Regenerative Medicine of Liver: Promises, Advances and Challenges
Abstract
1. Introduction
2. Treatment
2.1. Liver Transplantation
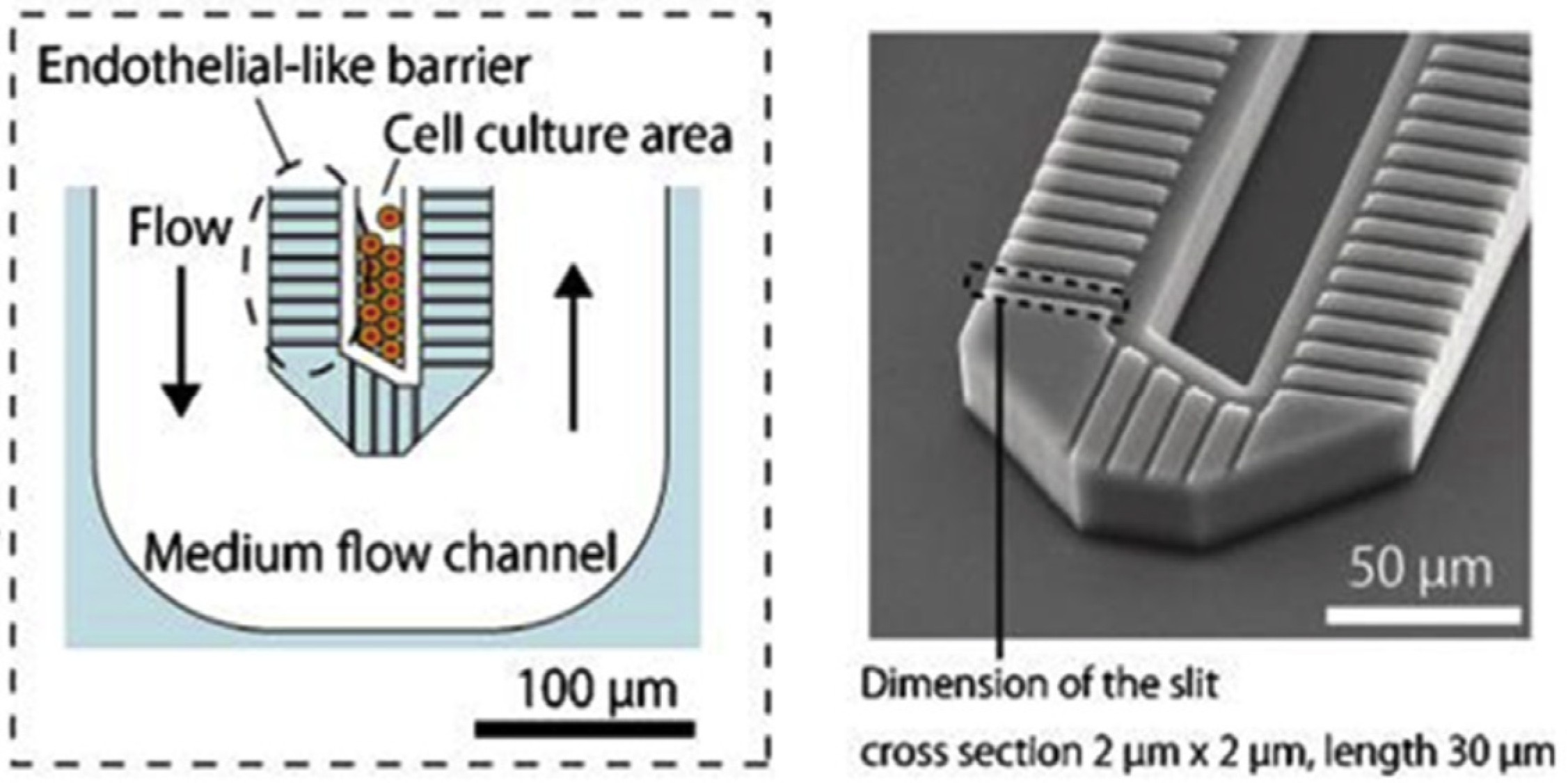
2.2. Liver-on-a-Chip
3. Bioengineered Scaffolds
4. Stem Cell Therapy
4.1. Hematopoietic Stem Cells (HSA)
