Abstract
The method of micro-arc oxidation has been utilized to synthesize a protective biocompatible coating for a bioresorbable orthopedic Mg implant. This paper presents the results of comprehensive research of micro-arc coatings based on diatomite—a biogenic material consisting of shells of diatom microalgae. The main focus of this study was the functionalization of diatomite-based micro-arc coatings by incorporating particles of titania (TiO2) into them. Various properties of the resulting coatings were examined and evaluated. XRD analysis revealed the formation of a new magnesium orthosilicate phase—forsterite (Mg2SiO4). It was established that the corrosion current density of the coatings decreased by 1–2 orders of magnitude after the inclusion of TiO2 particles, depending on the coating process voltage. The adhesion strength of the coatings increased following the particle incorporation. The processes of dissolution of both coated and uncoated samples in a sodium chloride solution were studied. The in vitro cell viability was assessed, which showed that the coatings significantly reduced the cytotoxicity of Mg samples.
1. Introduction
In recent years, a lot of scientific research has aimed to develop new materials and designs for the creation and improvement of bone implants [1,2]. Much attention is paid to ensuring mechanical strength, fatigue durability of the implant, and reducing its weight [3,4]. Magnesium and its alloys have become increasingly popular in the field of medical materials science. In particular, magnesium can be used as a material for the fabrication of bioresorbable orthopedic implants. This is feasible due to its low specific gravity (≈1.74 g/cm3), high biocompatibility, and elastic modulus close to that of human cortical bone (≈40–45 GPa) [4,5,6,7,8,9,10]. Such a modulus of elasticity virtually eliminates the risk of the stress-shielding effect, which can lead to undesirable consequences in the form of the onset of osteopenia at the implant site [11,12,13]. According to Wolff’s law, the bone in a healthy human or animal adapts to the stresses to which they are subjected [14]. If the load on the bone decreases, the bone becomes less dense and weaker due to the lack of incentive needed to continue remodeling. Therefore, magnesium is a very promising material in this respect.
However, magnesium also has certain disadvantages associated with its excessive susceptibility to corrosive media, as well as the active release of hydrogen at the site of contact between the implant and the surrounding tissues [15,16,17,18]. To address these challenges, this paper proposes modifying the implant surface with a protective, biologically active coating based on natural diatomite functionalized with titanium dioxide particles.
To synthesize the coatings, the micro-arc oxidation (MAO, also known as plasma electrolytic oxidation or PEO) method was implemented. This method is characterized by coating a valve metal substrate submerged in a bath of electrolytes. Under the influence of a strong electric field, numerous plasma micro-arc discharges are formed on the surface of the substrate, contributing to intensive electrochemical interaction of the electrolyte components with the substrate material, and consequently, the formation of a protective oxide coating [19,20]. This coating acts as a two-way solution. On the one hand, it protects the magnesium implant from being dissolved too quickly by creating a barrier between it and the aggressive environment [21,22,23,24]. On the other hand, it enables and facilitates the osseointegration process between the implant and the bone tissue [25,26,27,28,29].
The most widely used coatings in the field of micro-arc coating research are the ones synthesized in electrolytes based on phosphates, silicates, and, aluminates, although these are a little less common [30,31,32,33,34,35,36,37]. Aluminate-based electrolytes are typically used to produce wear-resistant MAO coatings due to the dominant formation of MgAl2O4 as a very solid phase. Phosphate- and silicate-based electrolytes are commonly used to synthesize coatings with improved corrosion resistance, which is due to the high corrosion resistance of Mg2SiO4 and Mg3(PO4)2, respectively. [38]. Such coatings can be further functionalized by the addition of insoluble particles to the composition of an electrolyte. These can include various pharmaceutical products, carbides, nitrides, oxides, etc. [39,40,41,42,43,44,45,46,47]. Altering the concentration and/or proportions of these particles makes it possible to control and tailor the structure and various properties of the resulting coatings.
Another promising direction is the use of materials of biogenic origin to increase the osteoinductive/osteoconductive properties of bone-contact implantable devices, which contributes to their further osseointegration. In previous studies, we developed coatings for a magnesium alloy based on natural diatomite [48], a sedimentary rock of biogenic origin consisting of skeletons of diatom algae. The mineralogical composition of diatomite is mainly represented by amorphous hydrated silica SiO2∙nH2O. Diatomite powder was introduced into the composition of the electrolyte to form micro-arc coatings. The obtained coatings were characterized by excellent biocompatibility and high corrosion resistance. However, the mechanical properties of these coatings were not outstanding.
In this work, an electrolyte based on biogenic diatomite was used as the base electrolyte. The distinction of this work is the modification of a diatomite-based coating with titanium oxide particles added to the base electrolyte in the form of a dispersed phase. Analysis of the scientific literature on this topic shows that the addition of titanium dioxide particles changes the structure and properties of micro-arc coatings, increasing their adhesive strength, corrosion resistance, wear resistance, and antibacterial properties [49,50,51,52,53,54]. Ergo, the addition of TiO2 particles is a promising direction in the field of the functionalization of micro-arc coatings. The novelty of this work lies in the fact that completely new composite coatings based on biogenic diatomite with the addition of TiO2 particles characterized by high biocompatibility, adhesive strength, and excellent corrosion resistance were developed.
The main objective of this study is to investigate the effect of adding refractory titanium oxide particles on the structure and various properties of protective, biologically active micro-arc coatings for bioresorbable orthopedic magnesium implants.
2. Materials and Methods
As a substrate material, high-purity magnesium alloy (Mg alloy) MA2-1hp (AZ31 alloy equivalent) (VILS, Moscow, Russia) was chosen. Its chemical composition is as follows (wt.%):
- 94.12 Mg;
- 4.05 Al;
- 1.1 Zn;
- 0.6 Mn
- >0.1 other elements combined.
The alloy has a Young’s modulus of 42 GPa and a density of 1.790 kg/m3, based on the manufacturer’s tests. Model samples for the coating deposition were cut from a solid piece of magnesium alloy by the method of electric discharge machining. After that, the samples were ground with 600-grit abrasive sandpaper (Hermes Schleifmittel GmbH, Germany) to achieve surface roughness Ra of 0.5–0.6 µm. Following this, the samples were deep-cleaned using an ultrasonic cleaner (Elmasonic S, Elma, Germany) filled with distilled water and ethanol sequentially for 30 min each. After this preparation, samples with a smooth surface were obtained. When examining uncoated samples with an optical or electron microscope, distinctive scratches were observed on their surface, which remained after the mechanical impact of the abrasive material.
The electrolyte for the coating deposition included NaOH, Na2SiO3, and NaF dissolved in distilled water and dispersed phases of diatomite (SiO2∙nH2O) and titania (TiO2). The diffractogram of TiO2 powder is shown in Figure 1. The diffractogram of diatomite powder was provided previously in [48].
