Analyzing Modeled Torque Profiles to Understand Scale-Dependent Active Muscle Responses in the Hip Joint
Abstract
1. Introduction
2. Materials and Methods
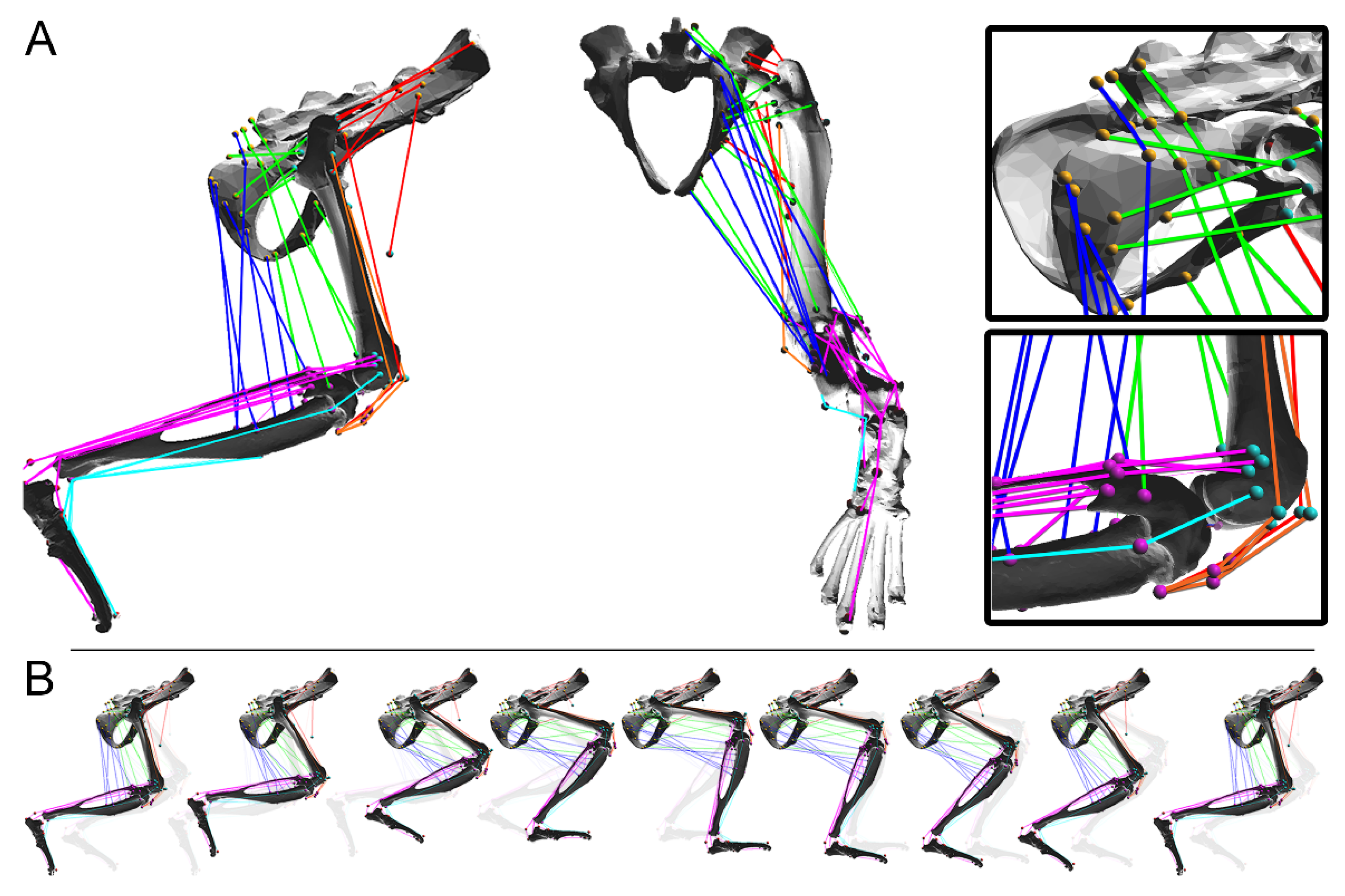
2.1. Limb Dynamics
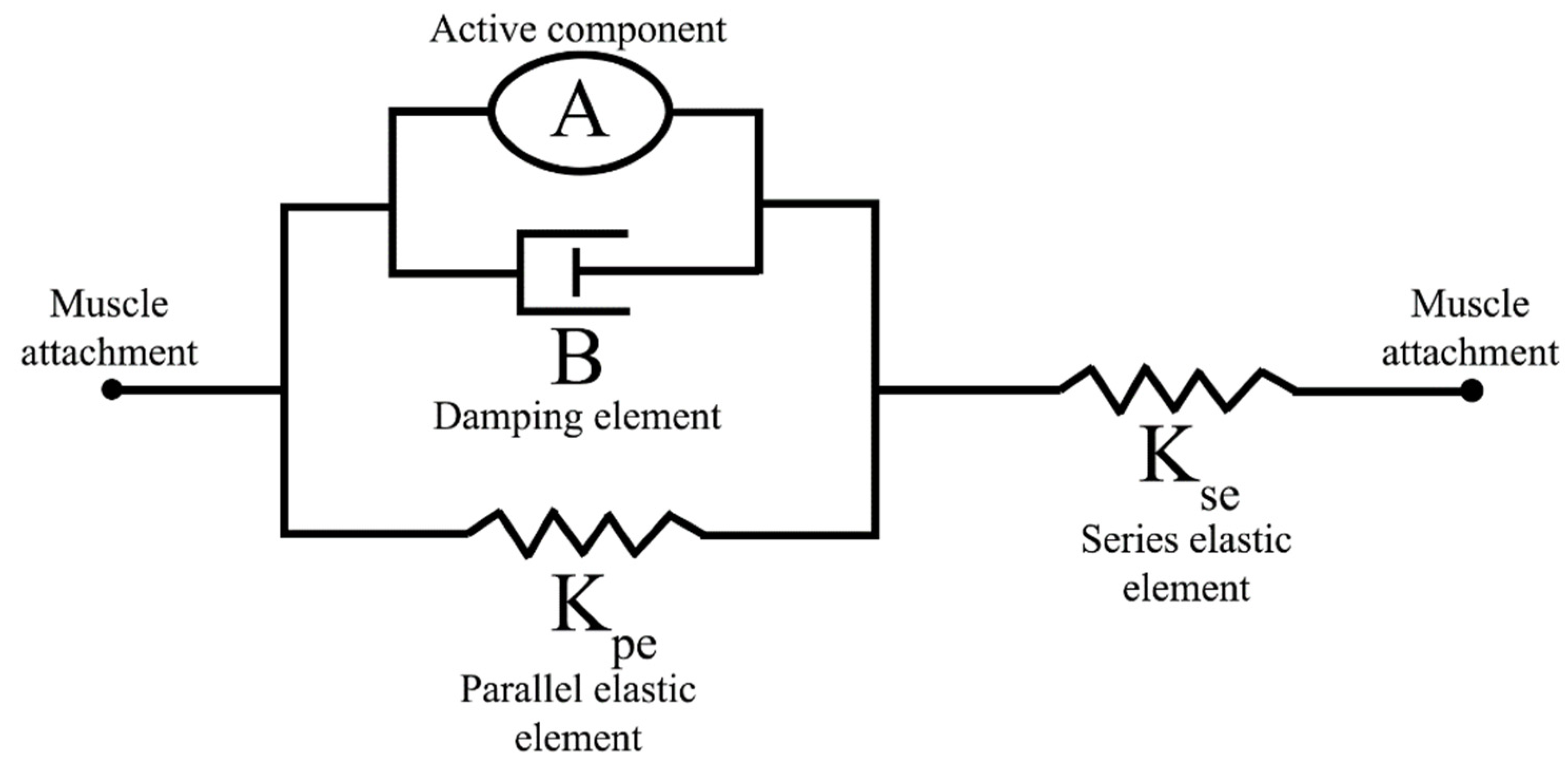
2.2. Muscle Dynamics
2.3. Limb Kinetics
2.4. Scaling Principles
3. Results
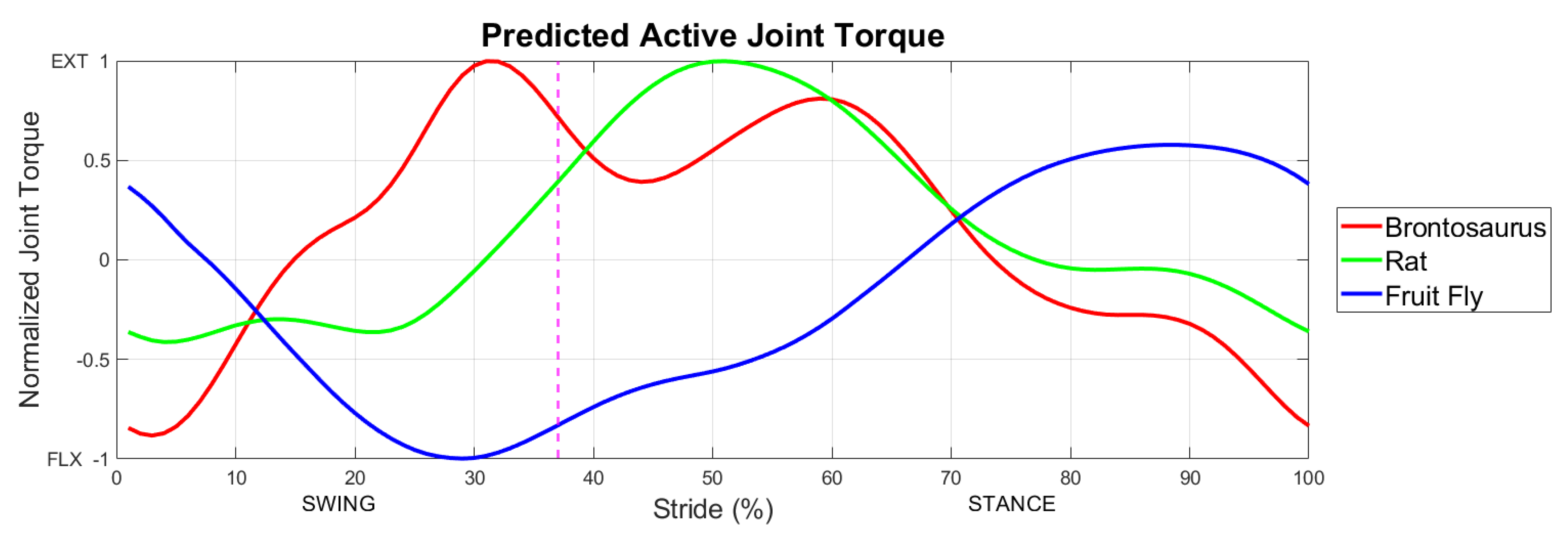
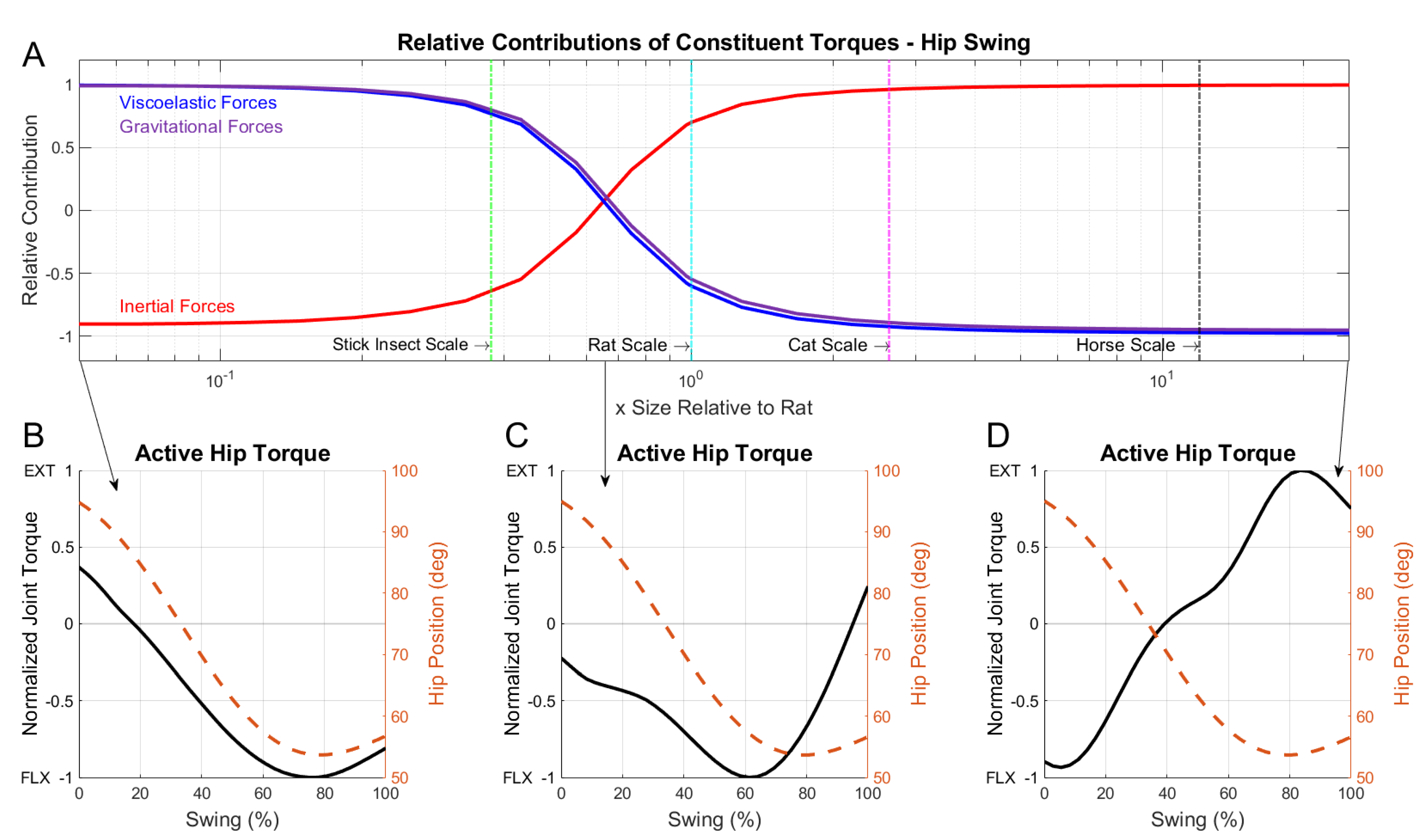
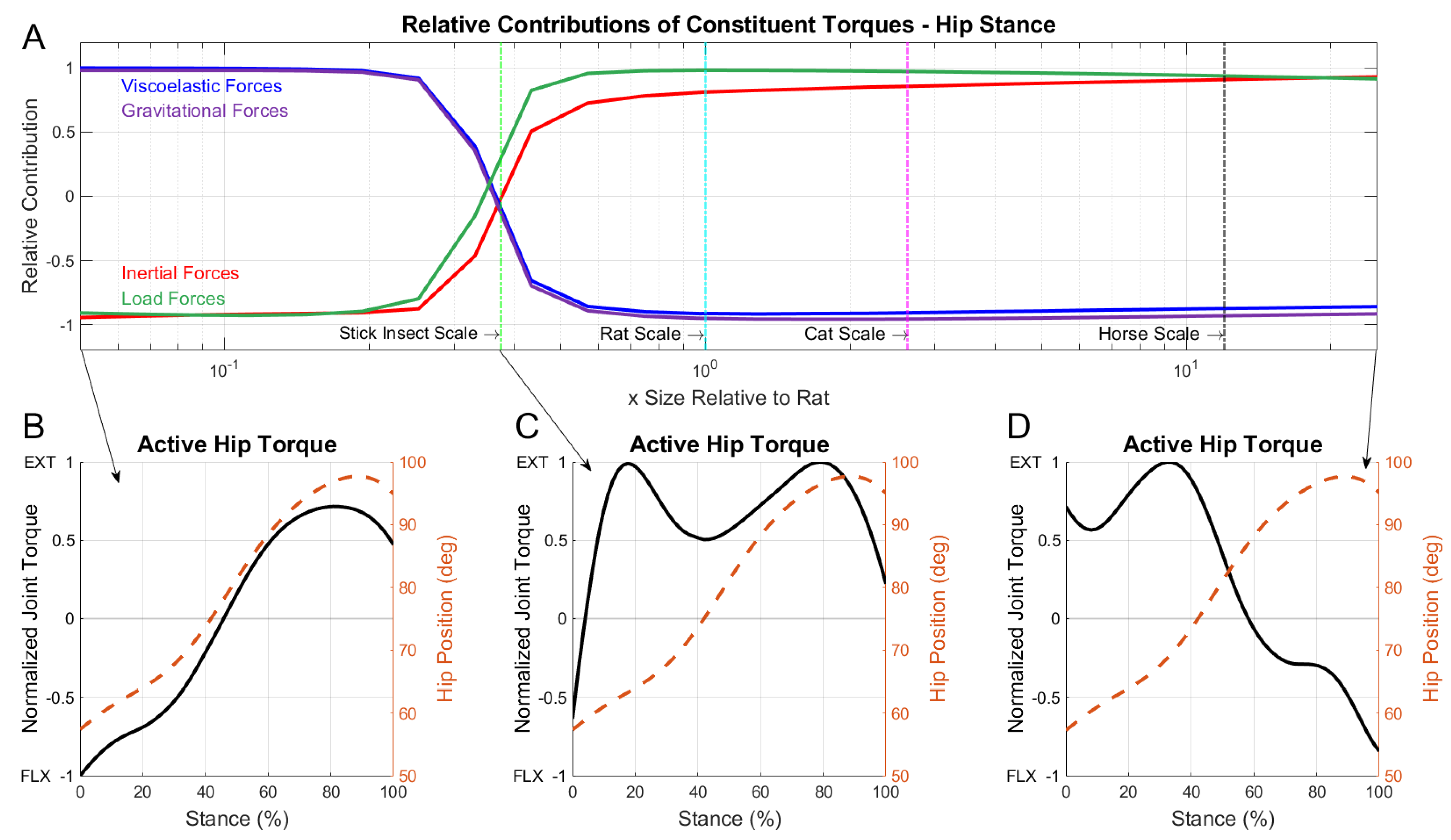
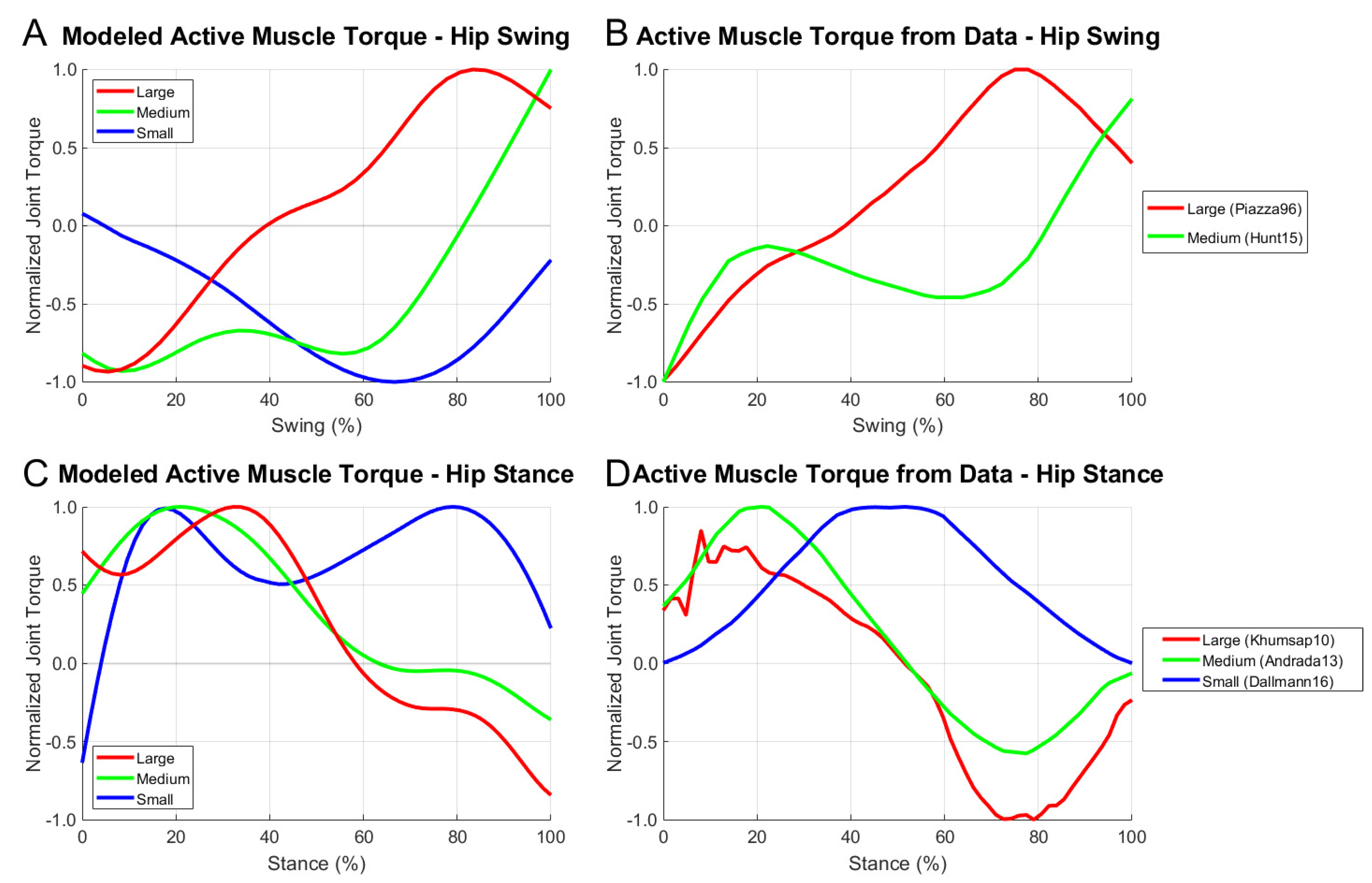
3.1. Active Torque across Scales
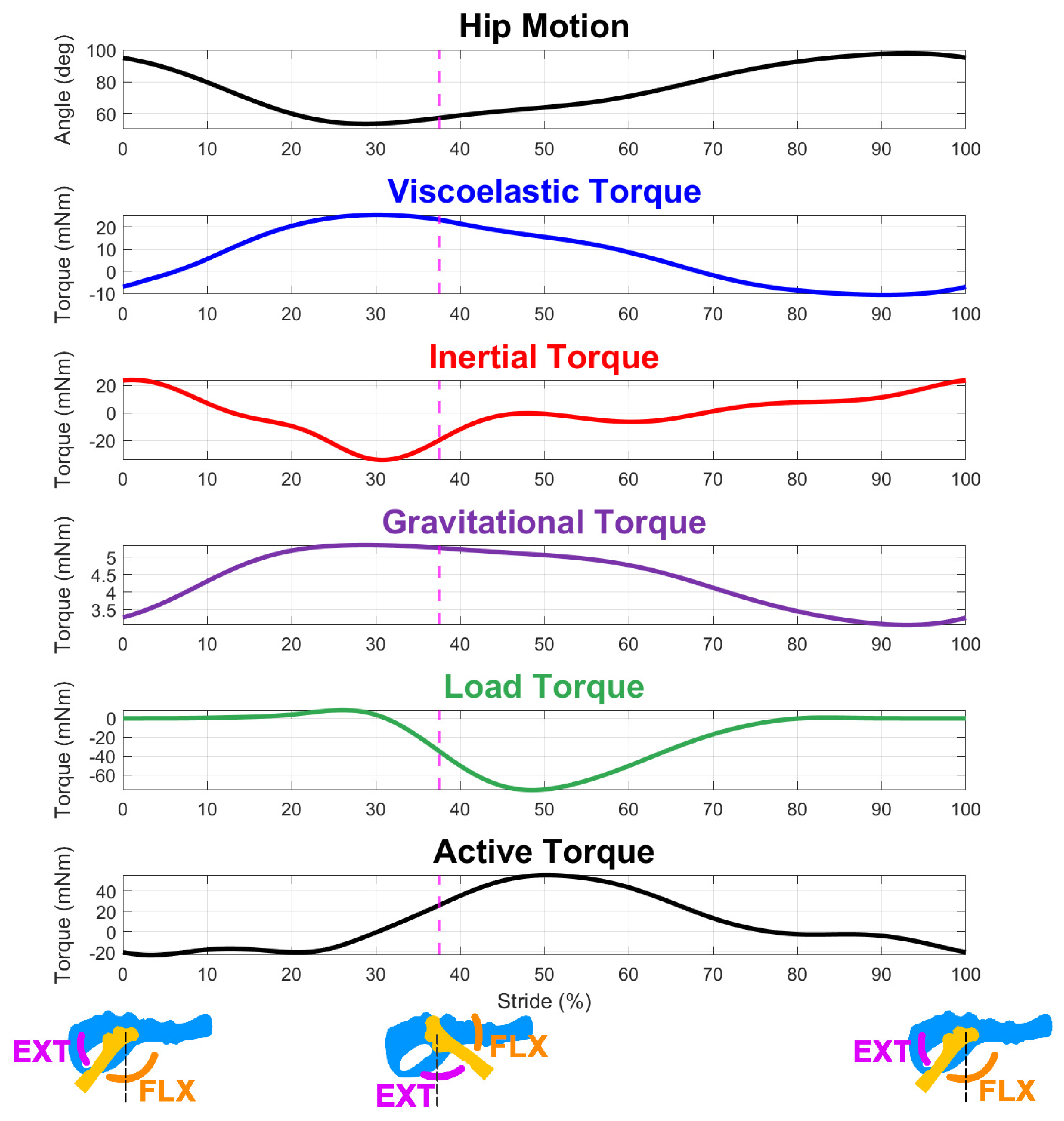
3.2. Dominant Forces during the Swing Phase
3.3. Dominant Forces during the Stance Phase
3.4. Comparison with the Data
4. Discussion
Model Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiel, H.J.; Beer, R.D. The brain has a body: Adaptive behavior emerges from interactions of nervous system, body and environment. Trends Neurosci. 1997, 20, 553–557. [Google Scholar] [CrossRef]
- Chiel, H.J.; Ting, L.H.; Ekeberg, Ö.; Hartmann, M.J.Z. The brain in its body: Motor control and sensing in a biomechanical context. J. Neurosci. 2009, 29, 12807–12814. [Google Scholar] [CrossRef]