4.2. Mesenchymal Stem Cells (MSC)
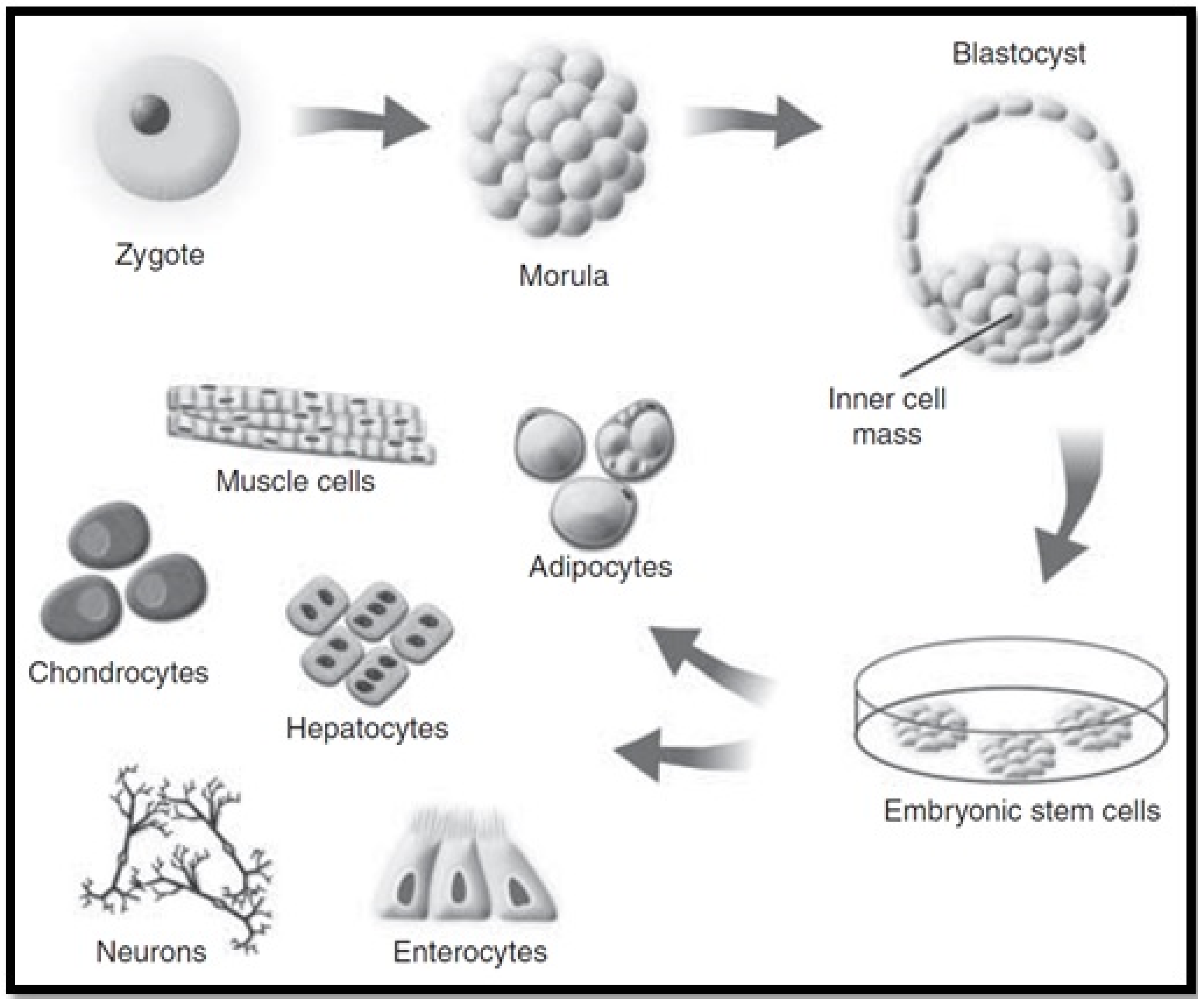
4.3. Embryonic Stem Cells (ESC)
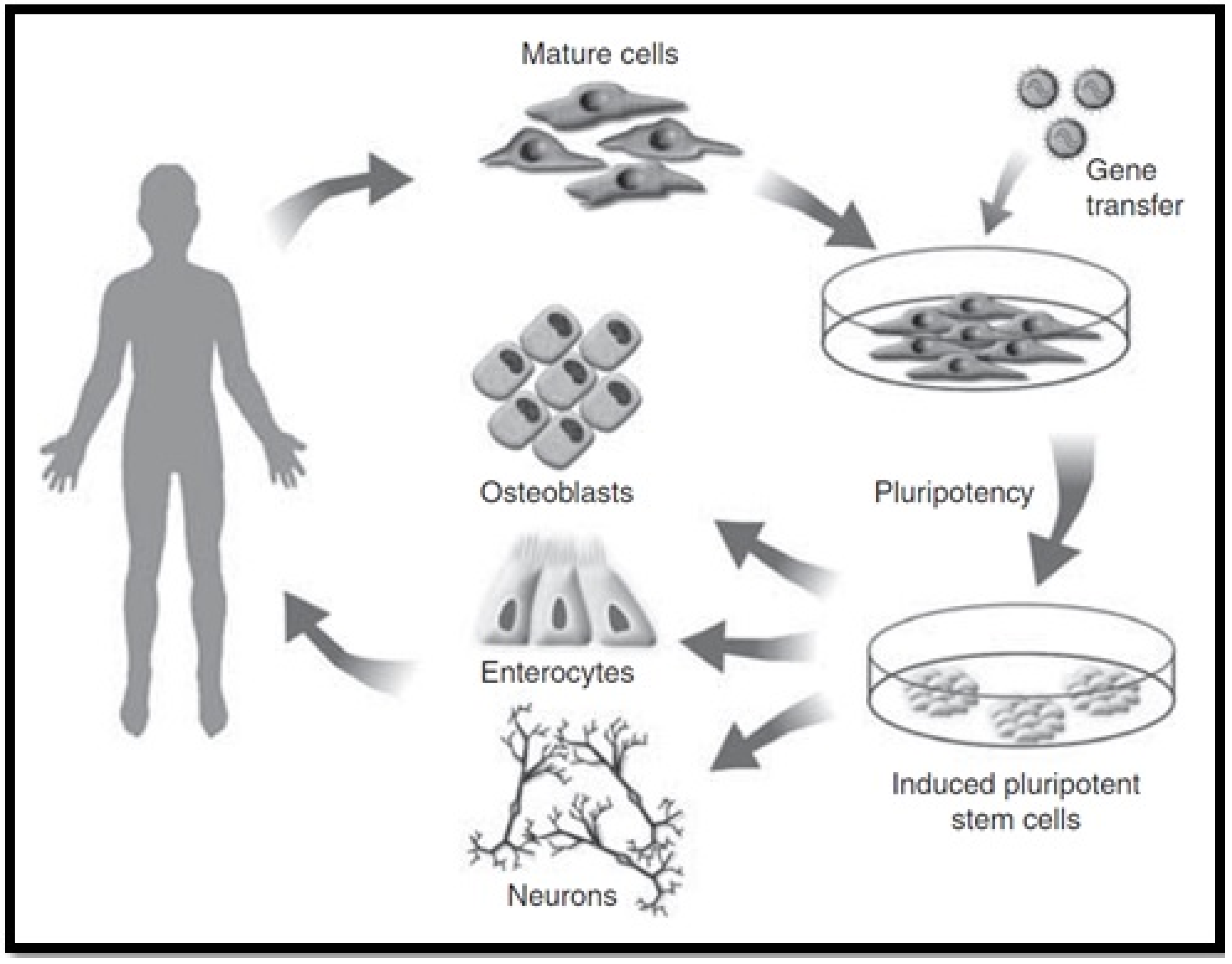
4.4. Induced Pluripotent Stem Cells (iPSC)
4.5. Endothelial Progenitor Cells (EPCs)