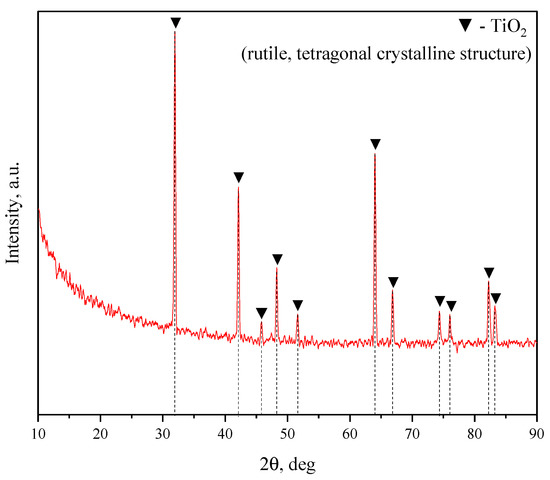
Figure 1.
X–ray diffraction pattern of the initial TiO2 powder.
The coatings were synthesized via the method of micro-arc oxidation. For the coating deposition process, the anodic potentiostatic mode was implemented with a pulse frequency and duration of 50 Hz and 100 µs, respectively. The coatings were deposited for 5 min at four different pulsed voltages: 350, 400, 450, and 500 V. To carry out the experiment, “Micro-Arc 3.0” equipment was used, which consisted of a direct current (DC) power supply, a water-cooled electrolyte bath, and counter electrodes. The monitoring of the parameters during the MAO process was carried out with the help of the equipment’s built-in software. A magnetic mixer stirred the electrolyte continuously during the process to agitate the solution and prevent particle aggregation. After MAO treatment, samples with a uniformly distributed coating layer were obtained.
The surface morphology and the elemental composition of the resulting coatings were studied using a LEO EVO 50 scanning electron microscope (Zeiss, Jena, Germany), equipped with an INCA x-act EDX spectrometer (Oxford Instruments, Abingdon, UK).
Changes in the phase composition of the coatings depending on the deposition voltage were investigated via an X-ray diffractometer (DRON–7, Burevestnik, St. Petersburg, Russia) with the following parameters: CoKα radiation; 35 kV voltage; 22 mA tube current; and 2θ angle range of 10° to 90° with 0.02° scanning step.
The adhesion strength of the coatings was assessed using a Revetest RST scratch tester (CSM Instruments SA, Peseux, Switzerland) equipped with a diamond indenter 200 µm in radius. The indenter scratched the surface of the sample, moving at a speed of 1 mm/min, while the load increased linearly. The length of the scratch was 5 mm. The peak indentation force was 40 N, and the loading rate was 3.9 N/min.
Corrosion resistance of the coatings in comparison with uncoated Mg alloy was determined using the “P-40X” electrochemical workstation (Electrochemical Instruments, Russia). A 0.9% NaCl solution was used as the electrolyte in a three-electrode cell, where a graphite rod served as a counter electrode and an Ag/AgCl electrode was used as a reference electrode. The exposed sample surface area equaled 1 cm2. The scan rate at which the potentiodynamic polarization curves were obtained equaled 2 mV/s. The electrode potential ranged from −1.9 V to +1.9 V. The temperature of the electrolyte was maintained at 37 °C to simulate the conditions under which a coated implant would function in a human body.
The method of immersion was used to determine the biodegradation rate of both the coated and uncoated Mg alloy specimens. The samples were placed in containers with 0.9% NaCl physiological saline solution and immersed at 37 °C in compliance with ISO 10993-5. The volume of the electrolyte was at least 1 mL per 1 cm2 of the sample surface and covered the sample completely. To obtain reliable results, the electrolyte was prepared under aseptic conditions. The experiment was conducted over the course of 11 days. The number of parallel experiments was not less than 3. Mass loss of the samples during dissolution was monitored on a daily basis. The following formula (1) was used to calculate the mass loss of the specimens:
where m0 is the sample mass before dissolution, mg, and mi is the sample mass after dissolution, mg.
The condition of the coatings at certain stages of the experiment was visually assessed via a MET 1MT optical microscope (Altami, Russia).
The biocompatibility of samples was examined in vitro using the MTT assay after direct contact of the cells with the testing material. The NIH/3T3 cell line was purchased from the State Research Center of Virology and Biotechnology “VECTOR” (Novosibirsk, Russia). The cells were seeded in a flask with DMEM (Lonza, Switzerland) with 10% fetal bovine serum (HyClone, Marlborough, MA, USA) and 5% penicillin/streptomycin-glutamine (HyClone, Marlborough, MA, USA) and incubated at 37 °C in a 5% CO2. After the 24 h incubation period, the attached cells were trypsinizated for 3–5 min and centrifuged at 1400 rpm for 5 min. The cell culture was seeded into 24-well culture plates (total volume of 2 mL) at 70,000 cells per well. Test samples were placed in each well. Cells were incubated for 24 h at 37 °C in 5% CO2. Cells that were incubated in the medium without the sample present were used as controls.
The viability of the cells after exposure was analyzed using the MTT assay [48]. In line with typical procedure, 200 µL of MTT [3-(4, 5-dimetheylthiazol-2)-2, 5 diphenyl tetrazolium bromide] solution was added to each well. The plates were incubated for 4 h at 37 °C in 5% CO2. Then, the MTT-containing medium was removed, and the cells were treated by adding 100 μL DMSO (HyClone, Marlborough, MA, USA) to dissolve the formazan crystal. In the final step, the optical density (OD) of the solution was recorded at a 570 nm wavelength using a microplate reader Thermo Scientific Multiskan FC (Thermo Fisher Scientific, Waltham, MA, USA). The measured OD value was used to calculate the relative cell viability (%) according to Equation (2):
Cell viability (%) = OD (test sample)/OD (control) × 100
Each experiment was carried out in duplicate. All statistical analyses were performed using the statistical software package STATISTICA 10.0. The normal distribution of the results was checked by the Kolmogorov–Smirnov test. The significance of differences in the mean values between groups was analyzed using the Mann–Whitney test. Differences were considered significant at p < 0.05.
3. Results and Discussion
3.1. Coating Thickness and Roughness
Figure 2 displays the thickness and roughness of the coatings in relation to the MAO process voltage. The thickness of deposited coatings increases exponentially, which correlates well with our previous studies related to diatomite-based coatings [48,55]. The surface roughness ranges from 2.5 to 5.0 µm, with a sharp increase at 450 V. This is due to the more intense course of the micro-arc oxidation process at higher voltages, which leads to a greater number of micro-arc plasma discharges on the substrate surface. This, in turn, leads to the merging of many small micro-discharges into one large electric arc, leading to more active intumescence of the surface and, accordingly, to the formation of a rougher topography.
