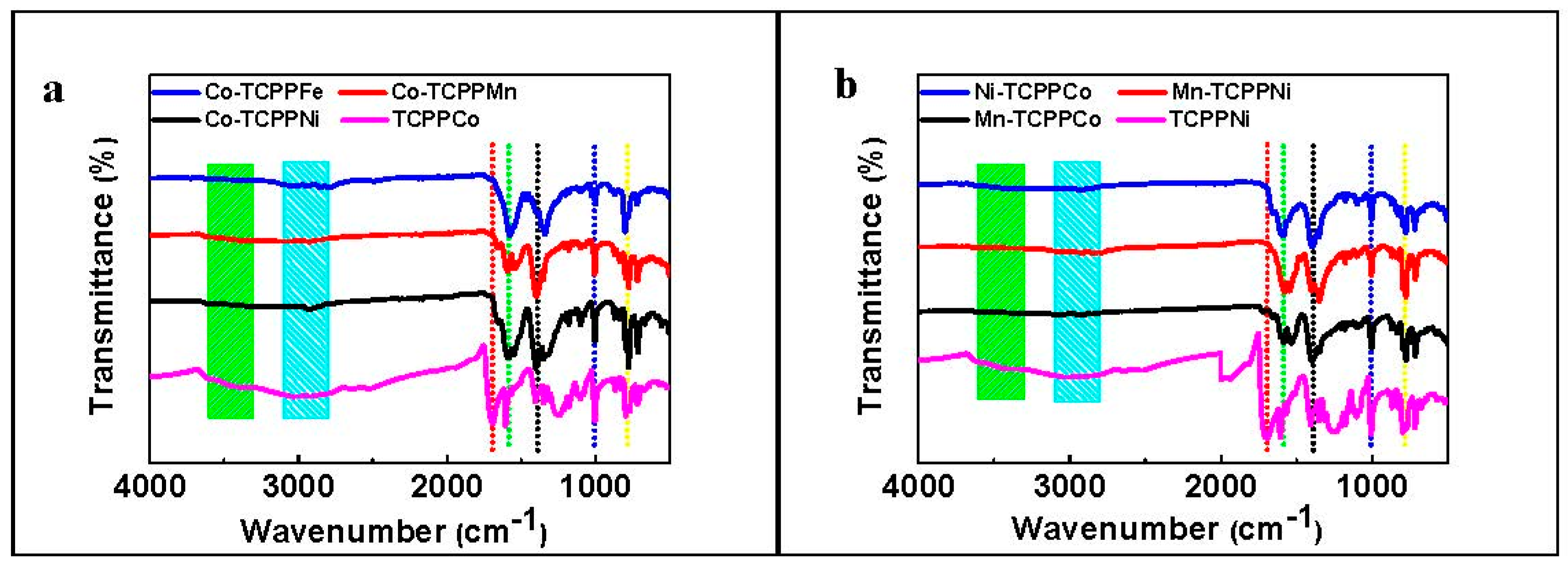
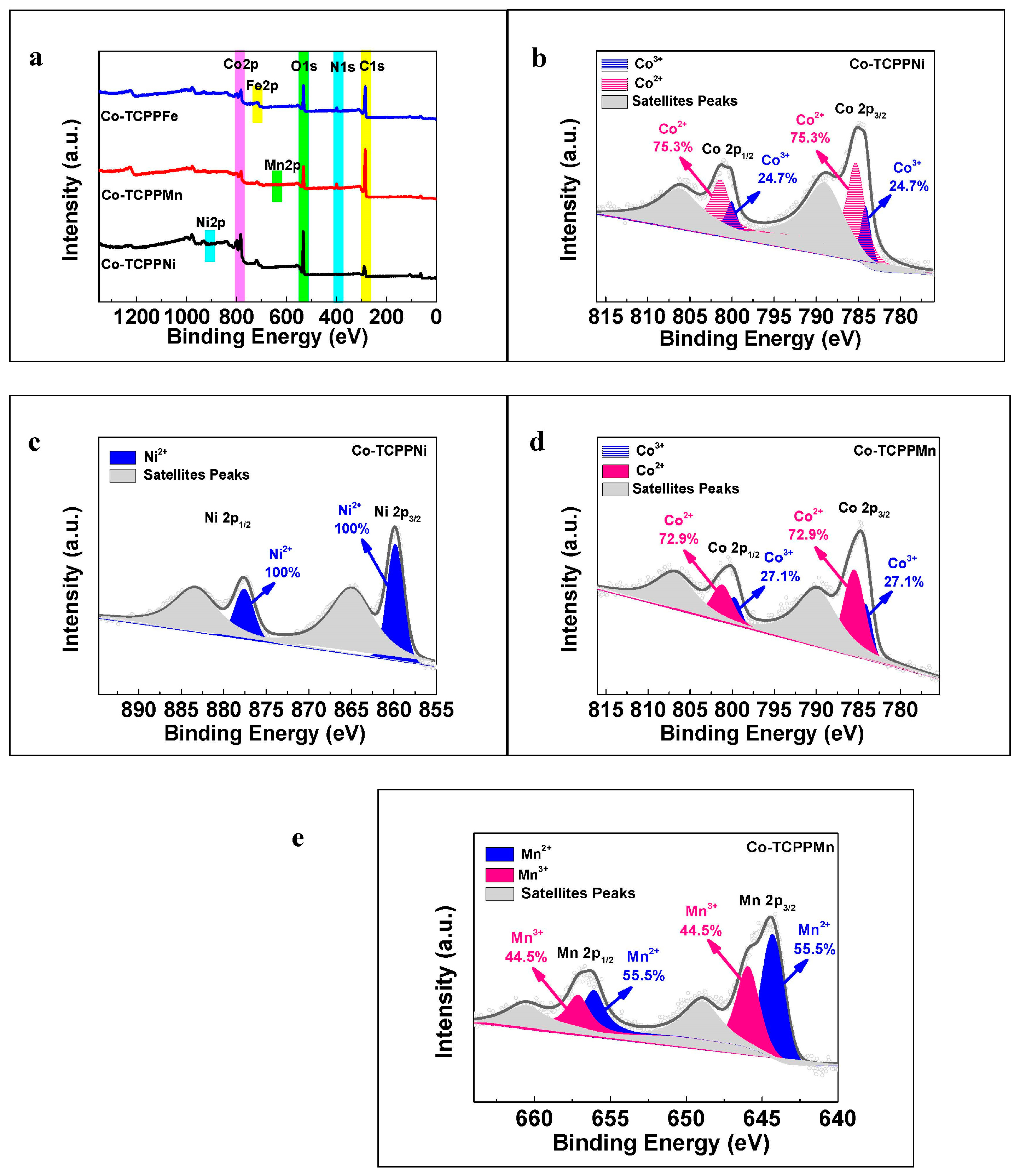
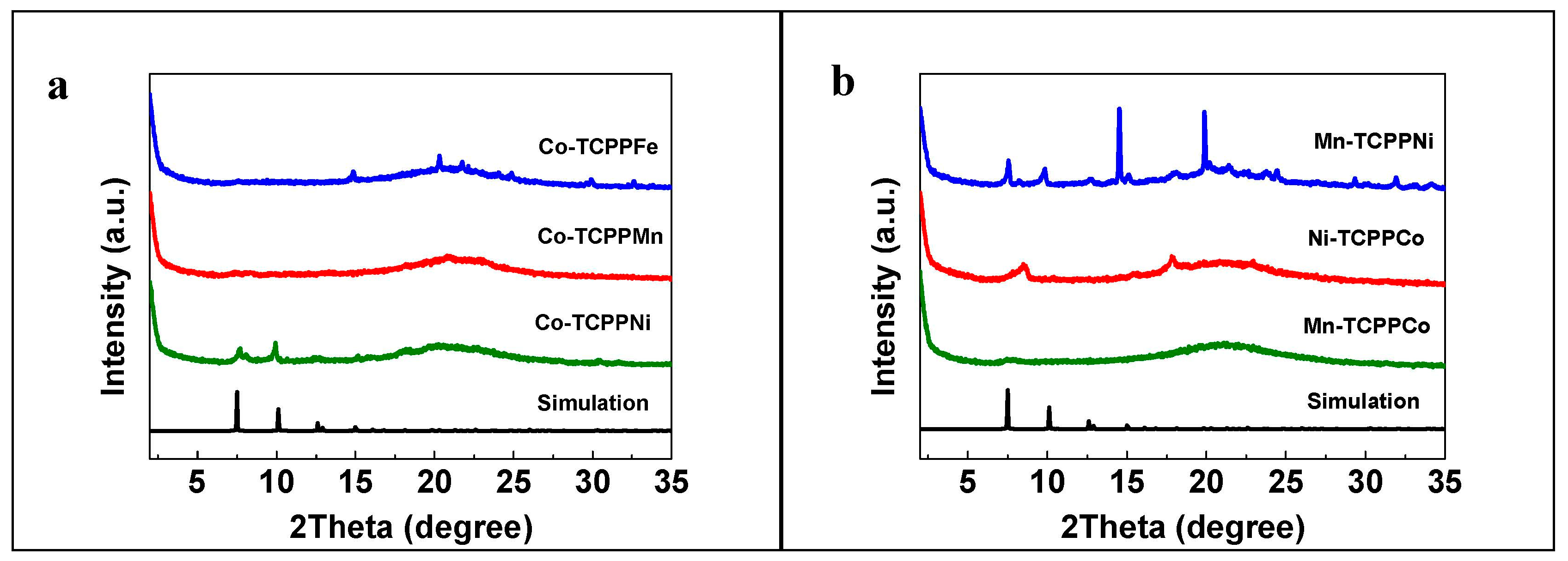
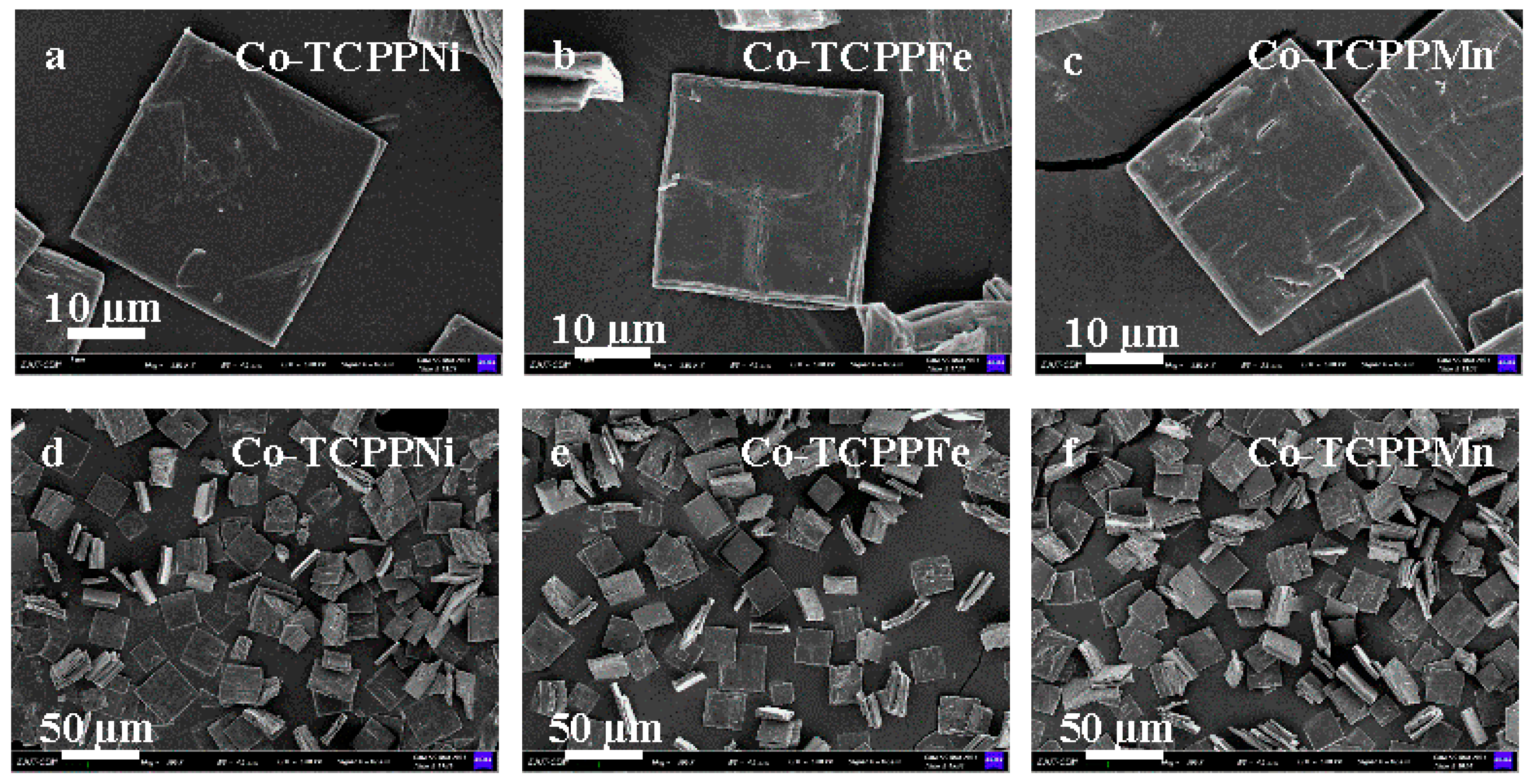
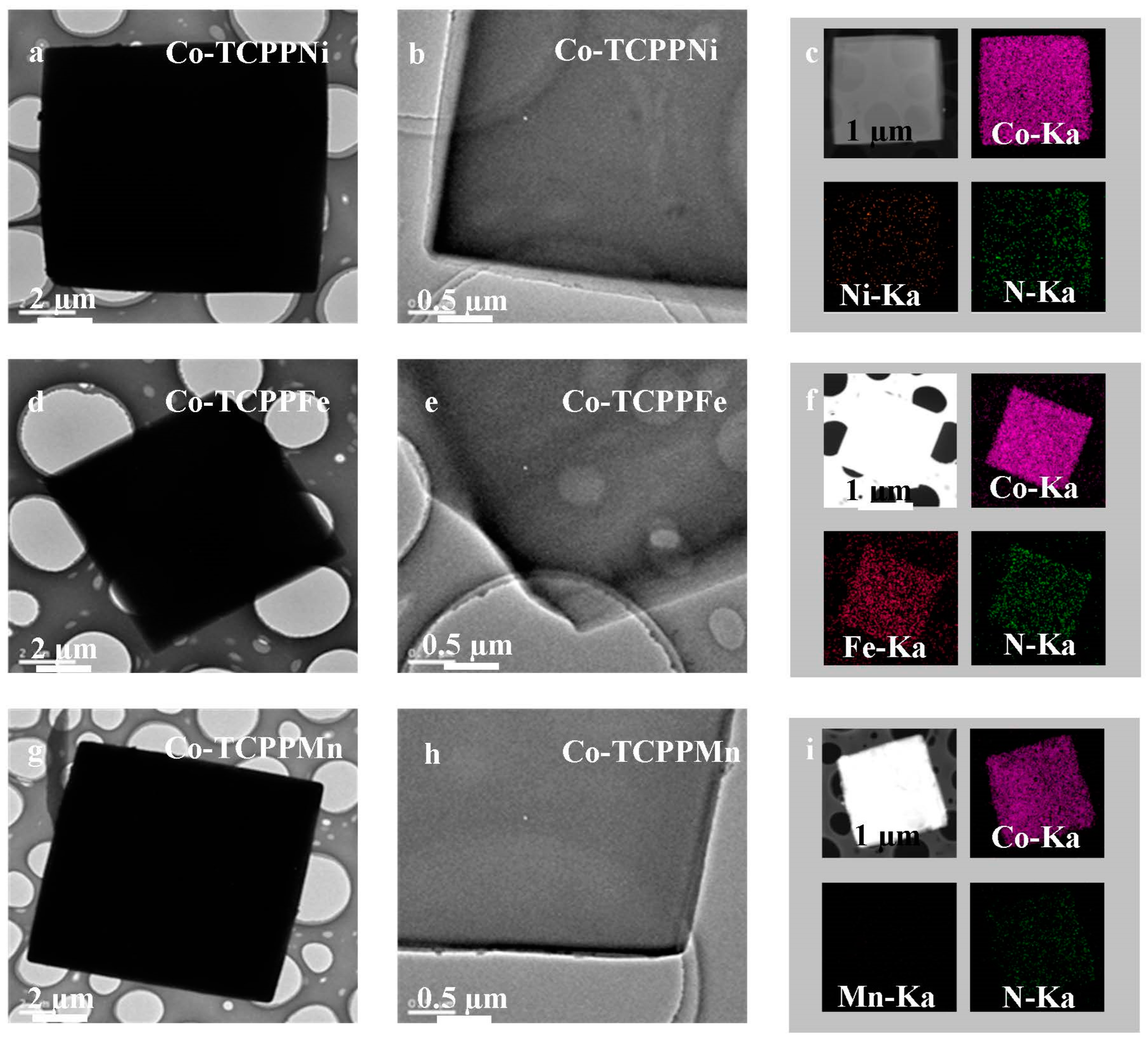
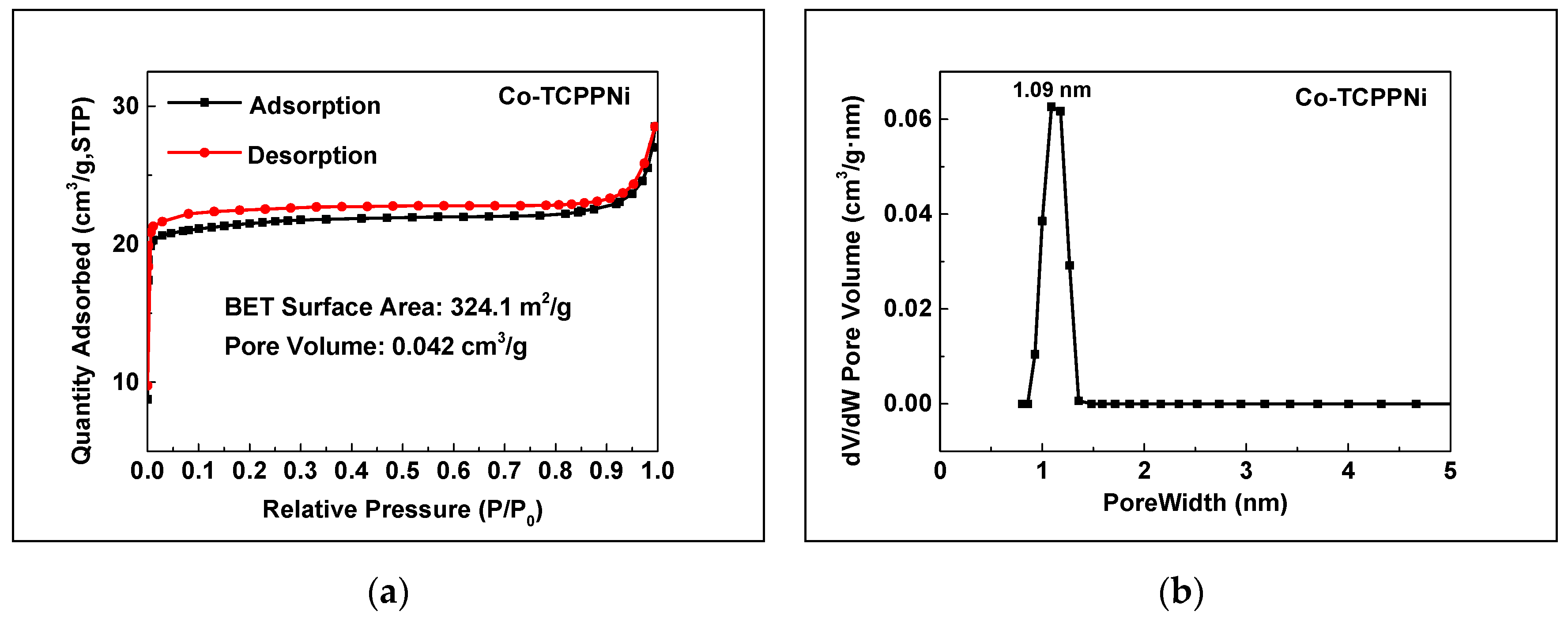
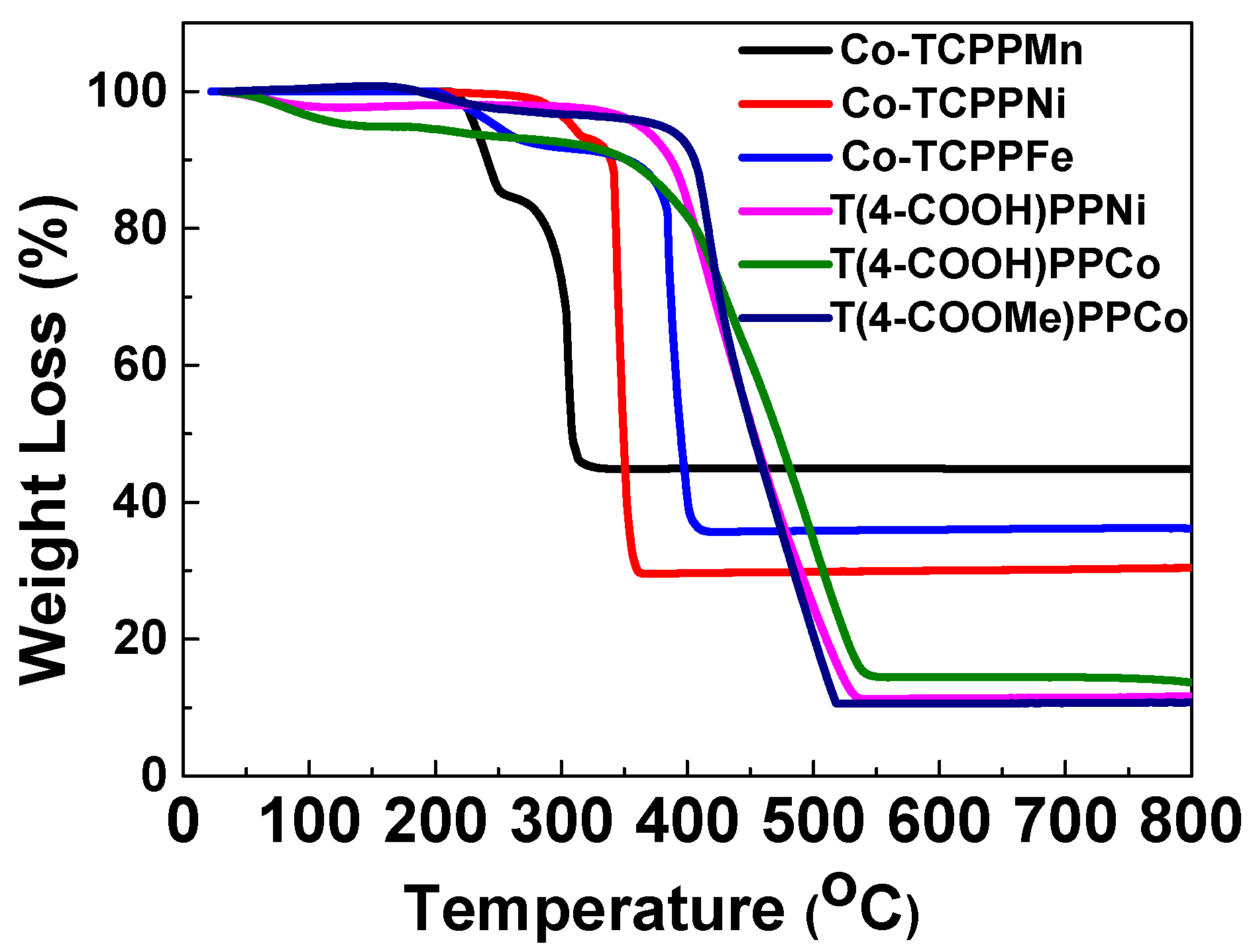
3.1. Catalyst Characterization
3.2. Preliminary Experiments for Cyclohexane Oxygenation as Model Reaction
3.3. Synergistic Catalytic Oxygenation of C–H Bonds with M1-TCPPM2
3.4. Further Optimizing Reaction Conditions
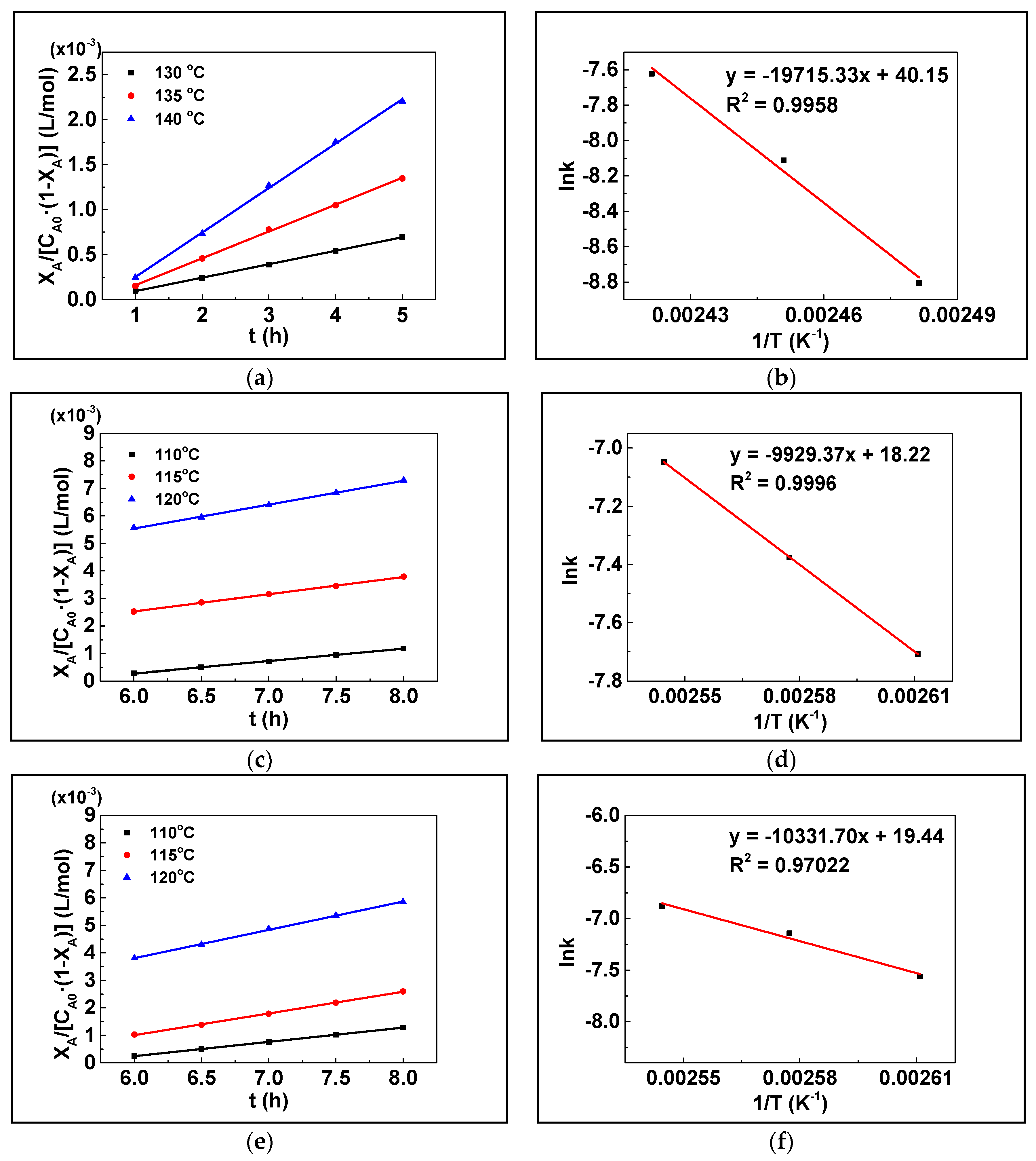
3.5. Apparent Kinetics Estimation
3.6. The Systematic Investigation into Substrate Scope
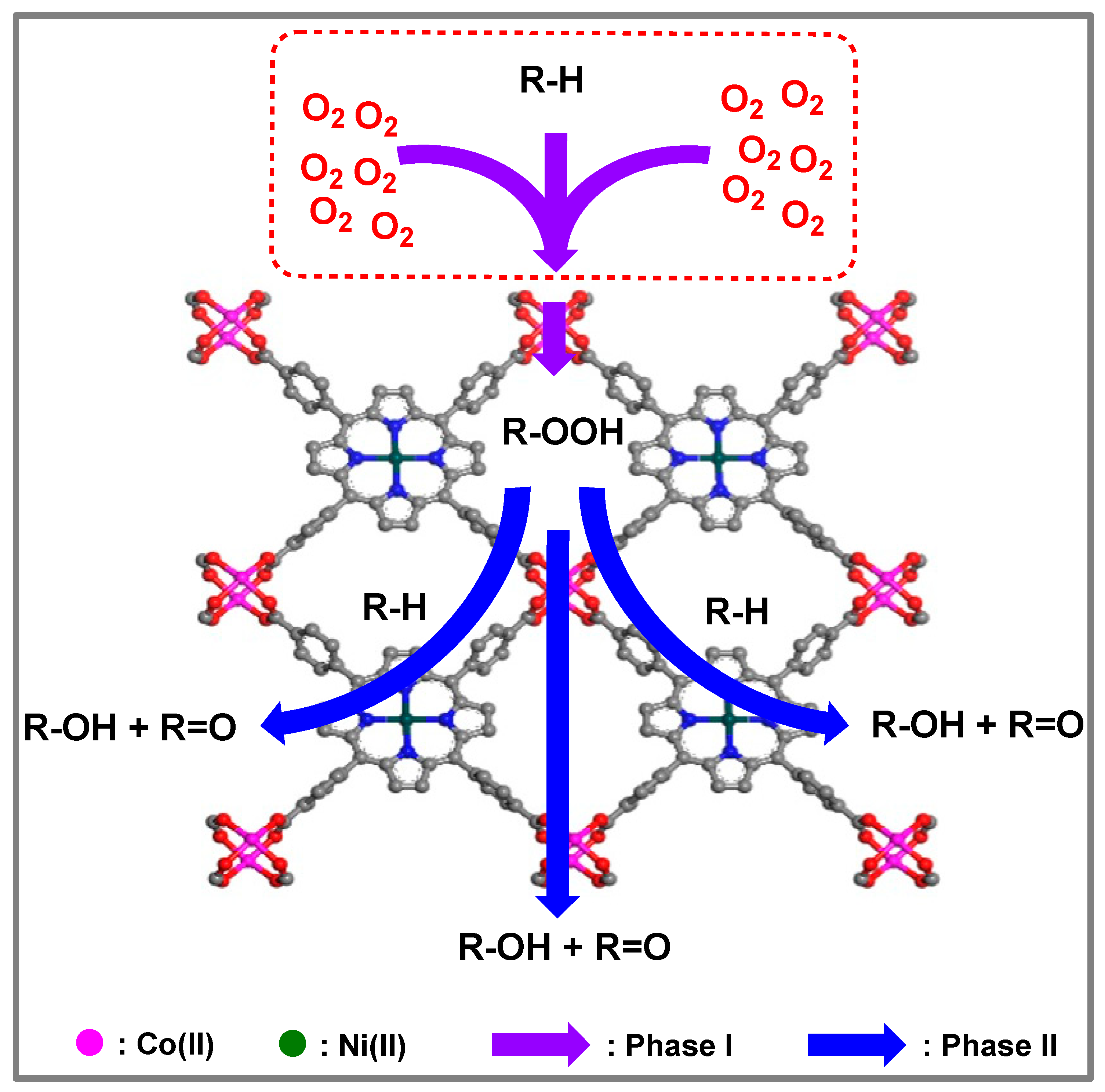
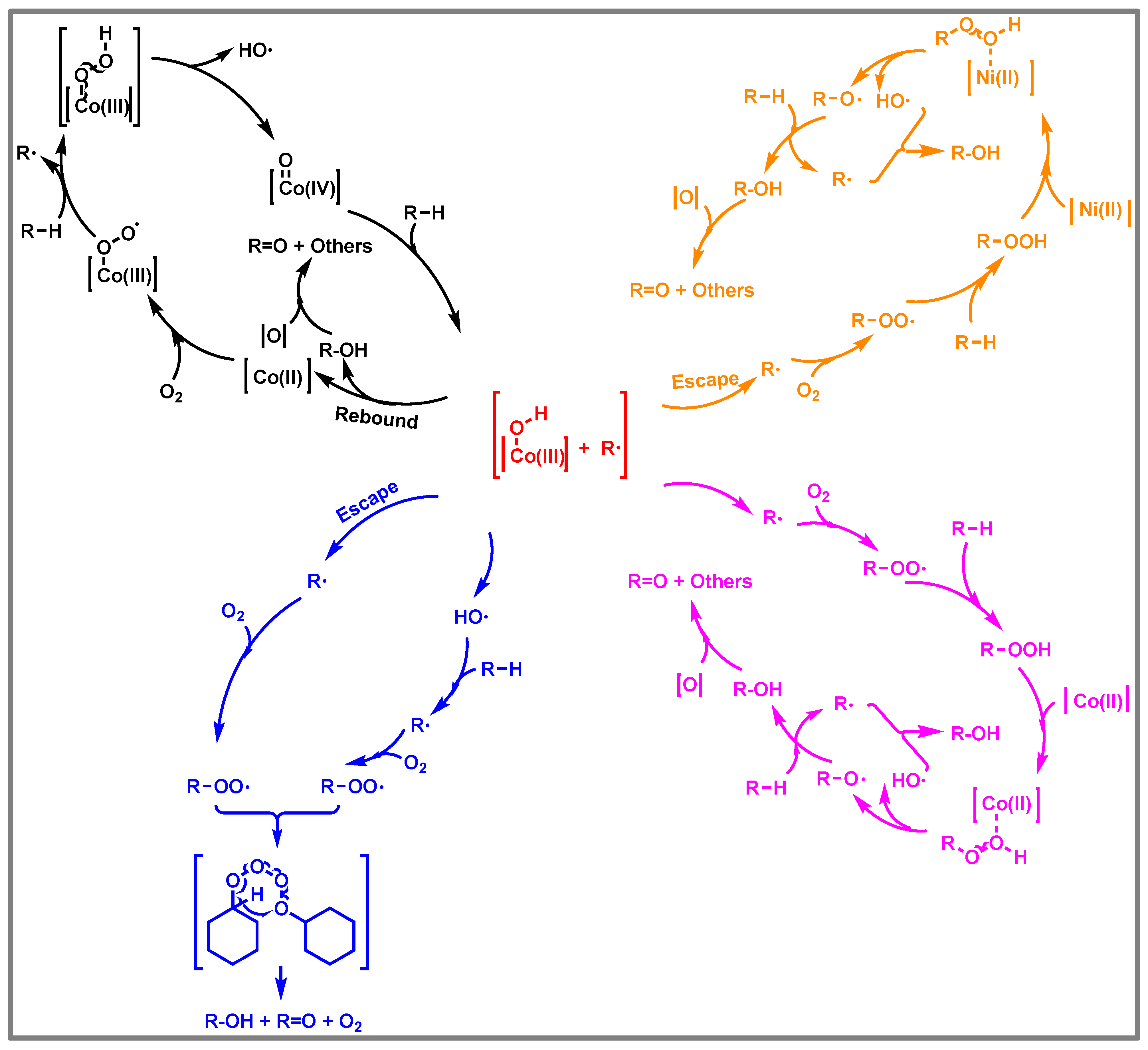
3.7. Synergistic Mechanism
3.8. Comparison with Other Catalytic Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, Y.H.; Hartwig, J.F. Mechanism of Ni-Catalyzed Oxidations of Unactivated C(sp3)-H Bonds. J. Am. Chem. Soc. 2020, 142, 19239–19248. [Google Scholar] [CrossRef]
- Muhumuza, E.; Wu, P.P.; Nan, T.; Zhao, L.M.; Bai, P.; Mintova, S.; Yan, Z.F. Perovskite-Type LaCoO3 as an Efficient and Green Catalyst for Sustainable Partial Oxidation of Cyclohexane. Ind. Eng. Chem. Res. 2020, 59, 21322–21332. [Google Scholar] [CrossRef]
- Milan, M.; Salamone, M.; Costas, M.; Bietti, M. The Quest for Selectivity in Hydrogen Atom Transfer Based Aliphatic C-H Bond Oxygenation. Acc. Chem. Res. 2018, 51, 1984–1995. [Google Scholar] [CrossRef]
- Zahedi, S.; Safaei, E. A tetra-cationic tetrapyridinoporphyrazinato Iron(II) grafted onto sulfonated SBA-15 as a novel heterogeneous catalyst for the aerobic oxidation of C(sp3)-H bonds in alkanes. Appl. Surf. Sci. 2021, 552, 149379. [Google Scholar] [CrossRef]
- Kiani, D.; Sourav, S.; Tang, Y.D.; Baltrusaitis, J.; Wachs, I.E. Methane activation by ZSM-5-supported transition metal centers. Chem. Soc. Rev. 2021, 50, 1251–1268. [Google Scholar] [CrossRef]
- Chen, L.Y.; Malollari, K.G.; Uliana, A.; Hartwig, J.F. Ruthenium-Catalyzed, Chemoselective and Regioselective Oxidation of Polyisobutene. J. Am. Chem. Soc. 2021, 143, 4531–4535. [Google Scholar] [CrossRef]
- Biswas, R.; Kanti, D.; Bhaduri, S.N.; Bhaumik, A.; Biswas, P. AgNPs Immobilized over Functionalized 2D Hexagonal SBA-15 for Catalytic C-H Oxidation of Hydrocarbons with Molecular Oxygen under Solvent-Free Conditions. ACS Sustain. Chem. Eng. 2020, 8, 5856–5867. [Google Scholar] [CrossRef]
- Janssen, M.; De Vos, D.E. Regioselective C-H hydroxylation of n-alkanes using Shilov-type Pt catalysis in perfluorinated micro-emulsions. Catal. Sci. Technol. 2020, 10, 1264–1272. [Google Scholar] [CrossRef]
- Deng, J.; Li, Y.H.; Cao, Y.H.; Wang, H.J.; Yu, H.; Zhang, Q.; Zuo, J.L.; Peng, F. Trace amounts of Cu(OAc)2 boost the efficiency of cumene oxidation catalyzed by carbon nanotubes washed with HCl. Catal. Sci. Technol. 2020, 10, 2523–2530. [Google Scholar] [CrossRef]
- Abazid, A.H.; Clamor, N.; Nachtsheim, B.J. An Enantioconvergent Benzylic Hydroxylation Using a Chiral Aryl Iodide in a Dual Activation Mode. ACS Catal. 2020, 10, 8042–8048. [Google Scholar] [CrossRef]
- Dou, Y.J.; Zhu, C.; Zhu, M.M.; Fu, Y.J.; Wang, H.B.; Shi, C.F.; Huang, H.; Liu, Y.; Kang, Z.H. Highly mesoporous carbon nitride photocatalysts for efficient and stable overall water splitting. Appl. Surf. Sci. 2020, 509, 144706. [Google Scholar] [CrossRef]
- Keum, C.; Cho, J.; Park, S.; Lee, S.Y. Copper-Coordinated Histidyl Bolaamphiphile Assembly as an Oxidative Catalyst: Coordination Structure and Catalytic Activity in Cyclohexane Oxidation. Chemcatchem 2019, 11, 4935–4943. [Google Scholar] [CrossRef]
- Doiuchi, D.; Nakamura, T.; Hayashi, H.; Uchida, T. Non-Heme-Type Ruthenium Catalyzed Chemo- and Site-Selective C-H Oxidation. Chem. Asian J. 2020, 15, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Antonangelo, A.R.; Bezzu, C.G.; McKeown, N.B.; Nakagaki, S. Highly active manganese porphyrin-based microporous network polymers for selective oxidation reactions. J. Catal. 2019, 369, 133–142. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, P.H.; Wang, J.H.; Liu, Z.F.; Wang, Y.; Lu, Y.; Liu, Y.; Duan, L.M.; Li, W.F.; Sarina, S.; et al. Visible-light photocatalytic selective oxidation of C(sp3)-H bonds by anion-cation dual-metal-site nanoscale localized carbon nitride. Catal. Sci. Technol. 2021, 11, 4429–4438. [Google Scholar] [CrossRef]
- Hu, X.; Liu, Y.C.; Huang, H.T.; Huang, B.B.; Chai, G.L.; Xie, Z.L. Template-free synthesis of graphene-like carbons as efficient carbocatalysts for selective oxidation of alkanes. Green Chem. 2020, 22, 1291–1300. [Google Scholar] [CrossRef]