- Ekeberg, Ö.; Pearson, K. Computer simulation of stepping in the hind legs of the cat: An examination of mechanisms regulating the stance-to-swing transition. J. Neurophysiol. 2005, 94, 4256–4268. [Google Scholar] [CrossRef] [PubMed]
- Whittlesey, S.N.; van Emmerik, R.E.; Hamill, J. The swing phase of human walking is not a passive movement. Mot. Control 2000, 4, 273–292. [Google Scholar] [CrossRef] [PubMed]
- von Twickel, A.; Guschlbauer, C.; Hooper, S.L.; Büschges, A. Swing velocity profiles of small limbs can arise from transient passive torques of the antagonist muscle alone. Curr. Biol. 2019, 29, 1–12.e7. [Google Scholar] [CrossRef] [PubMed]
- Fowler, E.G.; Gregor, R.J.; Hodgson, J.A.; Roy, R.R. Relationship between ankle muscle and joint kinetics during the stance phase of locomotion in the cat. J. Biomech. 1993, 26, 465–483. [Google Scholar] [CrossRef]
- Khumsap, S.; Clayton, H.M.; Lanovaz, J.L. Effect of walking velocity on hindlimb kinetics during stance in normal horses. Equine Vet. J. 2001, 33, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.L.; Guschlbauer, C.; Blümel, M.; Rosenbaum, P.; Gruhn, M.; Akay, T.; Büschges, A. Neural control of unloaded leg posture and of leg swing in stick insect, cockroach, and mouse differs from that in larger animals. J. Neurosci. 