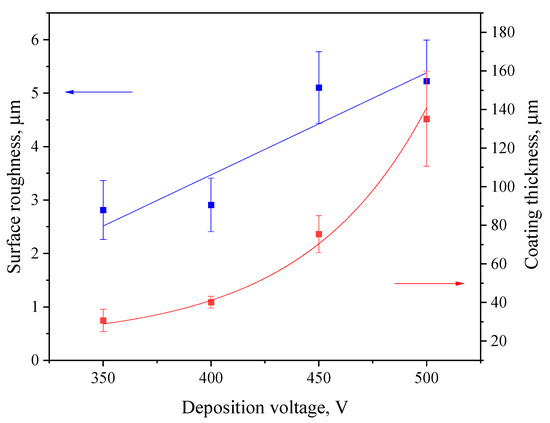
Figure 2.
Graphs of roughness (left axis, blue line) and thickness (right axis, red line) versus the MAO process voltage.
3.2. Morphology and Pore Structure
Visual analysis of the SEM micrographs of the coatings obtained at different process voltages was carried out (Figure 3). It was revealed that the coatings have a large number of variously shaped pores, ranging in size from 100 nm to 12 µm (Figure 3a,b,e,f). We can observe the fragments of lithified diatomeae shells both on the surface and within the bigger pores. Some of the fragments were partially melted into the matrix of the coating, thus giving it an ordered reticulated pore structure. Unmelted particles of both diatomite and titania are also present in substantial amounts on the surface of the coating. It is worth noting, however, that the number of unmelted particles decreases with the increase in the deposition process voltage. This can be explained by a larger quantity of more powerful micro-arc discharges being generated during the MAO process at higher voltages, which leads to a more active disintegration and fusion of the oxide particles into the coating matrix.
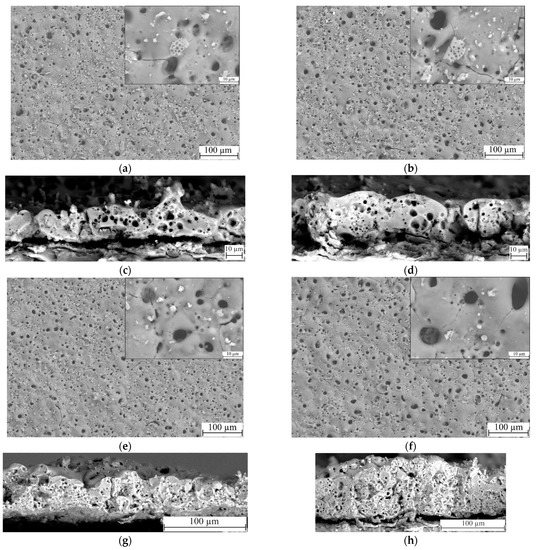
Figure 3.
SEM micrographs of the surfaces and cross-sections of TiO2-doped diatomite coatings synthesized at different process voltages: (a,c) 350 V; (b,d) 400 V; (e,g) 450 V; (f,h) 500 V. Mag.: 500×, 5000× for (a,b,e,f); 2000× for (c,d); 1000x for (g,h).
In addition, Figure 3c,d,g,h presents the micrographs of cross-sections of the coatings. The analysis of the microphotographs leads to the conclusion that the coatings have an internal porous structure, including pores of different types and sizes. The coatings deposited at MAO voltages of 350 and 400 V contain large pores, up to 5 μm in diameter, indicating single high-intensity micro-arc discharges (Figure 3c,d). Groups of small pores less than 1 μm in size are scattered around them, resulting from cascades of micro-arc discharges. As the voltage of the MAO process increases to 450–500 V, the thickness of the coatings increases significantly, but the nature of the internal pore structure does not change; both large and small pores, evenly distributed throughout the coating thickness, are present in the coatings (Figure 3g,h). The pores are mostly enclosed, but in some cases channeled pores resulting from a powerful breakdown of the coating are observed (Figure 3g). In addition, the presence of TiO2 particles can be observed in the cross-sections of the coatings.
3.3. Elemental Composition
To characterize the chemical composition of the synthesized coatings, the method of energy-dispersive X-ray microanalysis was used. Figure 4 shows the maps of element distribution, as well as the sum spectrum of the elements present in the coating. High localized concentrations of Ti and Si were detected via element mapping, correlating to titania and diatomite particles, respectively. Table 1 displays the amount of each element (in at. %) in relation to the coating deposition voltage. It can be concluded that the amount of most elements remains approximately the same regardless of the process voltage. The exceptions are silicon and titanium, the amount of which decreases as the MAO voltage increases. This can be partially attributed to fewer particles of both diatomite and titania remaining unmelted on the coatings surface at higher MAO voltages, as can be seen in Figure 3.
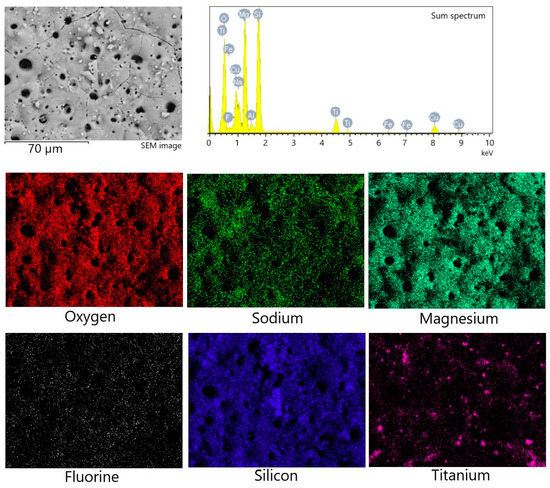
Figure 4.
Energy-dispersive X-ray analysis of the TiO2-incorporated diatomite coating formed at 500 V: SEM image, sum spectrum, and element mapping.

Table 1.
Chemical composition of the coatings, at. %.
EDX analysis of the element distribution in the cross-section of the coating (Figure 5) showed that the elements O and Si are evenly distributed throughout the coating thickness, while Mg and F are mainly concentrated closer to the substrate in the transition layer. Na concentration, on the contrary, is higher in the surface layer. In addition, isolated local Ti accumulations are observed, confirming the presence of TiO2 particles in the coating volume.
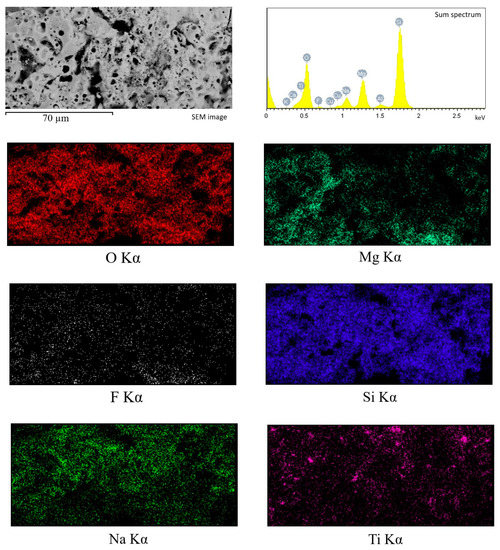
Figure 5.