- Gogoi, C.; Nagarjun, N.; Roy, S.; Mostakim, S.K.; Volkmer, D.; Dhakshinamoorthy, A.; Biswas, S. A Zr-Based Metal-Organic Framework with a DUT-52 Structure Containing a Trifluoroacetamido-Functionalized Linker for Aqueous Phase Fluorescence Sensing of the Cyanide Ion and Aerobic Oxidation of Cyclohexane. Inorg. Chem. 2021, 60, 4539–4550. [Google Scholar] [CrossRef]
- Hwang, K.C.; Sagadevan, A.; Kundu, P. The sustainable room temperature conversion of p-xylene to terephthalic acid using ozone and UV irradiation. Green Chem. 2019, 21, 6082–6088. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wang, H.; Li, M.; Han, J.Y.; Liu, X.; Gong, J.L. Molecular heterogeneous catalysts derived from bipyridine-based organosilica nanotubes for C-H bond activation. Chem. Sci. 2017, 8, 4489–4496. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z.X.; Zou, X.Y.; Zhang, Z.J.; Fu, G.Y.; Li, L.; Zhang, X.; Luo, F. Enhancing catalytic aerobic oxidation performance of cyclohexane via size regulation of mixed-valence V16 cluster-based metal-organic frameworks. New J. Chem. 2019, 43, 14527–14535. [Google Scholar] [CrossRef]
- Pokutsa, A.; Tkach, S.; Zaborovsky, A.; Bloniarz, P.; Paczesniak, T.; Muzart, J. Sustainable Oxidation of Cyclohexane and Toluene in the Presence of Affordable Catalysts: Impact of the Tandem of Promoter/Oxidant on Process Efficiency. ACS Omega 2020, 5, 7613–7626. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Iguchi, S.; Miseki, Y.; Sayama, K. Photo-Electrochemical C-H Bond Activation of Cyclohexane Using a WO3 Photoanode and Visible Light. Angew. Chem. Int. Ed. 2018, 57, 11238–11241. [Google Scholar] [CrossRef]
- Xiang, L.P.; Fan, J.J.; Zhong, W.Z.; Mao, L.Q.; You, K.Y.; Yin, D.L. Heteroatom-induced band-reconstruction of metal vanadates for photocatalytic cyclohexane oxidation towards KA-oil selectivity. Appl. Catal. A-Gen. 2019, 575, 120–131. [Google Scholar] [CrossRef]
- Liang, F.T.; Zhong, W.Z.; Xiang, L.P.; Mao, L.Q.; Xu, Q.O.; Kirk, S.R.; Yin, D.L. Synergistic hydrogen atom transfer with the active role of solvent: Preferred one-step aerobic oxidation of cyclohexane to adipic acid by N-hydroxyphthalimide. J. Catal. 2019, 378, 256–269. [Google Scholar] [CrossRef]
- Graca, I.; Chadwick, D. NH4-exchanged zeolites: Unexpected catalysts for cyclohexane selective oxidation. Micropor. Mesopor. Mater. 2020, 294, 109873. [Google Scholar] [CrossRef]
- Hong, Y.; Fang, Y.X.O.; Zhou, X.T.; Du, G.; Mai, J.J.; Sun, D.L.; Shao, Z.P. Ionic Liquid-Modified Co/ZSM-5 Catalyzed the Aerobic Oxidation of Cyclohexane: Toward Improving the Activity and Selectivity. Ind. Eng. Chem. Res. 2019, 58, 19832–19838. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Wu, C.; Xie, J.; Gu, X.; Yu, P.; Zong, M.; Gates, I.D.; Liu, H.; Rong, J. Electrophilic oxygen on defect-rich carbon nanotubes for selective oxidation of cyclohexane. Catal. Sci. Technol. 2020, 10, 332–336. [Google Scholar] [CrossRef]
- Tang, S.P.; Fu, Z.H.; Li, Y.; Li, Y.J. Study on boron and fluorine-doped C3N4 as a solid activator for cyclohexane oxidation with H2O2 catalyzed by 8-quinolinolato iron(III) complexes under visible light irradiation. Appl. Catal. A-Gen. 2020, 590, 117342. [Google Scholar] [CrossRef]
- Murayama, T.; Ishikawa, S.; Hiyoshi, N.; Goto, Y.; Zhang, Z.X.; Toyao, T.; Shimizu, K.; Lee, S.; Ueda, W. High dimensionally structured W-V oxides as highly effective catalysts for selective oxidation of toluene. Catal. Today 2021, 363, 60–66. [Google Scholar] [CrossRef]
- Betiha, M.A.; Kandile, N.G.; Badawi, A.M.; Solyman, S.M.; Afify, A.S. Oxidation of p-toluic acid to terephthalic acid via a bromine-free process using nano manganese and manganese-copper mixed oxides. New J. Chem. 2018, 42, 6343–6353. [Google Scholar] [CrossRef]
- Li, X.X.; Guo, L.L.; He, P.C.; Yuan, X.; Jiao, F.P. Co-SBA-15-Immobilized NDHPI as a New Composite Catalyst for Toluene Aerobic Oxidation. Catal. Lett. 2017, 147, 856–864. [Google Scholar] [CrossRef]
- Shahzeydi, A.; Ghiaci, M.; Farrokhpour, H.; Shahvar, A.; Sun, M.X.; Saraji, M. Facile and green synthesis of copper nanoparticles loaded on the amorphous carbon nitride for the oxidation of cyclohexane. Chem. Eng. J. 2019, 370, 1310–1321. [Google Scholar] [CrossRef]
- Xu, C.; Jin, L.L.; Wang, X.Z.; Chen, Y.Q.; Dai, L.Y. Honeycomb-like porous Ce-Cr oxide/N-doped carbon nanostructure: Achieving high catalytic performance for the selective oxidation of cyclohexane to KA oil. Carbon 2020, 160, 287–297. [Google Scholar] [CrossRef]
- Zhou, Q.Q.; Zhang, R.; Li, D.F.; Ding, B.J.; Zheng, A.N.; Yao, Y.F.; Gong, X.Q.; Hou, Z.S. Ionic liquid-stabilized vanadium oxo-clusters catalyzing alkane oxidation by regulating oligovanadates. Catal. Sci. Technol. 2020, 10, 7601–7612. [Google Scholar] [CrossRef]