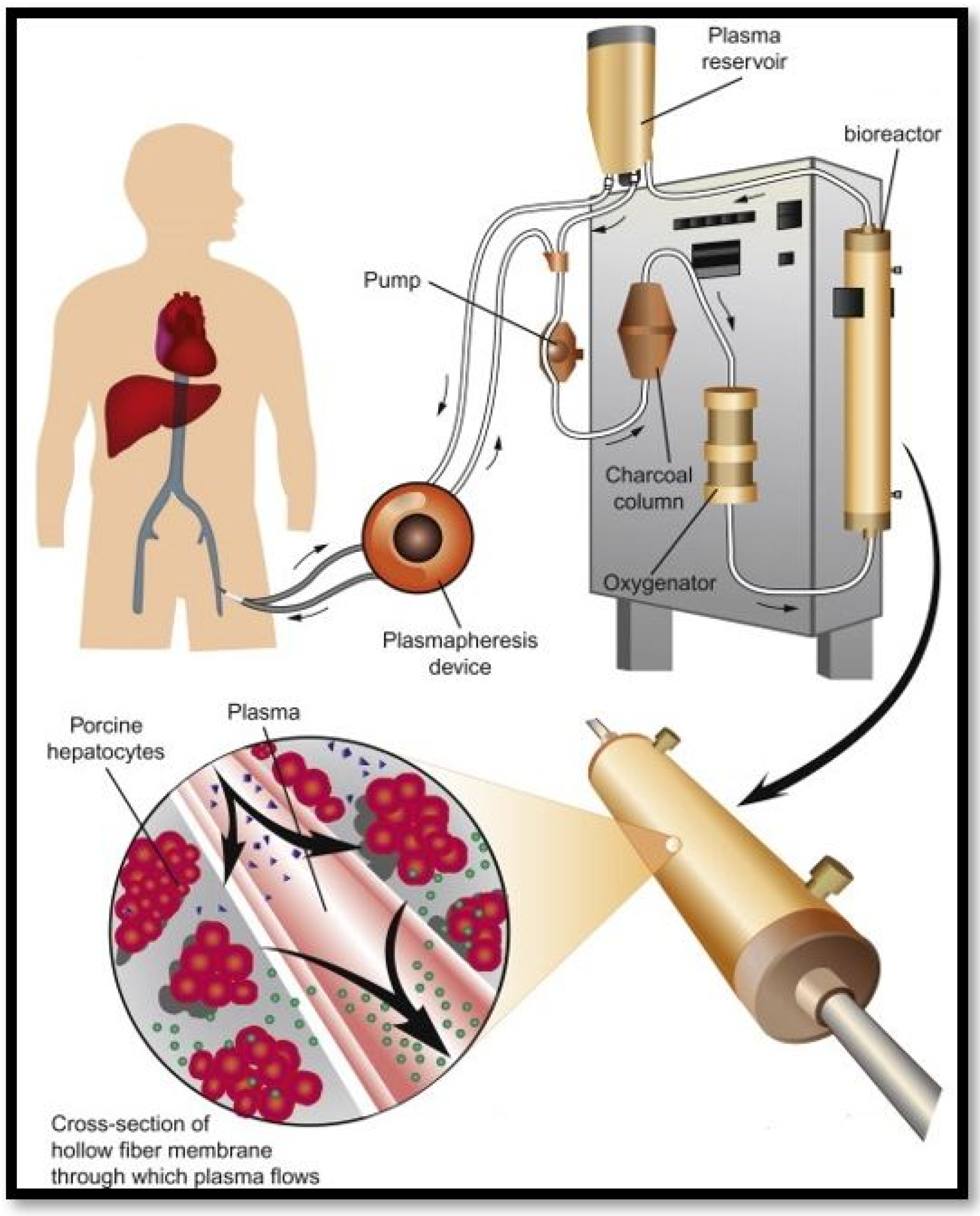
5. Bioartificial Livers
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffman, M. Picture of the Liver. 2017. Available online: https://www.webmd.com/digestive-disorders/picture-of-the-liver#1 (accessed on 18 October 2018).