Energy-dispersive X-ray analysis of the cross-sections of TiO2-incorporated diatomite coating formed at 450 V: SEM image, sum spectrum, and element mapping.
The chemical composition of the cross-sections of the coatings formed at different voltages of the MAO process (Table 2) is almost identical to the composition of the surface of these coatings presented in Table 1. In the cross-sections of the coatings applied at voltages of 350–400 V, a higher content of F is noted, but with an increase in voltage, its amount decreases. There is also a decrease in Mg concentration when the process voltage increases to 450–500 V.

Table 2.
Chemical composition of the coatings (cross-section), at. %.
X-ray Diffraction Analysis
Figure 6 showcases the X-ray diffraction patterns for the TiO2-augmented diatomite coatings formed at four different process voltages. The formation of a new predominant phase is detected in all four coatings, namely forsterite (Mg2SiO4) (ICDD #34-0189), which is a magnesium orthosilicate from the olivine group of minerals. We can also see the reflexes correlating to both magnesium (ICDD #35-0821) and its oxide, namely periclase (ICDD #45-0946). The incorporation of TiO2 particles into the coating results in a few peaks corresponding to rutile (ICDD #21-1276), which is the most common natural form of titanium dioxide.
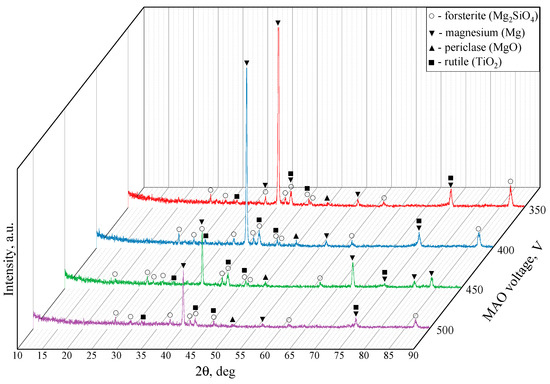
Figure 6.
X-ray diffraction patterns of the coatings formed at 350, 400, 450, 500 V.
3.4. Mechanical Properties
Scratch Testing
The results of the study of the mechanical properties of TiO2-augmented diatomite coatings, performed by scratch testing, showed that they have very high adhesion strength. Figure 7 shows the optical images of the coatings, applied at different voltages of the MAO process, after scratch testing. On scratches, it is possible to distinguish the zone of destruction of the coating at the moment of penetration of the indenter to the substrate—this is a solid light zone at the end of the scratch. But for the coatings applied at 500 V, this zone is practically absent, which indicates the highest adhesion strength of these coatings.
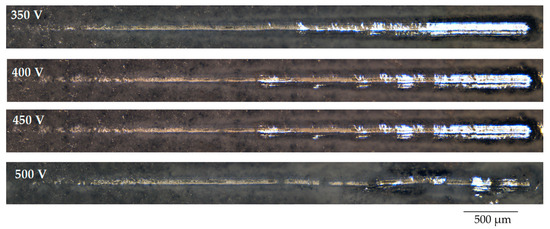
Figure 7.
Optical images of the scratches obtained as a result of testing the coatings deposited at different MAO voltages.
The determination of the critical load at failure confirmed these results. A diagram of the critical load versus voltage at which the coating was formed is shown in Figure 8a. The figure shows that the critical load values for coatings applied at 350–450 V are approximately the same, ranging from 24.0–25.4 N. However, the coating applied at 500 V is characterized by the highest critical load value of 35.2 N. The results obtained in previous studies show that diatomite-based coatings without TiO2 particles have much lower adhesion strength. The maximum critical load did not exceed 10 N [48].
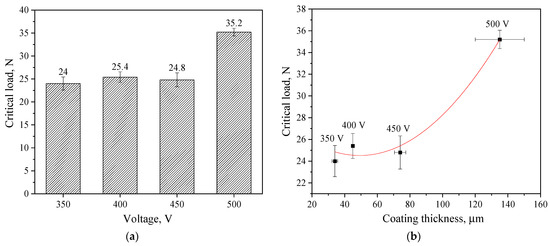
Figure 8.
Diagram of correlation between the critical load and the MAO voltage (a); graph of the correlation dependence of the critical load on the thickness of coatings formed at different voltages (b).
Because the thickness of the coatings increases exponentially with an increase in the stress of the MAO process, we assume that the adhesive strength of the coatings depends on their thickness.
Figure 8b shows a correlation graph of the dependence of the critical load on the thickness of the coatings, indicating that this dependence has an exponential character, which confirms our assumption.
3.5. Electrochemical Properties
One of the main objectives of this study was to improve the corrosion properties of diatomite-based coatings by incorporating titanium oxide particles into them. This is especially relevant for aggressive media, particularly the liquid component of the physiological medium of the human body. Figure 9 shows the potentiodynamic polarization curves for both pure Mg alloy samples and samples with coatings applied at different voltages. The values of polarization resistance and corrosion current density of the coatings varied in the ranges from 1.65 × 106 to 1.41 × 108 Ω cm2 and from 4.01 × 10−8 to 3.30 × 10−10 A cm–2, respectively, versus MAO process voltages (Table 3). Comparison with the literature data [56,57] shows that calcium-phosphate coatings obtained by the PEO method on magnesium alloys are characterized by lower values of polarization resistance in the range of 4.24–11.27 kΩ cm−2, and higher values of corrosion current density are characterized by the range of 1.25 × 10−6–8.54 × 10−6 A cm−2. This indicates that these coatings are inferior in corrosion resistance to diatomite-based coatings with TiO2 particles as presented in this paper.
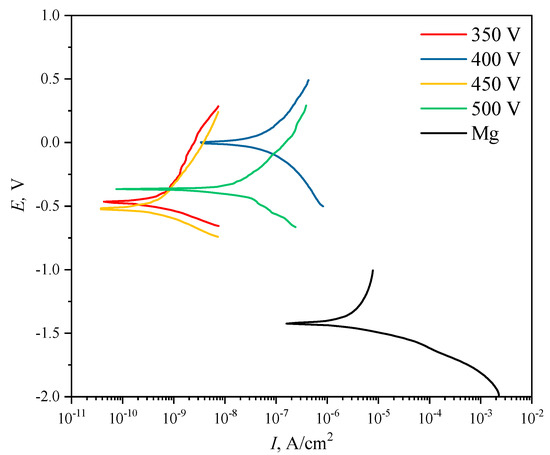
Figure 9.