- Tan, M.Y.; Zhu, L.; Liu, H.; Fu, Y.J.; Yin, S.F.; Yang, W.J. Microporous cobaltporphyrin covalent polymer mediated Co3O4@PNC nanocomposites for efficient catalytic C-H bond activation. Appl. Catal. A-Gen. 2021, 614, 118035. [Google Scholar] [CrossRef]
- Su, Y.Z.; Li, Y.H.; Chen, Z.C.; Huang, J.N.; Wang, H.J.; Yu, H.; Cao, Y.H.; Peng, F. New Understanding of Selective Aerobic Oxidation of Ethylbenzene Catalyzed by Nitrogen-doped Carbon Nanotubes. ChemCatChem 2021, 13, 646–655. [Google Scholar] [CrossRef]
- Wu, S.T.; He, Y.R.; Wang, C.H.; Zhu, C.M.; Shi, J.; Chen, Z.Y.; Wan, Y.; Hao, F.; Xiong, W.; Liu, P.L.; et al. Selective Cl-Decoration on Nanocrystal Facets of Hematite for High-Efficiency Catalytic Oxidation of Cyclohexane: Identification of the Newly Formed Cl-O as Active Sites. ACS Appl. Mater. Interf. 2020, 12, 26733–26745. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.T.; Poidevin, C.; Ochoa-Hernandez, C.; Auer, A.A.; Tuysuz, H. A Supported Bismuth Halide Perovskite Photocatalyst for Selective Aliphatic and Aromatic C-H Bond Activation. Angew. Chem. Int. Ed. 2020, 59, 5788–5796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Yang, Y.Y.; Wang, Y.Y.; Yang, L.L.; Li, Q.; Chen, L.X.; Xu, D.S. Revealing the A-Site Effect of Lead-Free A3Sb2Br9 Perovskite in Photocatalytic C(sp3)-H Bond Activation. Angew. Chem. Int. Ed. 2020, 59, 18136–18139. [Google Scholar] [CrossRef]
- Shen, H.M.; Zhang, L.; Deng, J.H.; Sun, J.; She, Y.B. Enhanced catalytic performance of porphyrin cobalt(II) in the solvent-free oxidation of cycloalkanes (C5~C8) with molecular oxygen promoted by porphyrin zinc(II). Catal. Commun. 2019, 132, 115809. [Google Scholar] [CrossRef]
- Shen, H.M.; Wang, X.; Guo, A.B.; Zhang, L.; She, Y.B. Catalytic oxidation of cycloalkanes by porphyrin cobalt(II) through efficient utilization of oxidation intermediates. J. Porphyr. Phthalocyanines 2020, 24, 1166–1173. [Google Scholar] [CrossRef]
- Shen, H.M.; Wang, X.; Ning, L.; Guo, A.B.; Deng, J.H.; She, Y.B. Efficient oxidation of cycloalkanes with simultaneously increased conversion and selectivity using O2 catalyzed by metalloporphyrins and boosted by Zn(AcO)2: A practical strategy to inhibit the formation of aliphatic diacids. Appl. Catal. A-Gen. 2021, 609, 117904. [Google Scholar] [CrossRef]
- Jeong, H.K. Metal-organic framework membranes: Unprecedented opportunities for gas separations. AlChE J. 2021, 67, e17258. [Google Scholar] [CrossRef]
- Fonseca, J.; Gong, T.H.; Jiao, L.; Jiang, H.L. Metal-organic frameworks (MOFs) beyond crystallinity: Amorphous MOFs, MOF liquids and MOF glasses. J. Mater. Chem. A 2021, 9, 10562–10611. [Google Scholar] [CrossRef]
- Xiong, Z.K.; Jiang, Y.N.; Wu, Z.L.; Yao, G.; Lai, B. Synthesis strategies and emerging mechanisms of metal-organic frameworks for sulfate radical-based advanced oxidation process: A review. Chem. Eng. J. 2021, 421, 127863. [Google Scholar] [CrossRef]
- Zulys, A.; Yulia, F.; Muhadzib, N.; Nasruddin. Biological Biological Metal-Organic Frameworks (Bio-MOFs) for CO2 Capture. Ind. Eng. Chem. Res. 2021, 60, 37–51. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Wu, Y.N.; Chen, G.Q.; Zheng, X.L.; Dai, M.; Peng, C.S. Metal-organic framework membranes: Recent development in the synthesis strategies and their application in oil-water separation. Chem. Eng. J. 2021, 405, 127004. [Google Scholar] [CrossRef]
- Biswas, S.; Maes, M.; Dhakshinamoorthy, A.; Feyand, M.; De Vos, D.E.; Garcia, H.; Stock, N. Fuel purification, Lewis acid and aerobic oxidation catalysis performed by a microporous Co-BTT (BTT3− = 1,3,5-benzenetristetrazolate) framework having coordinatively unsaturated sites. J. Mater. Chem. 2012, 22, 10200–10209. [Google Scholar] [CrossRef]
- Zheng, D.Y.; Chen, E.X.; Ye, C.R.; Huang, X.C. High-efficiency photo-oxidation of thioethers over C60@PCN-222 under air. J. Mater. Chem. A 2019, 7, 22084. [Google Scholar] [CrossRef]
- Wang, X.L.; Sun, Y.Y.; Xiao, Y.H.; Chen, X.X.; Huang, X.C.; Zhou, H.L. Facile Solution-Refluxing Synthesis and Photocatalytic Dye Degradation of a Dynamic Covalent Organic Framework. Molecules 2022, 27, 8002. [Google Scholar] [CrossRef]
- Dai, J.J.; Zhang, H.B. Recent Advances in Catalytic Confinement Effect within Micro/Meso-Porous Crystalline Materials. Small 2021, 17, 2005334. [Google Scholar] [CrossRef]
- Nie, R.F.; Tao, Y.W.; Nie, Y.Q.; Lu, T.L.; Wang, J.S.; Zhang, Y.S.; Lu, X.Y.; Xu, C.C. Recent Advances in Catalytic Transfer Hydrogenation with Formic Acid over Heterogeneous Transition Metal Catalysts. ACS Catal. 2021, 11, 1071–1095. [Google Scholar] [CrossRef]