2009, 29, 4109–4119. [Google Scholar] [CrossRef] [PubMed]
- Tokuriki, M.; Aoki, O. Electromyographic activity of the hindlimb muscles during the walk, trot and canter. Equine Vet. J. 1995, 27, 152–155. [Google Scholar] [CrossRef]
- Robert, C.; Valette, J.P.; Degueurce, C.; Denoix, J.M. Correlation between surface electromyography and kinematics of the hindlimb of horses at trot on a treadmill. CTO 1999, 165, 113–122. [Google Scholar] [CrossRef]
- Westerga, J.; Gramsbergen, A. The development of locomotion in the rat. Dev. Brain Res. 1990, 57, 163–174. [Google Scholar] [CrossRef]
- Carrano, M.T. Morphological indicators of foot posture in mammals: A statistical and biomechanical analysis. Zool. J. Linn. Soc. 1997, 121, 77–104. [Google Scholar] [CrossRef]
- Lewinson, R.T.; Stefanyshyn, D.J. A descriptive analysis of the climbing mechanics of a mountain goat (Oreamnos americanus). Zoology 2016, 119, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.C.; Pandy, M.G. Dynamic optimization of human walking. J. Biomech. Eng. 2001, 123, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.S.; Schilling, N.; Schmidt, M.; Haarhaus, D.; Witte, H. Basic limb kinematics of small therian mammals. J. Exp. Biol. 2002, 205, 1315–1338. [Google Scholar] [CrossRef] [PubMed]
- Goslow, G.E.; Reinking, R.M.; Stuart, D.G. The cat step cycle: Hind limb joint angles and muscle lengths during unrestrained locomotion. J. Morphol. 1973, 141, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Perell, K.L.; Gregor, R.J.; Buford, J.A.; Smith, J.L. Adaptive control for backward quadrupedal walking. IV. Hindlimb kinetics during stance and swing. J. Neurophysiol. 1993, 70, 2226–2240. [Google Scholar] [CrossRef]
- Song, H.; Polk, J.D.; Kersh, M.E. Rat bone properties and their relationship to gait during growth. J. Exp. Biol. 2019, 222, jeb203554. [Google Scholar] [CrossRef]
- Young, F.; Hunt, A.J.; Quinn, R.D. A neuromechanical rat model with a complete set of hind limb muscles. In Proceedings of the Conference on Biomimetic and Biohybrid Systems, Paris, France, 17–20 July 2018; Springer: Paris, France, 2018; pp. 527–537. [Google Scholar]