Potentiodynamic polarization (PDP) curves of the studied samples.

Table 3.
Electrochemical parameters of the Mg alloy and coated samples.
It can be seen that the coatings deposited at 350 and 450 V are the most resistant to corrosion. The values of polarization resistance Rp for these samples amounted to 1.22 × 108 A∙cm−2 and 1.41 × 108 A∙cm−2, respectively.
A comparative analysis of the corrosion resistance of diatomite coatings before [48] and after the addition of titanium dioxide particles showed that the addition of particles increases the polarization resistance of coatings by 2–3 orders of magnitude, depending on the coating deposition voltage. The corrosion current density in this case decreases by 1 to 2 orders of magnitude. Overall, the corrosion current density of TiO2-doped samples decreases by 2–4 orders of magnitude compared to uncoated Mg alloy samples, and the polarization resistance, respectively, increases by 2–4 orders of magnitude. The experimental data are given in Table 3.
3.6. Bioresorption Study
The processes of dissolution of both coated and uncoated alloy samples were studied for 11 days while kept in a 0.9% NaCl solution (Figure 10). Studies have shown that when a Mg alloy is dissolved, three periods can be distinguished. During the first period, i.e., during the first 4 days, the dissolution rate of the alloy is lower than the dissolution rate of samples with coatings. After 4 days, the dissolution rate increases sharply and exceeds the dissolution rate of the coated samples. After 8 days, the dissolution rate slightly decreases again. As described in previous studies, the dissolution rate of the Mg alloy directly depends on two processes: the first is the dissolution of alloy components, while the second is the process of reverse precipitation of dissolution products (magnesium hydroxide Mg(OH)2) on the alloy surface. If the first process prevails, the dissolution rate increases, but if the second process prevails, the alloy dissolves more slowly.
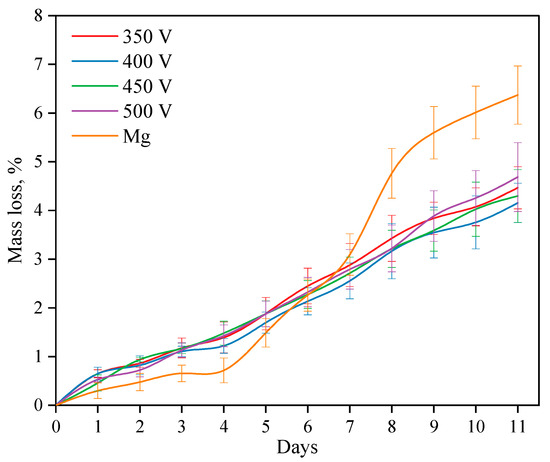
Figure 10.
Graph of the samples’ mass loss depending on the time of immersion in the 0.9% NaCl solution.
The rate of dissolution of coatings of all types is constant and linear. After 6 days of dissolution, the mass loss of the coated samples becomes less than that of the Mg alloy. The intensity of the dissolution of the coating does not depend on the voltage of the MAO process at which the coating is applied.
The optical images of the samples before dissolution and after 4, 8, and 11 days of dissolution were also analyzed (Figure 11). On the surface of the pure Mg alloy, the precipitation of dissolution products is observed after 4 days of incubation in the 0.9% NaCl solution. The amount of dissolution products on the surface of the Mg alloy increases with the increase in the duration of incubation. Similar patterns were observed and described in previous studies [48,55].
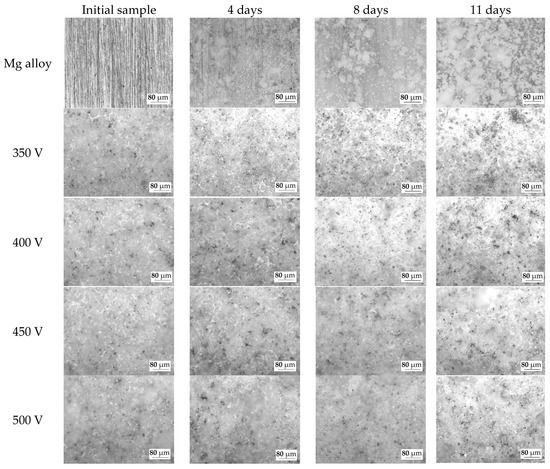
Figure 11.
Optical microscopic images of the samples at different stages of the bioresorption process.
As the optical images show, the dissolution of the coatings occurs in the area of surface structure defects (pores, cracks). An increase in the diameter and depth of the pores can be noted. But the morphology of the coatings surface does not change significantly, which indicates the resistance of this type of coatings to dissolution in the biological environment. This phenomenon is explained by the presence of insoluble compounds, such as magnesium silicate (forsterite) and oxides of magnesium and titanium, in the coatings.
3.7. In Vitro Cytotoxicity
Cell viability was assessed using the MTT assay and quantified according to Equation (2). Compared to the control, coating 400 V and coating 500 V-treated cells showed no statistically significant decrease in viability; cell viabilities were about 94% and 98% of that of the control, respectively (Figure 12). At the same time, the Mg alloy-treated NIH/3T3 cells showed decreased viability, and the cell viability was approximately 43% (high toxicity). Based on these results, it is safe to say that the coatings significantly reduce the toxicity of the samples.
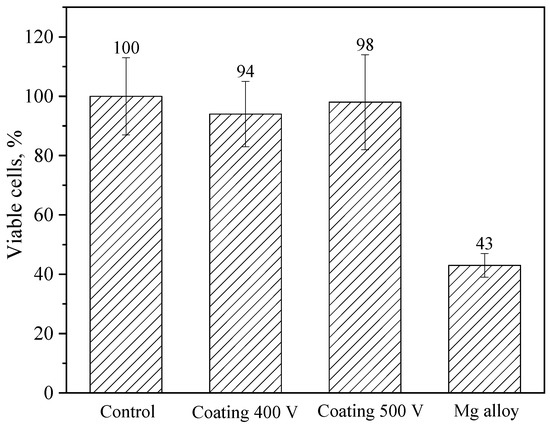
Figure 12.
Results of in vitro NIH/3T3 cell viability measured by an MTT assay after 24 h culturing with negative control (DMEM), as well as with Mg alloy (positive toxic control), or samples coated at 400 V and 500 V.
4. Conclusions
The coatings considered in this article are suitable for use as a biocompatible and bioactive protectant for magnesium-based bioresorbable orthopedic implants. The micro-arc oxidation method was used to synthesize coatings based on diatomite with the addition of titanium dioxide particles. A range of 350 to 500 V was chosen as the optimal voltage range of the MAO process. Depending on the MAO voltage, the thickness of the coating varied from approximately 30 to 140 µm, and the roughness values lay within a 2.5–5.0 µm range.