- Zhang, S.; Rui, Y.; Zhang, X.; Sa, R.J.; Zhou, F.; Wang, R.H.; Li, X.J. Ultrafine cobalt-ruthenium alloy nanoparticles induced by confinement effect for upgrading hydrogen evolution reaction in all-pH range. Chem. Eng. J. 2021, 417, 128047. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Catalysis in Confined Spaces of Metal Organic Frameworks. ChemCatChem 2020, 12, 4732–4753. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; López-Francés, A.; Navalon, S.; Garcia, H. Porous Metal Organic Frameworks as Multifunctional Catalysts for Cyclohexane Oxidation. ChemCatChem 2022, 14, e202201036. [Google Scholar] [CrossRef]
- Wu, J.X.; Hou, S.Z.; Zhang, X.D.; Xu, M.; Yang, H.F.; Cao, P.S.; Gu, Z.Y. Cathodized copper porphyrin metal-organic framework nanosheets for selective formate and acetate production from CO2 electroreduction. Chem. Sci. 2019, 10, 2199–2205. [Google Scholar] [CrossRef]
- Burnett, B.J.; Barron, P.M.; Hu, C.H.; Choe, W. Stepwise Synthesis of Metal-Organic Frameworks: Replacement of Structural Organic Linkers. J. Am. Chem. Soc. 2011, 133, 9984–9987. [Google Scholar] [CrossRef]
- Choi, E.Y.; Wray, C.A.; Hu, C.H.; Choe, W. Highly tunable metal-organic frameworks with open metal centers. CrystEngComm 2009, 11, 553–555. [Google Scholar] [CrossRef]
- Zhang, G.; Li, M.; Yu, K.; Chai, H.; Xu, S.; Xu, T.; Qu, L.; Zhang, X. Two-Dimensional Metalloporphyrinic Framework Nanosheet-Based Dual-Mechanism-Driven Ratiometric Electrochemiluminescent Biosensing of Protein Kinase Activity. ACS Appl. Bio Mater. 2021, 4, 1616–1623. [Google Scholar] [CrossRef]
- Barron, P.M.; Son, H.T.; Hu, C.H.; Choe, W. Highly Tunable Heterometallic Frameworks Constructed from Paddle-Wheel Units and Metalloporphyrins. Cryst. Growth Des. 2009, 9, 1960–1965. [Google Scholar] [CrossRef]
- Shen, H.M.; Wang, Y.; Deng, J.H.; Zhang, L.; She, Y.B. Catalyst-free and solvent-free oxidation of cycloalkanes (C5-C8) with molecular oxygen: Determination of autoxidation temperature and product distribution. Chin. J. Chem. Eng. 2018, 26, 1064–1070. [Google Scholar] [CrossRef]
- Shen, H.M.; Hu, M.Y.; Liu, L.; Qi, B.; Ye, H.L.; She, Y.B. Efficient and selective oxidation of tertiary benzylic C-H bonds with O2 catalyzed by metalloporphyrins under mild and solvent-free conditions. Appl. Catal. A-Gen. 2020, 599, 117599. [Google Scholar] [CrossRef]
- Shen, H.M.; Liu, L.; Qi, B.; Hu, M.Y.; Ye, H.L.; She, Y.B. Efficient and selective oxidation of secondary benzylic C-H bonds to ketones with O2 catalyzed by metalloporphyrins under solvent-free and additive-free conditions. Mol. Catal. 2020, 493, 111102. [Google Scholar] [CrossRef]
- Shen, H.M.; Qi, B.; Hu, M.Y.; Liu, L.; Ye, H.L.; She, Y.B. Selective Solvent-Free and Additive-Free Oxidation of Primary Benzylic C-H Bonds with O2 Catalyzed by the Combination of Metalloporphyrin with N-Hydroxyphthalimide. Catal. Lett. 2020, 150, 3096–3111. [Google Scholar] [CrossRef]
- Shen, H.M.; Ye, H.L.; Wang, Q.; Hu, M.Y.; Liu, L.; She, Y.B. Efficient oxidation of cumene to cumene hydroperoxide with ambient O2 catalyzed by metalloporphyrins. J. Porphyr. Phthalocyanines 2021, 25, 314–322. [Google Scholar] [CrossRef]
- Ou, J.H.; Xiang, J.; Liu, J.X.; Sun, L.C. Surface-Supported Metal-Organic Framework Thin-Film-Derived Transparent CoS1.097@N-Doped Carbon Film as an Efficient Counter Electrode for Bifacial Dye-Sensitized Solar Cells. ACS Appl. Mater. Interf. 2019, 11, 14862–14870. [Google Scholar] [CrossRef]
- Chen, L.Y.; Wang, Y.X.; Yu, F.Y.; Shen, X.S.; Duan, C.Y. A simple strategy for engineering heterostructures of Au nanoparticle-loaded metal-organic framework nanosheets to achieve plasmon-enhanced photocatalytic CO2 conversion under visible light. J. Mater. Chem. A 2019, 7, 11355–11361. [Google Scholar] [CrossRef]
- Xin, Z.F.; Wang, Y.R.; Chen, Y.F.; Li, W.L.; Dong, L.Z.; Lan, Y.Q. Metallocene implanted metalloporphyrin organic framework for highly selective CO2 electroreduction. Nano Energy 2020, 67, 104233. [Google Scholar] [CrossRef]
- Zhang, D.X.; Du, P.Y.; Chen, J.; Guo, H.X.; Lu, X.Q. Pyrazolate-based porphyrinic metal-organic frameworks as catechol oxidase mimic enzyme for fluorescent and colorimetric dual-mode detection of dopamine with high sensitivity and specificity. Sens. Actuators B 2021, 341, 130000. [Google Scholar] [CrossRef]
- Wang, L.; Jin, P.X.; Huang, J.W.; She, H.D.; Wang, Q.Z. Integration of Copper(II)-Porphyrin Zirconium Metal-Organic Framework and Titanium Dioxide to Construct Z-Scheme System for Highly Improved Photocatalytic CO2 Reduction. ACS Sustain. Chem. Eng. 2019, 7, 15660–15670. [Google Scholar] [CrossRef]
- Yan, T.T.; Guo, J.H.; Liu, Z.Q.; Sun, W.Y. Metalloporphyrin Encapsulation for Enhanced Conversion of CO2 to C2H4. ACS Appl. Mater. Interf. 2021, 13, 25937–25945. [Google Scholar] [CrossRef]
- Ang, H.X.; Hong, L. Polycationic Polymer-Regulated Assembling of 2D MOF Nanosheets for High-Performance Nanofiltration. ACS Appl. Mater. Interf. 2017, 9, 28079–28088. [Google Scholar] [CrossRef]