- Cofer, D.; Cymbalyuk, G.; Reid, J.; Zhu, Y.; Heitler, W.J.; Edwards, D.H. AnimatLab: A 3D graphics environment for neuromechanical simulations. J. Neurosci. Methods 2010, 187, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Greene, E.C. Anatomy of the Rat; Hafner Publishing Co.: New York, NY, USA, 1955. [Google Scholar]
- Andrada, E.; Mämpel, J.; Schmidt, A.; Fischer, M.S.; Karguth, A.; Witte, H. From biomechanics of rats’ inclined locomotion to a climbing robot. Int. J. Des. Nat. Ecodyn. 2013, 8, 192–212. [Google Scholar] [CrossRef]
- Hill, A.V. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1938, 126, 136–195. [Google Scholar] [CrossRef]
- Shadmehr, R.; Arbib, M.A. A mathematical analysis of the force-stiffness characteristics of muscles in control of a single joint system. Biol. Cybern. 1992, 66, 463–477. [Google Scholar] [CrossRef]
- Zajac, F.E. Muscle and tendon: Properties, models, scaling, and application to biomechanics and motor control. Crit. Rev. Biomed. Eng. 1989, 17, 359–411. [Google Scholar]
- Johnson, W.L.; Jindrich, D.L.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Application of a rat hindlimb model: A prediction of force spaces reachable through stimulation of nerve fascicles. IEEE Trans. Biomed. Eng. 2011, 58, 3328–3338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yeo, S.H.; Mullens, C.H.; Sandercock, T.G.; Pai, D.K.; Tresch, M.C. Estimation of musculoskeletal models from in situ measurements of muscle action in the rat hindlimb. J. Exp. Biol. 2011, 214, 735–746. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murray, R.M.; Li, Z.; Sastry, S.S. A Mathematical Introduction to Robotic Manipulation; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Young, F.; Rode, C.; Hunt, A.; Quinn, R. Analyzing moment arm profiles in a full-muscle rat hindlimb model. Biomimetics 2019, 4, 10. [Google Scholar] [CrossRef]
- Muir, G.D.; Whishaw, I.Q. Ground reaction forces in locomoting hemi-parkinsonian rats: A definitive test for impairments and compensations. Exp. Brain Res. 1999, 126, 307–314. [Google Scholar] [CrossRef]
- Biewener, A.A. Biomechanics of mammalian terrestrial locomotion. Science 1990, 250, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.M.; Bennet-Clark, H.C. Storage of elastic strain energy in muscle and other tissues. Nature 1977, 265, 114–117. [Google Scholar] [CrossRef]
- Ilton, M.; Bhamla, M.S.; Ma, X.; Cox, S.M.; Fitchett, L.L.; Kim, Y.; Koh, J.; Krishnamurthy, D.; Kuo, C.-Y.; Temel, F.Z.; et al. The principles of cascading power limits in small, fast biological and engineered systems. Science 2018, 360, eaao1082. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, K.; Mongeau, J.-M.; Mohapatra, A.; Birkmeyer, P.; Fearing, R.S.; Full, R.J. Transition by head-on collision: Mechanically mediated manoeuvres in cockroaches and small robots. J. R. Soc. Interface 2018, 15, 20170664. [Google Scholar] [CrossRef]
- Garcia, M.; Kuo, A.; Peattie, A.; Wang, P.; Full, R. Damping and size: Insights and biological inspiration. In International Symposium on Adaptive Motion of Animals and Machines; McGill University: Montreal, QC, Canada, 2000. [Google Scholar]
- Sutton, G.; Szczecinski, N.; Quinn, R.; Chiel, H. Neural Control of Rhythmic Limb Motion Is Shaped by Size and Speed. 2021. Available online: https://assets.researchsquare.com/files/rs-153297/v1_covered.pdf?c=1631852362 (accessed on 10 November 2021).