The resulting coatings were studied using a number of research methods, including scanning electron microscopy (SEM), energy-dispersive X-ray microanalysis (EDX), X-ray diffraction analysis (XRD), scratch testing, potentiodynamic polarization testing, in vitro immersion method, and in vitro MTT assay.
It has been found that the morphology of the coatings is comprised of a large number of pores of various shapes and sizes, fragments of lithified diatomeae shells, and unmelted particles of both diatomite and TiO2. Some diatom algae fragments were partially fused into the coating matrix, giving it a reticulated pore structure. The X-ray diffraction analysis revealed the formation of a new prevalent phase—forsterite (Mg2SiO4). Peaks corresponding to magnesium and periclase (MgO), as well as rutile (TiO2), were also observed in the diffraction patterns of the resulting coatings. The coatings demonstrated very high adhesion strength (critical load equaled to 24–35 N) compared to diatomite-based coatings without the addition of TiO2 particles (critical load <10 N). Diatomite-based coatings, the composition of which included TiO2 particles, had 2–3 orders of magnitude greater polarization resistance than coatings without the addition of titanium dioxide particles. The in vitro immersion method was implemented to study the dissolution rate of both the coated and uncoated samples, which showed that the dissolution rate of all coated samples, regardless of the deposition voltage, has a linear character. The mass loss rate of the uncoated Mg alloy samples exceeds that of the coated samples after 4 days of immersion and amounts to more than 6% at the end of the experiment. NIH/3T3 mouse fibroblasts were used to assess the in vitro cytotoxicity of the coatings, i.e., cell viability, during direct contact with the coating. The number of viable cells after direct contact with the coatings was 94–98%, which is not a statistically significant difference from the control samples. Meanwhile, the number of viable cells in contact with pure magnesium alloy was only 43%. Thus, modification of the surface of magnesium alloy samples by means of micro-arc coatings based on diatomite with TiO2 particles yielded an increase in their cytocompatibility by more than two-fold.
A comparative analysis of the properties of the coatings deposited at different stresses of the MAO process was carried out, and it was concluded that the coatings formed at 350 and 450 V are the most promising for practical application in terms of the set of optimal properties. These coatings are characterized not only by sufficient thickness and roughness and high adhesive strength, but also by the highest corrosion resistance, exceeding that in the works of other authors [56,57]. They had the highest values of polarization resistance at 1.22 × 108 Ω cm2 and 1.41 × 108 Ω cm2, respectively, and the lowest values of corrosion current density at 3.3 × 10−10 A cm–2 and 4.0 × 10−10 A cm−2, respectively.
Author Contributions
Conceptualization, M.B.S. and A.D.K.; investigation, A.D.K., P.V.U., N.A.L., A.V.U., M.A.K. and O.V.B.; validation, M.B.S., A.D.K. and O.V.B.; formal analysis, A.D.K., A.V.U., M.A.K. and P.V.U.; resources, Y.P.S.; data curation, P.V.U.; writing—original draft preparation, A.D.K.; writing—review and editing, M.B.S. and A.D.K.; visualization, A.D.K.; supervision, M.B.S. and Y.P.S.; project administration, M.B.S.; funding acquisition, M.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Russian Science Foundation, grant No. 23-29-00141, https://rscf.ru/en/project/23-29-00141/.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to A.I. Tolmachev from the Institute of Strength Physics and Materials Science SB RAS (Tomsk, Russia) for his assistance in the preparation of experimental materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ammarullah, M.I.; Hartono, R.; Supriyono, T.; Santoso, G.; Sugiharto, S.; Permana, M.S. Polycrystalline diamond as a potential material for the hard-on-hard bearing of total hip prosthesis: Von Mises stress analysis. Biomedicines 2023, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zeng, R.C.; Li, S.Q.; Cui, L.Y.; Zou, Y.H.; Guan, S.K.; Zheng, Y.F. Advance in antibacterial magnesium alloys and surface coatings on magnesium alloys: A Review. Acta Metall. Sin. 2020, 33, 615–629. [Google Scholar] [CrossRef]
- Salaha, Z.F.M.; Ammarullah, M.I.; Abdullah, N.N.A.A.; Aziz, A.U.A.; Gan, H.S.; Abdullah, A.H.; Kadir, M.R.A.; Ramlee, M.H. Biomechanical effects of the porous structure of Gyroid and Voronoi hip implants: A finite element analysis using an experimentally validated model. Materials 2023, 16, 3298. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of activity changes increases the fatigue life of the porous magnesium scaffold, as observed in dynamic immersion tests, over time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Chakraborty Banerjee, P.; Al-Saadi, S.; Choudhary, L.; Harandi, S.E.; Singh, R. Magnesium Implants: Prospects and Challenges. Materials 2019, 12, 136. [Google Scholar] [CrossRef]
- Jhamb, S.; Matai, J.; Marwaha, J.; Goyal, A.; Pandey, A. A comprehensive analysis on magnesium-based alloys and metal matrix composites for their in-vitro biocompatibility. Adv. Mater. Process. Technol. 2022, 1–34. [Google Scholar] [CrossRef]
- Uppal, G.; Thakur, A.; Chauhan, A.; Bala, S. Magnesium based implants for functional bone tissue regeneration—A review. J. Magnes. Alloys 2022, 10, 356–386. [Google Scholar] [CrossRef]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloys 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Shahin, M.; Munir, K.; Wen, C.; Li, Y. Magnesium matrix nanocomposites for orthopedic applications: A review from mechanical, corrosion, and biological perspectives. Acta Biomater. 2019, 96, 1–19. [Google Scholar] [CrossRef]
- Hassan, S.F.; Islam, M.T.; Saheb, N.; Baig, M.M.A. Magnesium for Implants: A Review on the Effect of Alloying Elements on Biocompatibility and Properties. Materials 2022, 15, 5669. [Google Scholar] [CrossRef]