- Cong, M.Y.; Chen, X.Y.; Xia, K.; Ding, X.; Zhang, L.L.; Jin, Y.; Gao, Y.; Zhang, L.X. Selective nitrogen reduction to ammonia on iron porphyrin-based single-site metal-organic frameworks. J. Mater. Chem. A 2021, 9, 4673–4678. [Google Scholar] [CrossRef]
- Huang, L.Y.; Hu, X.; Shan, H.Y.; Yu, L.; Gu, Y.X.; Wang, A.J.; Shan, D.; Yuan, P.X.; Feng, J.J. High-performance electrochemiluminescence emitter of metal organic framework linked with porphyrin and its application for ultrasensitive detection of biomarker mucin-1. Sens. Actuators B 2021, 344, 130300. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, S.C.; Fan, C.R.; Ye, Q. A photosensitive metal-organic framework having a flower-like structure for effective visible light-driven photodegradation of rhodamine B. RSC Adv. 2021, 11, 18565–18575. [Google Scholar] [CrossRef]
- Fang, X.Z.; Jiao, L.; Yu, S.H.; Jiang, H.L. Metal-Organic Framework-Derived FeCo-N-Doped Hollow Porous Carbon Nanocubes for Electrocatalysis in Acidic and Alkaline Media. ChemSusChem 2017, 10, 3019–3024. [Google Scholar] [CrossRef]
- Han, Q.A.; Wang, C.; Liu, P.K.; Zhang, G.; Song, L.; Fu, Y.Z. Three kinds of porphyrin dots as near-infrared electrochemiluminescence luminophores: Facile synthesis and biosensing. Chem. Eng. J. 2021, 421, 129761. [Google Scholar] [CrossRef]
- Westrup, K.C.M.; da Silva, R.M.; Mantovani, K.M.; Bach, L.; Stival, J.F.; Zamora, P.G.P.; Wypych, F.; Machado, G.S.; Nakagaki, S. Light-assisted cyclohexane oxidation catalysis by a manganese(III) porphyrin immobilized onto zinc hydroxide salt and zinc oxide obtained by zinc hydroxide salt hydrothermal decomposition. Appl. Catal. A-Gen. 2020, 602, 117708. [Google Scholar] [CrossRef]
- Mukherjee, M.; Dey, A. Electron Transfer Control of Reductase versus Monooxygenase: Catalytic C-H Bond Hydroxylation and Alkene Epoxidation by Molecular Oxygen. ACS Cent. Sci. 2019, 5, 671–682. [Google Scholar] [CrossRef]
- Kuznetsov, M.L.; Pombeiro, A.J.L. Metal-free and iron(II)-assisted oxidation of cyclohexane to adipic acid with ozone: A theoretical mechanistic study. J. Catal. 2021, 399, 52–66. [Google Scholar] [CrossRef]
- Lakk-Bogath, D.; Kripli, B.; Meena, B.I.; Speier, G.; Kaizer, J. Catalytic and stoichiometric C-H oxidation of benzylalcohols and hydrocarbons mediated by nonheme oxoiron(IV) complex with chiral tetrapyridyl ligand. Inorg. Chem. Commun. 2019, 104, 165–170. [Google Scholar] [CrossRef]
- Xie, C.J.; Xiong, Q.; Jiang, L.; Wang, Y.F.; Tang, Q.Y.; He, J.; Wang, J.Q. Carbon supported copper catalyst prepared in situ by one-pot pyrolysis of Bougainvillea glabra: An efficient and stable catalyst for selective oxidation of cyclohexane. Appl. Surf. Sci. 2022, 576, 151833. [Google Scholar] [CrossRef]
- Martins, N.M.R.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Green oxidation of cyclohexane catalyzed by recyclable magnetic transition-metal silica coated nanoparticles. Catal. Commun. 2019, 125, 15–20. [Google Scholar] [CrossRef]
- Gutierrez-Tarrino, S.; Gaona-Miguelez, J.; Ona-Burgos, P. Tailoring the electron density of cobalt oxide clusters to provide highly selective superoxide and peroxide species for aerobic cyclohexane oxidation. Dalton Trans. 2021, 50, 15370–15379. [Google Scholar] [CrossRef]
- Xie, C.J.; Wang, W.; Yang, Y.P.; Jiang, L.; Chen, Y.J.; He, J.; Wang, J.Q. Enhanced stability and activity for solvent-free selective oxidation of cyclohexane over Cu2O/CuO fabricated by facile alkali etching method. Mol. Catal. 2020, 495, 111134. [Google Scholar] [CrossRef]
- Xu, S.N.; Draksharapu, A.; Rasheed, W.; Que, L. Acid pKa Dependence in O-O Bond Heterolysis of a Nonheme FeIII-OOH Intermediate To Form a Potent FeV=O Oxidant with Herne Compound I-Like Reactivity. J. Am. Chem. Soc. 2019, 141, 16093–16107. [Google Scholar] [CrossRef]
- Pamin, K.; Tabor, E.; Gorecka, S.; Kubiak, W.W.; Rutkowska-Zbik, D.; Poltowicz, J. Three Generations of Cobalt Porphyrins as Catalysts in the Oxidation of Cycloalkanes. ChemSusChem 2019, 12, 684–691. [Google Scholar] [CrossRef]
- Wang, X.Y.; Feng, X.Y.; Liu, J.C.; Huang, Z.L.; Zong, S.; Liu, L.L.; Liu, J.R.; Fang, Y.X. Photo-thermo catalytic selective oxidation of cyclohexane by In-situ prepared nonstoichiometric Molybdenum oxide and Silver-palladium alloy composite. J. Colloid Interf. Sci. 2022, 607, 954–966. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhang, Y.B.; Yuan, H.R.; Du, R.F.; Yao, J.; Li, H.R. Selective Aerobic Oxidation of Secondary C (sp3)-H Bonds with NHPI/CAN Catalytic System. Catal. Lett. 2021, 151, 1663–1669. [Google Scholar] [CrossRef]