- Ye, Q.; Zou, B.; Yeo, Y.H.; Li, J.; Huang, D.Q.; Wu, Y.; Yang, H.; Liu, C.; Kam, L.Y.; Tan, X.X.E.; et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 739–752. [Google Scholar] [CrossRef]
- Heydari, Z.; Najimi, M.; Mirzaei, H.; Shpichka, A.; Ruoss, M.; Farzaneh, Z.; Montazeri, L.; Piryaei, A.; Timashev, P.; Gramignoli, R.; et al. Tissue Engineering in Liver Regenerative Medicine: Insights into Novel Translational Technologies. Cells 2020, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Pavlides, M.; Cobbold, J.F. Non-Alcoholic fatty liver disease. Medicine 2015, 43, 585–589. [Google Scholar] [CrossRef]
- Ellis, H. Anatomy of the liver. Surgery (Oxford) 2011, 29, 589–592. [Google Scholar] [CrossRef]
- Brunt, E.M.; Wong, V.W.-S.; Nobili, V.; Day, C.P.; Sookoian, S.; Maher, J.J.; Bugianesi, E.; Sirlin, C.B.; Neuschwander-Tetri, B.A.; Rinella, M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers 2015, 1, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Pappachan, J.M.; Babu, S.; Krishnan, B.; Ravindran, N.C. Non-Alcoholic Fatty Liver Disease: A Clinical Update. J. Clin. Transl. Hepatol. 2017, 5, 384. [Google Scholar] [CrossRef]
- Ahmad, J. Hepatitis, C. BMJ 2017, 358, j2861. [Google Scholar] [CrossRef]
- Jinga, M.; Balaban, V.D.; Bontas, E.; Tintoiu, I.C. Future Approaches in Liver Disorders: Regenerative Medicine. In Liver Diseases; Springer: Berlin/Heidelberg, Germany, 2020; pp. 811–827. [Google Scholar] [CrossRef]
- Munir, S.; Saleem, S.; Idrees, M.; Tariq, A.; Butt, S.; Rauff, B.; Hussain, A.; Badar, S.; Naudhani, M.; Fatima, Z.; et al. Hepatitis C Treatment: Current and future perspectives. Virol. J. 2010, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Burns, A.; Rodden, D.; Chang, F.; Chaum, M.; Garcia, N.; Bollipalli, N.; Niemz, A. Diagnosis and Management of Hepatitis C Virus Infection. J. Lab. Autom. 2015, 20, 519–538. [Google Scholar] [CrossRef]
- Taguchi, T.; Kijima, S.; Kuroki, M.; Ishii, A.; Yoshimaru, K.; Matsuura, T. Future Prospects of Biliary Atresia. In Introduction to Biliary Atresia; Springer: Berlin/Heidelberg, Germany, 2021; pp. 329–339. [Google Scholar]
- Hartley, J.; Harnden, A.; Kelly, D. Biliary atresia. BMJ 2010, 340, c2383. [Google Scholar] [CrossRef]
- Couturier, L.; Jarvis, C.; Rousseau, H.; Jimenez, V. Biliary atresia. Can. Fam. Physician 2015, 61, 965–968. [Google Scholar]
- Hartley, J.L.; Davenport, M.; Kelly, D.A. Biliary atresia. Lancet 2009, 374, 1704–1713. [Google Scholar] [CrossRef]
- Huster, D. Wilson disease. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 531–539. [Google Scholar] [CrossRef]
- Clinic, M. Wilson’s Disease. 2018. Available online: https://www.mayoclinic.org/diseases-conditions/wilsons-disease/symptoms-causes/syc-20353251 (accessed on 24 October 2018).
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017, 152, 745–761. [Google Scholar] [CrossRef]
- Karin, M.; Dhar, D. Liver carcinogenesis: From naughty chemicals to soothing fat and the surprising role of NRF2. Carcinogenesis 2016, 37, 541–546. [Google Scholar] [CrossRef]
- Shiani, A.; Narayanan, S.; Pena, L.; Friedman, M. The Role of Diagnosis and Treatment of Underlying Liver Disease for the Prognosis of Primary Liver Cancer. Cancer Control. 2017, 24, 1073274817729240. [Google Scholar] [CrossRef]
- Bayraktar, U.D.; Seren, S.; Bayraktar, Y. Hepatic venous outflow obstruction: Three similar syndromes. World J. Gastroenterol. WJG 2007, 13, 1912. [Google Scholar] [CrossRef]
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Clinic, M. Liver Transplant. 2018. Available online: https://www.mayoclinic.org/tests-procedures/liver-transplant/about/pac-20384842 (accessed on 1 November 2018).