- Baskerville, S.J.J. Effects of Orthopedic Implant and Host Bone Properties on Stress-Shielding Induced Osteopenia. Ph.D. Thesis, Florida Institute of Technology, Melbourne, FL, USA, May 2022. [Google Scholar]
- Schwartz, C. How to reduce osteopenia in total knee arthroplasty? Eur. J. Orthop. Surg. Traumatol. 2019, 29, 139–145. [Google Scholar] [CrossRef]
- Savio, D.; Bagno, A. When the Total Hip Replacement Fails: A Review on the Stress-Shielding Effect. Processes 2022, 10, 612. [Google Scholar] [CrossRef]
- Wolff, J. The classic: On the inner architecture of bones and its importance for bone growth. 1870. Clin. Orthop. Relat. Res. 2010, 468, 1056–1065. [Google Scholar] [CrossRef]
- Wang, J.; Dou, J.; Wang, Z.; Hu, C.; Yu, H.; Chen, C. Research progress of biodegradable magnesium-based biomedical materials: A review. J. Alloys Compd. 2022, 923, 166377. [Google Scholar] [CrossRef]
- Atrens, A.; Shi, Z.; Mehreen, S.U.; Johnston, S.; Song, G.-L.; Chen, X.; Pan, F. Review of Mg alloy corrosion rates. J. Magnes. Alloys 2020, 8, 989–998. [Google Scholar] [CrossRef]
- Feliu, S., Jr. Electrochemical Impedance Spectroscopy for the Measurement of the Corrosion Rate of Magnesium Alloys: Brief Review and Challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Khalili, M.A.; Tamjid, E. Controlled biodegradation of magnesium alloy in physiological environment by metal organic framework nanocomposite coatings. Sci. Rep. 2021, 11, 8645. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation—An Overview of the Process and Applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (PEO) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Gnedenkov, S.V. Hydroxyapatite-containing PEO-coating design for biodegradable Mg-0.8Ca alloy: Formation and corrosion behavior. J. Magnes. Alloys 2022, in press. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Sharifi-Asl, S.; Mozafari, M. Improved corrosion performance of biodegradable magnesium in simulated inflammatory condition via drug-loaded plasma electrolytic oxidation coatings. Mater. Chem. Phys. 2020, 239, 122003. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Enhanced corrosion resistance and in-vitro biodegradation of plasma electrolytic oxidation coatings prepared on AZ91 Mg alloy using ZnO nanoparticles-incorporated electrolyte. Surf. Coat. Technol. 2019, 360, 153–171. [Google Scholar] [CrossRef]
- Alateyah, A.I.; Aljohani, T.A.; Alawad, M.O.; Elkatatny, S.; El-Garaihy, W.H. Improving the Corrosion Behavior of Biodegradable AM60 Alloy through Plasma Electrolytic Oxidation. Metals 2021, 11, 953. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, J.; Zhang, J.; Huang, H.; Xie, Z.; Xie, X. Silver-Releasing Micro-/Nanoporous Coating on Additively Manufactured Macroporous Ti-Ta-Nb-Zr Scaffolds with High Osseointegration and Antibacterial Properties. Coatings 2021, 11, 716. [Google Scholar] [CrossRef]
- He, J.; Zhang, B.; Shao, L.; Feng, W.; Jiang, L.; Zhao, B. Biomechanical and histological studies of the effects of active zinc-coated implants by plasma electrolytic oxidation method on osseointegration in rabbit osteoporotic jaw. Surf. Coat. Technol. 2020, 396, 125848. [Google Scholar] [CrossRef]
- He, X.; Zhang, G.; Zhang, H.; Hang, R.; Huang, X.; Yao, X.; Zhang, X. Cu and Si co-doped microporous TiO2 coating for osseointegration by the coordinated stimulus action. Appl. Surf. Sci. 2020, 503, 144072. [Google Scholar] [CrossRef]
- Lu, X.; Wu, Z.; Xu, K.; Wang, X.; Wang, S.; Qiu, H.; Li, X.; Chen, J. Multifunctional Coatings of Titanium Implants Toward Promoting Osseointegration and Preventing Infection: Recent Developments. Front. Bioeng. Biotechnol. 2021, 9, 783816. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.E.; Golozar, M.; Best, S.M.; Brooks, R.A. Cell response to plasma electrolytic oxidation surface-modified low-modulus β-type titanium alloys. Colloids Surf. B: Biointerfaces 2019, 176, 176–184. [Google Scholar] [CrossRef]
- Kaseem, M.; Hussain, T.; Ur Rehman, Z.; Ko, Y.G. Stabilization of AZ31 Mg alloy in sea water via dual incorporation of MgO and WO3 during micro-arc oxidation. J. Alloys Compd. 2021, 853, 157036. [Google Scholar] [CrossRef]
- Hao, G.; Zhang, D.; Lou, L.; Yin, L. High-temperature oxidation resistance of ceramic coatings on titanium alloy by micro-arc oxidation in aluminate solution. Prog. Nat. Sci. Mater. Int. 2022, 32, 401–406. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Ivanov, K.V.; Ugodchikova, A.V.; Kashin, A.D.; Uvarkin, P.V.; Sharkeev, Y.; Tolkacheva, T.V.; Tolmachev, A.I.; Schmidt, J.; Egorkin, V.S.; et al. The effect of pulsed electron irradiation on the structure, phase composition, adhesion and corrosion properties of calcium phosphate coating on Mg0.8Ca alloy. Mater. Chem. Phys. 2023, 294, 126996. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Dudek, Ł. Phosphate Porous Coatings Enriched with Selected Elements via PEO Treatment on Titanium and Its Alloys: A Review. Materials 2020, 13, 2468. [Google Scholar] [CrossRef]
- Wu, T.; Blawert, C.; Serdechnova, M.; Karlova, P.; Dovzhenko, G.; Wieland, D.C.F.; Stojadinovic, S.; Vasilic, R.; Wang, L.; Wang, C.; et al. Role of phosphate, silicate and aluminate in the electrolytes on PEO coating formation and properties of coated Ti6Al4V alloy. Appl. Surf. Sci. 2022, 595, 153523. [Google Scholar] [CrossRef]
- Toulabifard, A.; Rahmati, M.; Raeissi, K.; Hakimizad, A.; Santamaria, M. The Effect of Electrolytic Solution Composition on the Structure, Corrosion, and Wear Resistance of PEO Coatings on AZ31 Magnesium Alloy. Coatings 2020, 10, 937. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Sharkeev, Y.P.; Tolkacheva, T.V.; Uvarkin, P.V.; Chebodaeva, V.V.; Prosolov, K.A.; Bakina, O.V.; Kashin, A.D.; Shcheglova, N.A.; Panchenko, A.A.; et al. Additively manufactured porous titanium 3D–scaffolds with antibacterial Zn-, Ag- calcium phosphate biocoatings. Mater. Charact. 2022, 186, 111782. [Google Scholar] [CrossRef]