- Reisi, B.; Chermahini, A.N.; Rodriguez-Padron, D.; Munoz-Batista, M.J.; Luque, R. Synthesis and characterization of Pd-Ni catalysts supported on KIT-6 and their application in cyclohexane oxidation using molecular oxygen. J. Ind. Eng. Chem. 2021, 102, 103–111. [Google Scholar] [CrossRef]
- Ye, J.H.; Tang, J.J.; Zhao, Y.J.; Wu, C.D. Synthesis and Catalytic Properties of Porous Metal Silica Materials Templated and Functionalized by Extended Coordination Cages. Inorg. Chem. 2020, 59, 767–776. [Google Scholar] [CrossRef]
- Guo, Y.; Ying, T.; Liu, X.H.; Shi, B.F.; Wang, Y.Q. A partially graphitic carbon catalyst for aerobic oxidation of cyclohexane. Mol. Catal. 2019, 479, 170–175. [Google Scholar] [CrossRef]
- Yuan, E.X.; Gu, M.Q.; Jian, P.M. Aerobic oxidation of cyclohexane over metal-organic framework-derived Ce, Ni-modified Co3O4. Korean J. Chem. Eng. 2020, 37, 1137–1148. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, J.; Jia, Y.; Li, W.Q.; Tan, X.L.; Zhang, D.; Xu, S.N.; Zhang, P.P.; Wei, C.D.; Miao, S.D. Encapsulating spinel nancrystals in Laponite cages and applications in molecular oxidation of cyclohexane. Appl. Clay Sci. 2019, 181, 105226. [Google Scholar] [CrossRef]
- Mo, L.Q.; Huang, X.F.; Huang, G.; Yuan, G.P.; Wei, S.J. Highly Active Catalysis of Cobalt Tetrakis(pentafluorophenyl)porphyrin Promoted by Chitosan for Cyclohexane Oxidation in Response-Surface-Methodology-Optimized Reaction Conditions. ChemistryOpen 2019, 8, 104–113. [Google Scholar] [CrossRef]
- Wang, X.C.; Jie, S.S.; Liu, Z.G. The influence of encapsulated cobalt content within N-doped bamboo-like carbon nanotubes catalysts for arylalkanes oxidation. Mater. Chem. Phys. 2019, 232, 393–399. [Google Scholar] [CrossRef]
- Yuan, E.X.; Zhou, M.X.; Gu, M.Q.; Jian, P.M.; Xia, L.; Xiao, J.R. Boosting Creation of Oxygen Vacancies in Co-Co3O4 Homogeneous Hybrids for Aerobic Oxidation of Cyclohexane. Catal. Lett. 2022, 152, 282–298. [Google Scholar] [CrossRef]
- Jian, J.; Kuang, D.N.; Wang, X.H.; Zhou, H.; Gao, H.X.; Sun, W.J.; Yuan, Z.Q.; Zeng, J.X.; You, K.Y.; Luo, H.A. Highly dispersed Co/SBA-15 mesoporous materials as efficient and stable catalyst for partial oxidation of cyclohexane with molecular oxygen. Mater. Chem. Phys. 2020, 246, 122814. [Google Scholar] [CrossRef]
- Liu, P.; You, K.Y.; Deng, R.J.; Chen, Z.P.; Jian, J.; Zhao, F.F.; Liu, P.L.; Ai, Q.H.; Luo, H.A. Hydrotalcite-derived Co-MgAlO mixed metal oxides as efficient and stable catalyst for the solvent-free selective oxidation of cyclohexane with molecular oxygen. Mol. Catal. 2019, 466, 130–137. [Google Scholar] [CrossRef]
- Huang, N.; Yuan, S.; Drake, H.; Yang, X.Y.; Pang, J.D.; Qin, J.S.; Li, J.L.; Zhang, Y.M.; Wang, Q.; Jiang, D.L.; et al. Systematic engineering of single substitution in Zirconium metal–organic frameworks towards high-performance catalysis. J. Am. Chem. Soc. 2017, 139, 18590–18597. [Google Scholar] [CrossRef]
- Feng, D.W.; Chung, W.C.; Wei, Z.W.; Gu, Z.Y.; Jiang, H.L.; Chen, Y.P.; Darensbourg, D.J.; Zhou, H.C. Construction of ultrastable porphyrin Zr metal–organic frameworks through linker elimination. J. Am. Chem. Soc. 2013, 135, 17105–17110. [Google Scholar] [CrossRef]
- Feng, D.W.; Gu, Z.Y.; Li, J.R.; Jiang, H.L.; Wei, Z.W.; Zhou, H.C. Zirconium-metalloporphyrin PCN-222: Mesoporous metal–organic frameworks with ultrahigh stability as biomimetic catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef]
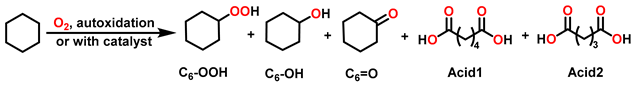 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Catalysts | Conv. (%) | Selectivity (%) | |||||
| C6-OOH | C6-OH | C6=O | Acid1 | Acid2 | Total b | |||
| 1 | / | <0.2 | / | / | / | / | / | / |
| 2 | Porp. c | 0.2 | 82.3 | / | 17.7 | / | / | >99 |
| 3 | Porp.Co | 4.4 | 3.1 | 41.2 | 41.1 | 13.0 | 1.6 | 85.4 |
| 4 | Porp.Mn | 2.5 | 29.3 | 41.5 | 18.9 | 8.2 | 2.1 | 89.7 |
| 5 | Porp.Fe | 1.2 | 70.7 | / | 29.3 | / | / | >99 |
| 6 | Porp.Ni | <0.2 | 86.6 | / | 13.4 | / | / | >99 |
| 7 d | Porp.Co and Porp.Fe | 4.6 | 12.1 | 38.4 | 35.8 | 11.3 | 2.4 | 86.3 |
| 8 | Porp.Co and Porp.Ni | 4.6 | 8.2 | 41.2 | 39.1 | 10.1 | 1.4 | 88.5 |
| 9 | Porp.Co and Porp.Mn | 4.4 | 12.2 | 42.1 | 31.2 | 12.1 | 2.4 | 85.5 |
| 10 e | Co-TCPPNi | 5.6 | 3.0 | 41.1 | 48.8 | 6.7 | 0.4 | 92.9 |
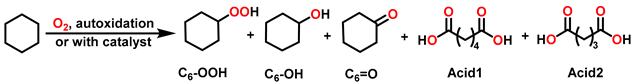 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Catalysts | Conv. (%) | Selectivity (%) | |||||
| C6-OOH | C6-OH | C6=O | Acid1 | Acid2 | Total b | |||
| 1 | / | <0.2 | / | / | / | / | / | / |
| 2 | Ni-TCPPNi | 0.2 | 70.9 | / | 29.1 | / | / | >99 |
| 3 | Mn-TCPPMn | 2.2 | 21.6 | 41.5 | 27.3 | 8.2 | 1.5 | 90.3 |
| 4 | Mn-TCPPNi | 2.6 | 16.0 | 42.3 | 33.8 | 6.8 | 1.2 | 92.0 |
| 5 | Mn-TCPPFe | 2.8 | 16.1 | 42.4 | 35.2 | 5.7 | 0.6 | 93.7 |
| 6 | Mn-TCPPCo | 3.0 | 17.5 | 39.5 | 36.4 | 5.6 | 1.0 | 93.4 |
| 7 | Mn-TCPPCu | 2.5 | 18.2 | 40.0 | 35.5 | 5.8 | 0.5 | 93.7 |