- Rashid, S.T.; Gimson, A.E. General Considerations. In Liver Transplantation: Clinical Assessment and Management; Neuberger, J., Ferguson, J., Newsome, P.N., Lucey, M.R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 1–26. [Google Scholar]
- Karunakaran, M.; Kaur, R. Textbook Outcomes in Liver Transplantation. World J. Surg. 2021, 45, 1257–1258. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Karam, V.; Delvart, V.; O’Grady, J.; Mirza, D.; Klempnauer, J.; Castaing, D.; Neuhaus, P.; Jamieson, N.; Salizzoni, M.; et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J. Hepatol. 2012, 57, 675–688. [Google Scholar] [CrossRef] [PubMed]
- NHS. Overview Liver Transplant. 2018. Available online: https://www.nhs.uk/conditions/liver-transplant/ (accessed on 1 November 2018).
- Bhatia, S.N.; Underhill, G.H.; Zaret, K.S.; Fox, I.J. Cell and tissue engineering for liver disease. Sci. Transl. Med. 2014, 6, 245sr2. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Moonka, D.K. Liver transplantation. Curr. Opin. Gastroenterol. 1999, 15, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Yu-Qing, C.; Guo-An, L.; Zhang, M.; Zhang, H.-Y.; Yue-Rong, W.; Ping, H. Organs-on-Chips and Its Applications. Chin. J. Anal. Chem. 2016, 44, 533–541. [Google Scholar]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/Body-on-a-Chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Beckwitt, C.H.; Clark, A.M.; Wheeler, S.; Taylor, D.L.; Stolz, D.B.; Griffith, L.; Wells, A. Liver ‘organ on a chip’. Exp. Cell Res. 2018, 363, 15–25. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Xin, H.; Deng, G. Single cell isolation and analysis. Front. Cell Dev. Biol. 2016, 4, 116. [Google Scholar] [CrossRef] [PubMed]
- Usta, O.; McCarty, W.; Bale, S.; Hegde, M.; Jindal, R.; Bhushan, A.; Golberg, I.; Yarmush, M. Microengineered cell and tissue systems for drug screening and toxicology applications: Evolution of in-vitro liver technologies. Technology 2015, 3, 1–26. [Google Scholar] [CrossRef]
- Ortega-Ribera, M.; Yeste, J.; Villa, R.; Gracia-Sancho, J. Nanoengineered Biomaterials for the treatment of liver diseases. In Nanoengineered Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 417–441. [Google Scholar]
- Elaut, G.; Henkens, T.; Papeleu, P.; Snykers, S.; Vinken, M.; Vanhaecke, T.; Rogiers, V. Molecular Mechanisms Underlying the Dedifferentiation Process of Isolated Hepatocytes and Their Cultures. Curr. Drug Metab. 2006, 7, 629–660. [Google Scholar] [CrossRef]
- Wilkening, S.; Stahl, F.; Bader, A. Comparison of primary human hepatocytes and hepatoma cell line hepg2 with regard to their biotransformation properties. Drug Metab. Dispos. 2003, 31, 1035–1042. [Google Scholar] [CrossRef]
- Natarajan, V.; Berglund, E.J.; Chen, D.X.; Kidambi, S. Substrate stiffness regulates primary hepatocyte functions. RSC Adv. 2015, 5, 80956–80966. [Google Scholar] [CrossRef] [PubMed]
- Mauriac, H.; Pannetier, C.; Casquillas, G.V. Organs on chip review. Elveflow 2020. [Google Scholar]
- Lee, S.-A.; No, D.Y.; Kang, E.; Ju, J.; Kim, D.-S.; Lee, S.-H. Spheroid-Based three-dimensional liver-on-a-chip to investigate hepatocyte–hepatic stellate cell interactions and flow effects. Lab Chip 2013, 13, 3529–3537. [Google Scholar] [CrossRef]
- Mazza, G.; Al-Akkad, W.; Rombouts, K.; Pinzani, M. Liver tissue engineering: From implantable tissue to whole organ engineering. Hepatol. Commun. 2017, 2, 131–141. [Google Scholar] [CrossRef]
- Rad, A.T.; Ali, N.; Kotturi, H.S.R.; Yazdimamaghani, M.; Smay, J.; Vashaee, D.; Tayebi, L. Conducting scaffolds for liver tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 4169–4181. [Google Scholar] [CrossRef]
- Hammond, J.S.; Beckingham, I.J.; Shakesheff, K.M. Scaffolds for liver tissue engineering. Expert Rev. Med Devices 2006, 3, 21–27. [Google Scholar] [CrossRef]