- Hooper, S.L. Body size and the neural control of movement. Curr. Biol. 2012, 22, R318–R322. [Google Scholar] [CrossRef]
- Dallmann, C.J.; Dürr, V.; Schmitz, J. Joint torques in a freely walking insect reveal distinct functions of leg joints in propulsion and posture control. Proc. R. Soc. B Biol. Sci. 2016, 283, 20151708. [Google Scholar] [CrossRef]
- Hunt, A.; Szczecinski, N.S.; Andrada, E.; Fischer, M.; Quinn, R.D. Using animal data and neural dynamics to reverse engineer a neuromechanical rat model. In Proceedings of the Conference on Biomimetic and Biohybrid Systems, Barcelona, Spain, 28–31 July 2015; Wilson, S.P., Verschure, P.F.M.J., Mura, A., Prescott, T.J., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 211–222. [Google Scholar]
- Tsai, H.P.; Middleton, K.M.; Hutchinson, J.R.; Holliday, C.M. More than one way to be a giant: Convergence and disparity in the hip joints of saurischian dinosaurs. Evolution 2020, 74, 1654–1681. [Google Scholar] [CrossRef]
- Klinkhamer, A.J.; Mallison, H.; Poropat, S.F.; Sinapius, G.H.K.; Wroe, S. Three-dimensional musculoskeletal modeling of the sauropodomorph hind limb: The effect of postural change on muscle leverage. Anat. Rec. 2018, 301, 2145–2163. [Google Scholar] [CrossRef]
- Bishop, P.J.; Falisse, A.; Groote, F.D.; Hutchinson, J.R. Predictive simulations of running gait reveal a critical dynamic role for the tail in bipedal dinosaur locomotion. Sci. Adv. 2021, 7, eabi7348. [Google Scholar] [CrossRef]
- Pearson, K.G. Role of sensory feedback in the control of stance duration in walking cats. Brain Res. Rev. 2008, 57, 222–227. [Google Scholar] [CrossRef]
- Brassey, C.A.; Kitchener, A.C.; Withers, P.J.; Manning, P.L.; Sellers, W.I. The role of cross-sectional geometry, curvature, and limb posture in maintaining equal safety factors: A computed tomography study. Anat. Rec. 2013, 296, 395–413. [Google Scholar] [CrossRef]
- Biewener, A.A. Musculoskeletal design in relation to body size. J. Biomech. 1991, 24, 19–29. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, F.R.; Chiel, H.J.; Tresch, M.C.; Heckman, C.J.; Hunt, A.J.; Quinn, R.D. Analyzing Modeled Torque Profiles to Understand Scale-Dependent Active Muscle Responses in the Hip Joint. Biomimetics 2022, 7, 17. https://doi.org/10.3390/biomimetics7010017
Young FR, Chiel HJ, Tresch MC, Heckman CJ, Hunt AJ, Quinn RD. Analyzing Modeled Torque Profiles to Understand Scale-Dependent Active Muscle Responses in the Hip Joint. Biomimetics. 2022; 7(1):17. https://doi.org/10.3390/biomimetics7010017
Chicago/Turabian StyleYoung, Fletcher R., Hillel J. Chiel, Matthew C. Tresch, Charles J. Heckman, Alexander J. Hunt, and Roger D. Quinn. 2022. "Analyzing Modeled Torque Profiles to Understand Scale-Dependent Active Muscle Responses in the Hip Joint" Biomimetics 7, no. 1: 17. https://doi.org/10.3390/biomimetics7010017
APA StyleYoung, F. R., Chiel, H. J., Tresch, M. C., Heckman, C. J., Hunt, A. J., & Quinn, R. D. (2022). Analyzing Modeled Torque Profiles to Understand Scale-Dependent Active Muscle Responses in the Hip Joint. Biomimetics, 7(1), 17. https://doi.org/10.3390/biomimetics7010017