| 8 | Mn-TCPPZn | 3.0 | 17.3 | 40.0 | 34.0 | 6.1 | 2.6 | 91.3 |
| 9 | Co-TCPPFe | 5.4 | 6.7 | 40.1 | 47.0 | 6.1 | 0.1 | 93.8 |
| 10 | Co-TCPPNi | 5.6 | 3.0 | 41.0 | 48.8 | 6.7 | 0.5 | 92.9 |
| 11 | Co-TCPPMn | 4.5 | 13.4 | 40.7 | 32.1 | 12.1 | 1.7 | 86.2 |
| 12 | Co-TCPPCo | 4.5 | 4.1 | 43.1 | 39.1 | 11.1 | 2.6 | 86.3 |
| 13 c | Porp.Co | 4.4 | 3.1 | 41.2 | 41.1 | 13.0 | 1.6 | 85.4 |
| 14 c | Porp.Ni | <0.20 | 86.6 | / | 13.4 | / | / | >99 |
| 15 d | Porp.Co and Porp.Ni | 4.6 | 8.2 | 41.2 | 39.1 | 10.1 | 1.4 | 88.5 |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Catalyst Dosage (mg/mol) | Conv. (%) | Selectivity (%) | |||||
| C6-OOH | C6-OH | C6=O | Acid1 | Acid2 | Total b | |||
| 1 c | 0.04 | 3.5 | 12.2 | 40.1 | 33.0 | 12.6 | 2.1 | 85.3 |
| 2 c | 0.06 | 4.2 | 12.3 | 40.0 | 33.0 | 12.1 | 2.5 | 85.4 |
| 3 c | 0.08 | 4.5 | 13.4 | 40.7 | 32.1 | 12.1 | 1.7 | 86.2 |
| 4 c | 0.10 | 3.7 | 10.1 | 45.0 | 31.1 | 11.0 | 2.8 | 86.2 |
| 5 c | 0.12 | 3.3 | 9.1 | 44.1 | 32.0 | 12.0 | 2.8 | 85.2 |
| 6 c | 0.14 | 2.2 | 10.1 | 44.0 | 31.0 | 12.9 | 2.0 | 85.1 |
| 7 d | 0.04 | 4.2 | 3.1 | 41.0 | 45.9 | 8.8 | 1.2 | 90.0 |
| 8 d | 0.06 | 5.0 | 2.1 | 40.1 | 48.0 | 8.3 | 1.5 | 90.2 |
| 9 d | 0.08 | 5.6 | 3.1 | 41.0 | 48.8 | 6.7 | 0.4 | 92.9 |
| 10 d | 0.10 | 4.5 | 3.4 | 48.0 | 40.0 | 8.2 | 0.4 | 91.4 |
| 11 d | 0.12 | 3.2 | 6.2 | 53.1 | 31.1 | 8.1 | 1.5 | 90.4 |
| 12 d | 0.14 | 2.4 | 5.9 | 50.1 | 34.0 | 8.7 | 1.3 | 90.0 |
| 13 e | 0.04 | 3.4 | 9.1 | 47.6 | 34.0 | 8.2 | 1.1 | 90.7 |
| 14 e | 0.06 | 4.8 | 8.4 | 45.3 | 37.0 | 7.9 | 1.4 | 90.7 |
| 15 e | 0.08 | 5.4 | 6.7 | 40.1 | 47.0 | 6.1 | 0.1 | 93.8 |
| 16 e | 0.10 | 4.1 | 8.1 | 42.3 | 40.0 | 8.4 | 1.2 | 90.4 |
| 17 e | 0.12 | 3.2 | 9.1 | 44.1 | 37.0 | 8.7 | 1.0 | 90.3 |
| 18 e | 0.14 | 2.0 | 11.2 | 45.0 | 31.2 | 10.4 | 2.2 | 87.4 |
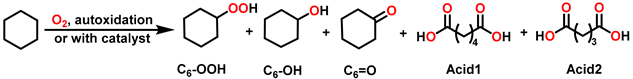 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Pressure (MPa) | Conv. (%) | Selectivity (%) | |||||
| C6-OOH | C6-OH | C6=O | Acid1 | Acid2 | Total b | |||
| 1 c | 0.60 | 5.2 | 11.0 | 44.1 | 39.0 | 5.0 | 0.8 | 94.2 |
| 2 c | 0.80 | 5.3 | 7.8 | 41.8 | 43.5 | 6.0 | 0.9 | 93.1 |
| 3 c | 1.00 | 5.4 | 6.7 | 40.1 | 47.0 | 6.1 | 0.1 | 93.8 |
| 4 c | 1.20 | 5.4 | 2.4 | 39.5 | 49.5 | 7.8 | 0.8 | 91.4 |
| 5 c | 1.40 | 5.3 | 2.1 | 38.7 | 49.5 | 8.8 | 0.9 | 90.3 |
| 6 d | 0.60 | 5.1 | 14.1 | 45.0 | 36.1 | 4.6 | 0.1 | 95.3 |
| 7 d | 0.80 | 5.3 | 7.6 | 43.7 | 42.6 | 5.7 | 0.4 | 93.9 |
| 8 d | 1.00 | 5.6 | 3.0 | 41.0 | 48.8 | 6.7 | 0.4 | 92.9 |
| 9 d | 1.20 | 5.5 | 3.0 | 38.1 | 49.2 | 8.4 | 1.2 | 90.4 |
| 10 d | 1.40 | 5.4 | 3.1 | 34.3 | 50.1 | 10.2 | 2.4 | 87.4 |
| Entry | Catalysts | Temperature (°C) | k (L mol−1 h−1) | R2 | Ea (kJ/mol) |
|---|---|---|---|---|---|
| 1 | / | 130 | 0.00015 | 0.9996 | 163.9 |
| 2 | 135 | 0.00030 | 0.9991 | ||
| 3 | 140 | 0.00049 | 0.9989 | ||
| 4 | Co-TCPPNi | 110 | 0.00045 | 0.99924 | 82.55 |
| 5 | 115 | 0.00063 | 0.99917 | ||
| 6 | 120 | 0.00087 | 0.99864 | ||
| 7 | Co-TCPPFe | 110 | 0.00052 | 0.99990 | 85.91 |
| 8 | 115 | 0.00079 | 0.99890 | ||
| 9 | 120 | 0.00103 | 0.99906 | ||
| 10 | Co-TCPPMn | 110 | 0.00040 | 0.99352 | 133.08 |
| 11 | 115 | 0.00071 | 0.99414 | ||
| 12 | 120 | 0.00117 | 0.99941 |
| Entry | Cycloalkanes | Conv. (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|---|
| -OOH | -OH | =O | Acid1 | Acid2 | Total b | |||
| 1 |  | 2.4 | 19.1 | 8.0 | 68.9 | 0.3 | 3.7 | 96.0 |
| 2 |  | 5.6 | 3.0 | 41.0 | 48.8 | 6.7 | 0.4 | 92.9 |
| 3 | 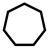 | 13.7 | 25.0 | 11.0 | 58.0 | 1.1 | 4.9 | 94.0 |
| 4 | 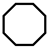 | 27.8 | 27.9 | 11.2 | 56.5 | 0.4 | 3.9 | 95.7 |
| 5 | 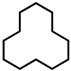 | 30.7 | 24.2 | 16.3 | 59.5 | / | / | >99 |
| Entry | Substrates | Products | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|
| 1 a,b |  | 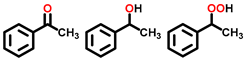 | 37.1 | 65, 21, 10 |
| 2 a | 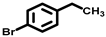 | 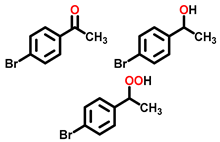 | 59.2 | 89, 1, 7 |
| 3 a | 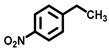 |  | 61.2 | 85, 4, 8 |
| 4 a,b | 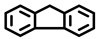 | 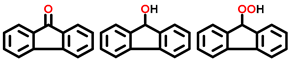 | 51.0 | 87, 5, 5 |
| 5 a,b |  | 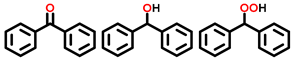 | 36.3 | 77, 13, 7 |
| 6 c |  | 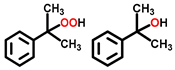 | 33.5 | 87, 7 |
| 7 c | 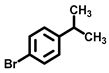 | 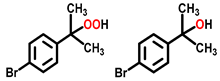 | 35.4 | 75, 18 |
| 8 c | 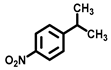 | 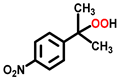 | 12.2 | >99 |
| 9 c,d | 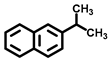 | 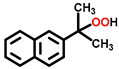 | 14.3 | >99 |
| Entry | Ref. | Reaction Conditions | Quantitative Methods | Conversion, Selectivity a (%) |