- François, S.; Chakfé, N.; Durand, B.; Laroche, G. A poly(l-lactic acid) nanofibre mesh scaffold for endothelial cells on vascular prostheses. Acta Biomater. 2009, 5, 2418–2428. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-S.; Harn, H.-J.; Hsieh, D.-K.; Wen, T.-C.; Subeq, Y.-M.; Sun, L.-Y.; Lin, S.-Z.; Chiou, T.-W. Cells and Materials for Liver Tissue Engineering. Cell Transplant. 2013, 22, 685–700. [Google Scholar] [CrossRef]
- Jain, E.; Damania, A.; Kumar, A. Biomaterials for liver tissue engineering. Hepatol. Int. 2013, 8, 185–197. [Google Scholar] [CrossRef]
- Guillaume, O.; Daly, A.; Lennon, K.; Gansau, J.; Buckley, S.F.; Buckley, C.T. Shape-Memory porous alginate scaffolds for regeneration of the annulus fibrosus: Effect of TGF-β3 supplementation and oxygen culture conditions. Acta Biomater. 2014, 10, 1985–1995. [Google Scholar] [CrossRef]
- Edgar, L.; McNamara, K.; Wong, T.; Tamburrini, R.; Katari, R.; Orlando, G. Heterogeneity of Scaffold Biomaterials in Tissue Engineering. Materials 2016, 9, 332. [Google Scholar] [CrossRef]
- Abbott, R.D.; Kaplan, D.L. Engineering Biomaterials for Enhanced Tissue Regeneration. Curr. Stem Cell Rep. 2016, 2, 140–146. [Google Scholar] [CrossRef]
- Shick, T.M.; Kadir, A.Z.A.; Ngadiman, N.H.A.; Ma’Aram, A. A review of biomaterials scaffold fabrication in additive manufacturing for tissue engineering. J. Bioact. Compat. Polym. 2019, 34, 415–435. [Google Scholar] [CrossRef]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kazemnejad, S. Hepatic Tissue Engineering Using Scaffolds: State of the Art. Avicenna J. Med. Biotechnol. 2009, 1, 135–145. [Google Scholar] [PubMed]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Kwak, K.-A.; Cho, H.-J.; Yang, J.-Y.; Park, Y.-S. Current Perspectives Regarding Stem Cell-Based Therapy for Liver Cirrhosis. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1–19. [Google Scholar] [CrossRef]
- Almeida-Porada, G.; Zanjani, E.D.; Porada, C.D. Bone marrow stem cells and liver regeneration. Exp. Hematol. 2010, 38, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Irfan, A.; Ahmed, I. Could stem cell therapy be the cure in liver cirrhosis? J. Clin. Exp. Hepatol. 2015, 5, 142–146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.Y.; Hong, S.-H. Hematopoietic Stem Cells and Their Roles in Tissue Regeneration. Int. J. Stem Cells 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Hombach-Klonisch, S.; Panigrahi, S.; Rashedi, I.; Seifert, A.; Alberti, E.; Pocar, P.; Kurpisz, M.; Schulze-Osthoff, K.; Mackiewicz, A.; Los, M.J. Adult stem cells and their trans-differentiation potential—perspectives and therapeutic applications. J. Mol. Med. 2008, 86, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Menichella, G.; Lai, M.; Serafini, R.; Pierelli, L.; Vittori, M.; Ciarli, M.; Rumi, C.; Puggioni, P.; Scambia, G.; Sica, S.; et al. Large Volume Leukapheresis for Collecting Hemopoietic Progenitors: Role of CD 34+ Precount in Predicting Successful Collection. Int. J. Artif. Organs 1999, 22, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Bryder, D.; Rossi, D.J.; Weissman, I.L. Hematopoietic Stem Cells: The Paradigmatic Tissue-Specific Stem Cell. Am. J. Pathol. 2006, 169, 338–346. [Google Scholar] [CrossRef]
- Jang, Y.-Y.; Collector, M.I.; Baylin, S.B.; Diehl, A.M.; Sharkis, S.J. Hematopoietic stem cells convert into liver cells within days without fusion. Nature 2004, 6, 532–539. [Google Scholar] [CrossRef]
- Lagasse, E.; Connors, H.; Al-Dhalimy, M.; Reitsma, M.; Dohse, M.; Osborne, L.; Wang, X.; Finegold, M.; Weissman, I.L.; Grompe, M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000, 6, 1229–1234. [Google Scholar] [CrossRef]