- Wang, J.; Pan, Y.; Feng, R.; Cui, H.; Gong, B.; Zhang, L.; Gao, Z.; Cui, X.; Zhang, H.; Jia, Z. Effect of electrolyte composition on the microstructure and bio-corrosion behavior of micro-arc oxidized coatings on biomedical Ti6Al4V alloy. J. Mater. Res. Technol. 2020, 9, 1477–1490. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Wang, J.; Jiang, B.; Zhang, D.; Serdechnova, M.; Shulha, T.; Blawert, C.; Zheludkevich, M.L.; Pan, F. Micro-arc oxidation of magnesium alloys: A review. J. Mater. Sci. Technol. 2022, 118, 158–180. [Google Scholar] [CrossRef]
- Sedelnikova, M.; Bakina, O.; Ugodchikova, A.; Tolkacheva, T.; Khimich, M.; Uvarkin, P.; Kashin, A.; Miller, A.; Egorkin, V.; Schmidt, J.; et al. The Role of Microparticles of β-TCP and Wollastonite in the Creation of Biocoatings on Mg0.8Ca Alloy. Metals 2022, 12, 1647. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Xie, E.; Wang, F.; Gan, Q.; Ping, S.; Wei, J.; Li, F.; Wang, Z. Simultaneous incorporation of gallium oxide and tantalum microparticles into micro-arc oxidation coating of titanium possessing antibacterial effect and stimulating cellular response. Biomater. Adv. 2022, 135, 212736. [Google Scholar] [CrossRef]
- Li, Z.; Cai, Z.; Ding, Y.; Cui, X.-J.; Yang, Z.; Zhu, M. Characterization of graphene oxide/ZrO2 composite coatings deposited on zirconium alloy by micro-arc oxidation. Appl. Surf. Sci. 2020, 506, 144928. [Google Scholar] [CrossRef]
- Askarnia, R.; Sobhani, M.; Zare, M.; Aghamohammadi, H.; Staji, H. Incorporation of Al2O3 and ZrO2 ceramics to AZ31 magnesium alloys composite coating using micro-arc oxidation method. J. Mech. Behav. Biomed. Mater. 2023, 141, 105784. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wu, S.; Xu, D.; Gao, W.; Yao, Y.; Guo, Q.; Chen, J. Preparation and performance of alumina ceramic coating doped with aluminum nitride by micro arc oxidation. Ceram. Int. 2020, 46, 17112–17116. [Google Scholar] [CrossRef]
- Molaeipour, P.; Allahkaram, S.R.; Akbarzadeh, S. Corrosion inhibition of Ti6Al4V alloy by a protective plasma electrolytic oxidation coating modified with boron carbide nanoparticles. Surf. Coat. Technol. 2022, 430, 127987. [Google Scholar] [CrossRef]
- Chebodaeva, V.V.; Sedelnikova, M.B.; Kashin, A.D.; Bakina, O.V.; Khlusov, I.A.; Zharin, A.L.; Egorkin, V.S.; Vyaliy, I.E.; Sharkeev, Y. Structure and electrical potential of calcium phosphate coatings modified with aluminum oxyhydroxide nanoparticles. Lett. Mater. 2022, 12, 336–342. [Google Scholar] [CrossRef]
- Wang, X.; Ju, P.; Lu, X.; Chen, Y.; Wang, F. Influence of Cr2O3 particles on corrosion, mechanical and thermal control properties of green PEO coatings on Mg alloy. Ceram. Int. 2022, 48, 3615–3627. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Lamaka, S.V.; Blawert, C.; Serdechnova, M.; Scharnagl, N.; Karlova, P.; Wieland, D.C.F.; Zheludkevich, M.L. Exploring the corrosion inhibition mechanism of 8-hydroxyquinoline for a PEO-coated magnesium alloy. Corros. Sci. 2022, 203, 110344. [Google Scholar] [CrossRef]
- Kashin, A.D.; Sedelnikova, M.B.; Chebodaeva, V.V.; Uvarkin, P.V.; Luginin, N.A.; Dvilis, E.S.; Kazmina, O.V.; Sharkeev, Y.; Khlusov, I.A.; Miller, A.A.; et al. Diatomite-based ceramic biocoating for magnesium implants. Ceram. Int. 2022, 48, 28059–28071. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Babaei, K. Effect of particles addition to solution of plasma electrolytic oxidation (PEO) on the properties of PEO coatings formed on magnesium and its alloys: A review. J. Magnes. Alloys 2020, 8, 799–818. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Imshinetskiy, I.M.; Nadaraia, K.V.; Gnedenkov, A.S.; Suchkov, S.N.; Opra, D.P.; Pustovalov, E.V.; Yu Ustinov, A.; Sinebryukhov, S.L.; Gnedenkov, S.V. Effect of TiO2 nanoparticles on the photocatalytic properties of PEO coatings on Mg alloy. J. Magnes. Alloys 2023, 11, 735–752. [Google Scholar] [CrossRef]
- Mozafarnia, H.; Fattah-Alhosseini, A.; Chaharmahali, R.; Nouri, M.; Keshavarz, M.K.; Kaseem, M. Corrosion, Wear, and Antibacterial Behaviors of Hydroxyapatite/MgO Composite PEO Coatings on AZ31 Mg Alloy by Incorporation of TiO2 Nanoparticles. Coatings 2022, 12, 1967. [Google Scholar] [CrossRef]
- Molaei, M.; Fattah-alhosseini, A.; Nouri, M.; Mahmoodi, P.; Nourian, A. Incorporating TiO2 nanoparticles to enhance corrosion resistance, cytocompatibility, and antibacterial properties of PEO ceramic coatings on titanium. Ceram. Int. 2022, 48, 21005–21024. [Google Scholar] [CrossRef]
- Ignjatović, S.; Blawert, C.; Serdechnova, M.; Karpushenkov, S.; Damjanović, M.; Karlova, P.; Wieland, D.C.F.; Starykevich, M.; Stojanović, S.; Damjanović-Vasilić, L.; et al. Formation of multi-functional TiO2 surfaces on AA2024 alloy using plasma electrolytic oxidation. Appl. Surf. Sci. 2021, 544, 148875. [Google Scholar] [CrossRef]
- Hashemzadeh, M.; Raeissi, K.; Ashrafizadeh, F.; Hakimizad, A.; Santamaria, M. Incorporation mechanism of colloidal TiO2 nanoparticles and their effect on properties of coatings grown on 7075 Al alloy from silicate-based solution using plasma electrolytic oxidation. Trans. Nonferrous Met. Soc. China 2021, 31, 3659–3676. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Kashin, A.D.; Uvarkin, P.V.; Tolmachev, A.I.; Sharkeev, Y.P.; Ugodchikova, A.V.; Luginin, N.A.; Bakina, O.V. Porous Biocoatings Based on Diatomite with Incorporated ZrO2 Particles for Biodegradable Magnesium Implants. J. Funct. Biomater. 2023, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Chaharmahali, R.; Fattah-alhosseini, A.; Babaei, K. Surface characterization and corrosion behavior of calcium phosphate (Ca-P) base composite layer on Mg and its alloys using plasma electrolytic oxidation (PEO): A review. J. Magnes. Alloys 2021, 9, 21–40. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, S.D.; Zhou, L.; Lian, J.B.; He, J.; Li, X.W. Preparation and characterization of calcium phosphate containing coating on plasma electrolytic oxidized magnesium and its corrosion behavior in simulated body fluids. J. Alloys Compd. 2022, 896, 163042. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).