|---|---|---|---|---|
| 1 | [35] | 110 °C, oxidant (oxygen 1.0 MPa), catalyst (Co3O4 on nitrogen-doped carbon), substrate (cyclohexane) | GC | 16.5, 90.5 |
| 2 | [89] | 45 °C, dichloroethane, oxidant (oxygen 0.1 MPa), catalyst (NHPI/cerium ammonium nitrate), substrate (cyclohexane) | GC | 9.0, 79.0 |
| 3 | [90] | 140 °C, acetonitrile, oxidant (oxygen 1.0 MPa), catalyst (Pd-Ni on mesoporous silica), substrate (cyclohexane) | GC | 10.9, 95.5 |
| 4 | [27] | 130 °C, acetone, oxidant (oxygen 1.5 MPa), catalyst (oxidized carbon nanotube), substrate (cyclohexane) | Online GC | 8.6, 76.2 |
| 5 | [91] | 120 °C, acetonitrile, oxidant (oxygen 1.0 MPa), catalyst (Co in porous metal silica), substrate (cyclohexane) | GC | 14.0, 91.0 |
| 6 | [92] | 125 °C, acetonitrile, oxidant (oxygen 1.5 MPa), catalyst (partially graphitic carbon), substrate (cyclohexane) | GC | 54.8, 46.5 |
| 7 | [2] | 150 °C, oxidant (oxygen 0.8 MPa), catalyst (perovskite-type LaCoO3), substrate (cyclohexane) | GC | 8.3, 90.0 |
| 8 | [93] | 130 °C, oxidant (oxygen 0.8 MPa), catalyst (Ce, Ni-modified Co3O4), substrate (cyclohexane) | GC | 12.8, 95.5 |
| 9 | [94] | 150 °C, oxidant (oxygen 2.0 MPa), catalyst (Co-based spinel nanocrystal), substrate (cyclohexane) | GC | 17.2, 95.3 |
| 10 | [95] | 165 °C, oxidant (oxygen 0.8 MPa), catalyst (TPFPPCo), substrate (cyclohexane) | GC | 25.7, 72.0 |
| 11 | [95] | 165 °C, oxidant (oxygen 0.8 MPa), catalyst (TPFPPCo), substrate (cyclohexane) | GC | 26.6, 70.4 |
| 12 | [26] | 150 °C, oxidant (oxygen 1.5 MPa), catalyst (ionic liquid-modified Co/ZSM-5 zeolite), substrate (cyclohexane) | GC | 9.7, 92.2 |
| 13 | [96] | 140 °C, oxidant (oxygen 0.8 MPa), catalyst (Co within N-doped carbon nanotube), substrate (cyclohexane) | GC | 19.8, 65.4 |
| 14 | [97] | 130 °C, oxidant (oxygen 0.8 MPa), catalyst (Co-Co3O4 hybrids on nitrogen-doped carbon), substrate (cyclohexane) | GC | 16.3, 96.5 |
| 15 | [37] | 150 °C, oxidant (oxygen 1.0 MPa), catalyst (Cl decorated rhombohedral α-Fe2O3), substrate (cyclohexane) | GC + Titration | 11.5, 82.2 |
| 16 | [98] | 135 °C, oxidant (oxygen 0.6 MPa), catalyst (Co in SBA-15 mesoporous material), substrate (cyclohexane) | GC + Titration | 9.6, 82.3 |
| 17 | [99] | 150 °C, oxidant (oxygen 0.6 MPa), catalyst (Hydrotalcite-derived Co-MgAlO), substrate (cyclohexane) | GC + Titration | 9.1, 84.8 |
| 18 | [41] | 120 °C, oxidant (oxygen 1.4 MPa), catalyst (T(2 Cl)PPCo and T(4 Cl)PPCu), substrate (cyclohexane) | GC + HPLC | 4.4, 97.2 |
| 19 | [40] | 120 °C, oxidant (oxygen 1.4 MPa), catalyst (T(4-Cl)PPCo and T(4-Cl)PPZn), substrate (cyclohexane) | GC + HPLC | 4.2, 96.4 |
| 20 | [32] | 140 °C, oxidant (oxygen 2.5 MPa), catalyst (Copper nanoparticle in carbon nitride), substrate (cyclohexane) | GC + HPLC | 25.5, 10.0 |
| 21 | This study | 120 °C, oxidant (oxygen 1.0 MPa), catalyst (T(4-COOCH3)PPCo), substrate (cyclohexane) | GC + HPLC | 4.4, 85.4 |
| 22 | This study | 120 °C, oxidant (oxygen 1.0 MPa), catalyst (Co-TCPPNi), substrate (cyclohexane) | GC + HPLC | 5.6, 92.9 |
| 23 | This study | 120 °C, oxidant (oxygen 1.0 MPa), catalyst (Co-TCPPFe), substrate (cyclohexane) | GC + HPLC | 5.4, 93.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.-Y.; Fu, B.; Jin, W.-D.; Wang, X.; Wang, K.-K.; Wang, M.; She, Y.-B.; Shen, H.-M. Efficient and Selective Oxygenation of Cycloalkanes and Alkyl Aromatics with Oxygen through Synergistic Catalysis of Bimetallic Active Centers in Two-Dimensional Metal-Organic Frameworks Based on Metalloporphyrins. Biomimetics 2023, 8, 325. https://doi.org/10.3390/biomimetics8030325
Zhou X-Y, Fu B, Jin W-D, Wang X, Wang K-K, Wang M, She Y-B, Shen H-M. Efficient and Selective Oxygenation of Cycloalkanes and Alkyl Aromatics with Oxygen through Synergistic Catalysis of Bimetallic Active Centers in Two-Dimensional Metal-Organic Frameworks Based on Metalloporphyrins. Biomimetics. 2023; 8(3):325. https://doi.org/10.3390/biomimetics8030325
Chicago/Turabian StyleZhou, Xin-Yan, Bo Fu, Wen-Dong Jin, Xiong Wang, Ke-Ke Wang, Mei Wang, Yuan-Bin She, and Hai-Min Shen. 2023. "Efficient and Selective Oxygenation of Cycloalkanes and Alkyl Aromatics with Oxygen through Synergistic Catalysis of Bimetallic Active Centers in Two-Dimensional Metal-Organic Frameworks Based on Metalloporphyrins" Biomimetics 8, no. 3: 325. https://doi.org/10.3390/biomimetics8030325
APA StyleZhou, X.-Y., Fu, B., Jin, W.-D., Wang, X., Wang, K.-K., Wang, M., She, Y.-B., & Shen, H.-M. (2023). Efficient and Selective Oxygenation of Cycloalkanes and Alkyl Aromatics with Oxygen through Synergistic Catalysis of Bimetallic Active Centers in Two-Dimensional Metal-Organic Frameworks Based on Metalloporphyrins. Biomimetics, 8(3), 325. https://doi.org/10.3390/biomimetics8030325