- Tsolaki, E.; Athanasiou, E.; Gounari, E.; Zogas, N.; Siotou, E.; Yiangou, M.; Anagnostopoulos, A.; Yannaki, E. Hematopoietic stem cells and liver regeneration: Differentially acting hematopoietic stem cell mobilization agents reverse induced chronic liver injury. Blood Cells Mol. Dis. 2014, 53, 124–132. [Google Scholar] [CrossRef]
- Shammaa, R.; El-Kadiry, A.E.-H.; Abusarah, J.; Rafei, M. Mesenchymal Stem Cells Beyond Regenerative Medicine. Front. Cell Dev. Biol. 2020, 8, 72. [Google Scholar] [CrossRef]
- Anker, P.S.I.; Scherjon, S.A.; der Keur, C.K.-V.; de Groot-Swings, G.M.; Claas, F.H.; Fibbe, W.E.; Kanhai, H.H. Isolation of Mesenchymal Stem Cells of Fetal or Maternal Origin from Human Placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Shiota, G.; Itaba, N. Progress in stem cell-based therapy for liver disease. Hepatol. Res. 2016, 47, 127–141. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Fan, L.; Zhang, F.; Li, L. The clinical application of mesenchymal stem cells in liver disease: The current situation and potential future. Ann. Transl. Med. 2020, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Castro-Manrreza, M.E.; Montesinos, J.J. Immunoregulation by Mesenchymal Stem Cells: Biological Aspects and Clinical Applications. J. Immunol. Res. 2015, 2015, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xie, N.; Li, W.; Yuan, B.; Shi, Y.; Wang, Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2013, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Yoshida, Y.; Akechi, Y.; Sakabe, T.; Nishio, R.; Ikeda, R.; Terabayashi, K.; Matsumi, Y.; Gonda, K.; Okamoto, H.; et al. Hepatic differentiation of human bone marrow-derived mesenchymal stem cells by tetracycline-regulated hepatocyte nuclear factor 3β. Hepatology 2008, 48, 597–606. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, L.; Zhang, Y.; Sun, C.; Chen, X.; Wang, Y. Adipose-derived mesenchymal stem cells promote liver regeneration and suppress rejection in small-for-size liver allograft. Transpl. Immunol. 2017, 45, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Li, J.; Wang, J.; Wang, X.; Zhang, Y.; Yuan, X.; Zhu, W.; Shi, X. Mesenchymal Stem Cells Enhance Liver Regeneration via Improving Lipid Accumulation and Hippo Signaling. Stem Cells Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Chagastelles, P.C.; Nardi, N.B. Biology of stem cells: An overview. Kidney Int. Suppl. 2011, 1, 63–67. [Google Scholar] [CrossRef]
- Tsolaki, E.; Yannaki, E. Stem cell-based regenerative opportunities for the liver: State of the art and beyond. World J. Gastroenterol. 2015, 21, 12334. [Google Scholar] [CrossRef]
- Tolosa, L.; Caron, J.; Hannoun, Z.; Antoni, M.; López, S.; Burks, D.; Castell, J.V.; Weber, A.; Gomez-Lechon, M.-J.; Dubart-Kupperschmitt, A. Transplantation of hESC-derived hepatocytes protects mice from liver injury. Stem Cell Res. Ther. 2015, 6, 1–17. [Google Scholar] [CrossRef]
- Nicolas, C.; Wang, Y.; Luebke-Wheeler, J.; Nyberg, S.L. Stem Cell Therapies for Treatment of Liver Disease. Biomedicines 2016, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.J.; Gupta, S.; Dhawan, A. Cell therapy for liver disease: From liver transplantation to cell factory. J. Hepatol. 2015, 62, S157–S169. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yu, Y.; Nyberg, S.L. Induced Pluripotent Stem Cells for the Treatment of Liver Diseases: Novel Concepts. Cells Tissues Organs 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, X.; Nyberg, S.L. Potential and Challenges of Induced Pluripotent Stem Cells in Liver Diseases Treatment. J. Clin. Med. 2014, 3, 997–1017. [Google Scholar] [CrossRef]
- Nakamura, T.; Torimura, T.; Sakamoto, M.; Hashimoto, O.; Taniguchi, E.; Inoue, K.; Sakata, R.; Kumashiro, R.; Murohara, T.; Ueno, T.; et al. Significance and Therapeutic Potential of Endothelial Progenitor Cell Transplantation in a Cirrhotic Liver Rat Model. Gastroenterology 2007, 133, 91–107. [Google Scholar] [CrossRef]
- Rautou, P.-E. Endothelial progenitor cells in cirrhosis: The more, the merrier? J. Hepatol. 2012, 57, 1163–1165. [Google Scholar] [CrossRef][Green Version]
- Selden, C.; Bundy, J.; Erro, E.; Puschmann, E.; Miller, M.; Kahn, D.; Hodgson, H.; Fuller, B.; Gonzalez-Molina, J.; Le Lay, A.; et al. A clinical-scale BioArtificial Liver, developed for GMP, improved clinical parameters of liver function in porcine liver failure. Sci. Rep. 2017, 7, 1–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J.; Ji, F.; Wang, J.; Pan, X.; Li, L. Bio-Artificial Liver. In Artificial Liver; Springer: Berlin/Heidelberg, Germany, 2021; pp. 479–504. [Google Scholar]
- Park, J.-K.; Lee, D.-H. Bioartificial liver systems: Current status and future perspective. J. Biosci. Bioeng. 2005, 99, 311–319. [Google Scholar] [CrossRef]
- Kumar, A.; Tripathi, A.; Jain, S. Extracorporeal Bioartificial Liver for Treating Acute Liver Diseases. J. Extra Corpor. Technol. 2011, 43, 195–206. [Google Scholar]
- Stocum, D.L. Regenerative Therapies for Digestive, Respiratory and Urinary Tissues. In Regenerative Biology and Medicine, 2nd ed.; Stocum, D.L., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 325–354. [Google Scholar]
- Sakiyama, R.; Blau, B.J.; Miki, T. Clinical translation of bioartificial liver support systems with human pluripotent stem cell-derived hepatic cells. World J. Gastroenterol. 2017, 23, 1974–1979. [Google Scholar] [CrossRef]
- Stutchfield, B.M.; Simpson, K.; Wigmore, S.J. Systematic review and meta-analysis of survival following extracorporeal liver support. BJS 2011, 98, 623–631. [Google Scholar] [CrossRef]
- Starokozhko, V.; Groothuis, G.M. Challenges on the road to a multicellular bioartificial liver. J. Tissue Eng. Regen. Med. 2017, 12, e227–e236. [Google Scholar] [CrossRef] [PubMed]
- van Wenum, M.; Chamuleau, R.A.; van Gulik, T.M.; Siliakus, A.; Seppen, J.; Hoekstra, R. Bioartificial livers in vitro and in vivo: Tailoring biocomponents to the expanding variety of applications. Expert Opin. Biol. Ther. 2014, 14, 1745–1760. [Google Scholar] [CrossRef]
- Zhao, L.-F.; Pan, X.-P.; Li, L.-J. Key challenges to the development of extracorporeal bioartificial liver support systems. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 243–249. [Google Scholar] [CrossRef]
- Chen, H.S.; Yang, J.; Nyberg, S.L. Acute Liver Failure and Bioartificial Liver Support. In Shackelford’s Surgery of the Alimentary Tract; Elsevier: Amsterdam, The Netherlands, 2019; Volume 2, pp. 1508–1516. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.J.; Choi, D. Cell Sources, Liver Support Systems and Liver Tissue Engineering: Alternatives to Liver Transplantation. Int. J. Stem Cells 2015, 8, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, X.; Liang, L.; Li, J.; Demirci, U.; Wang, S. A decade of progress in liver regenerative medicine. Biomaterials 2017, 157, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Figaro, S.; Pereira, U.; Dumé, A.-S.; Rada, H.; Capone, S.; Bengrine, A.; Baze, A.; Rabenirina, E.; Semenzato, N.; Herpe, Y.-E.; et al. SUPPLIVER: Bioartificial supply for liver failure. IRBM 2015, 36, 101–109. [Google Scholar] [CrossRef]
- Ziogas, I.A.; Zein, N.N.; Quintini, C.; Miller, C.M.; Tsoulfas, G. Chapter 7—Three-dimensional (3D) printing and liver transplantation. In 3D Printing: Applications in Medicine and Surgery; Tsoulfas, G., Bangeas, P.I., Suri, J.S., Eds.; Elsevier: St. Louis, MO, USA, 2020; pp. 97–116. [Google Scholar]
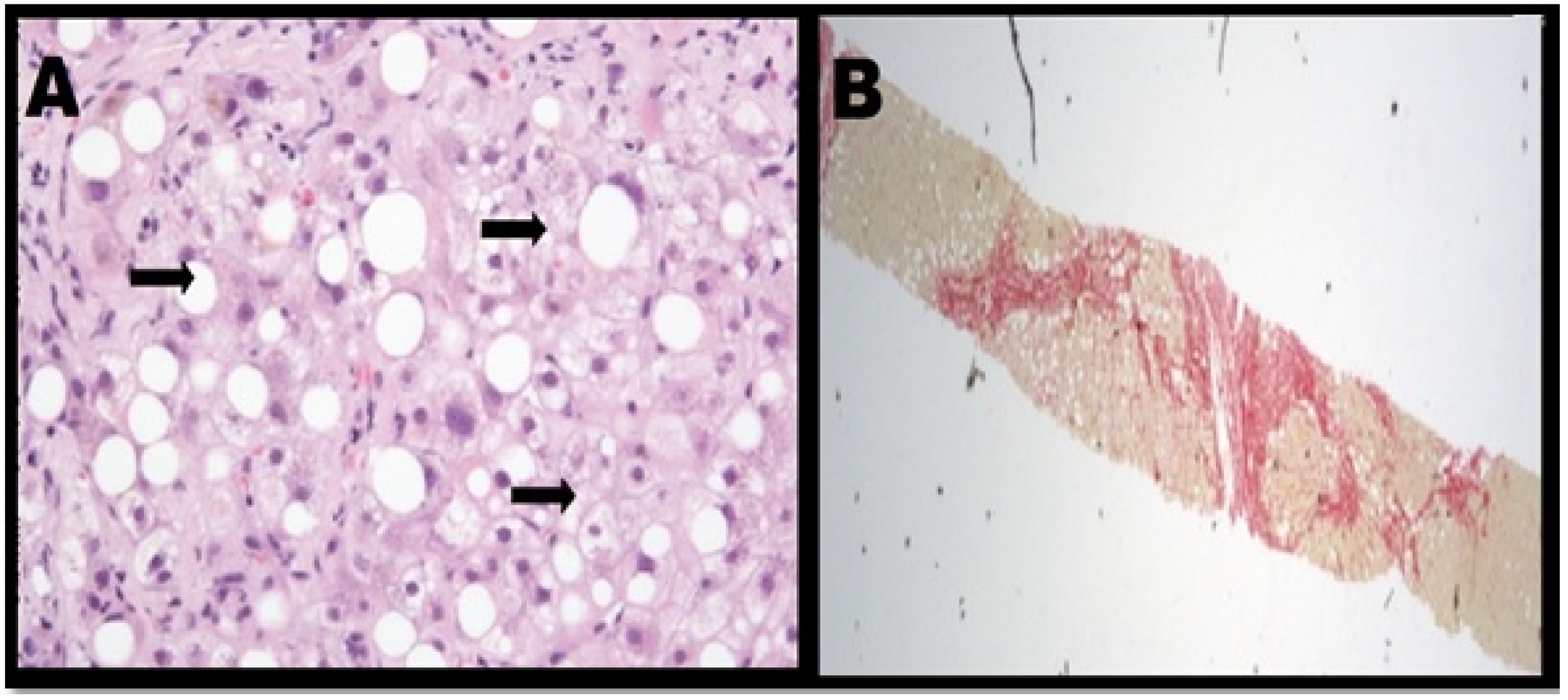
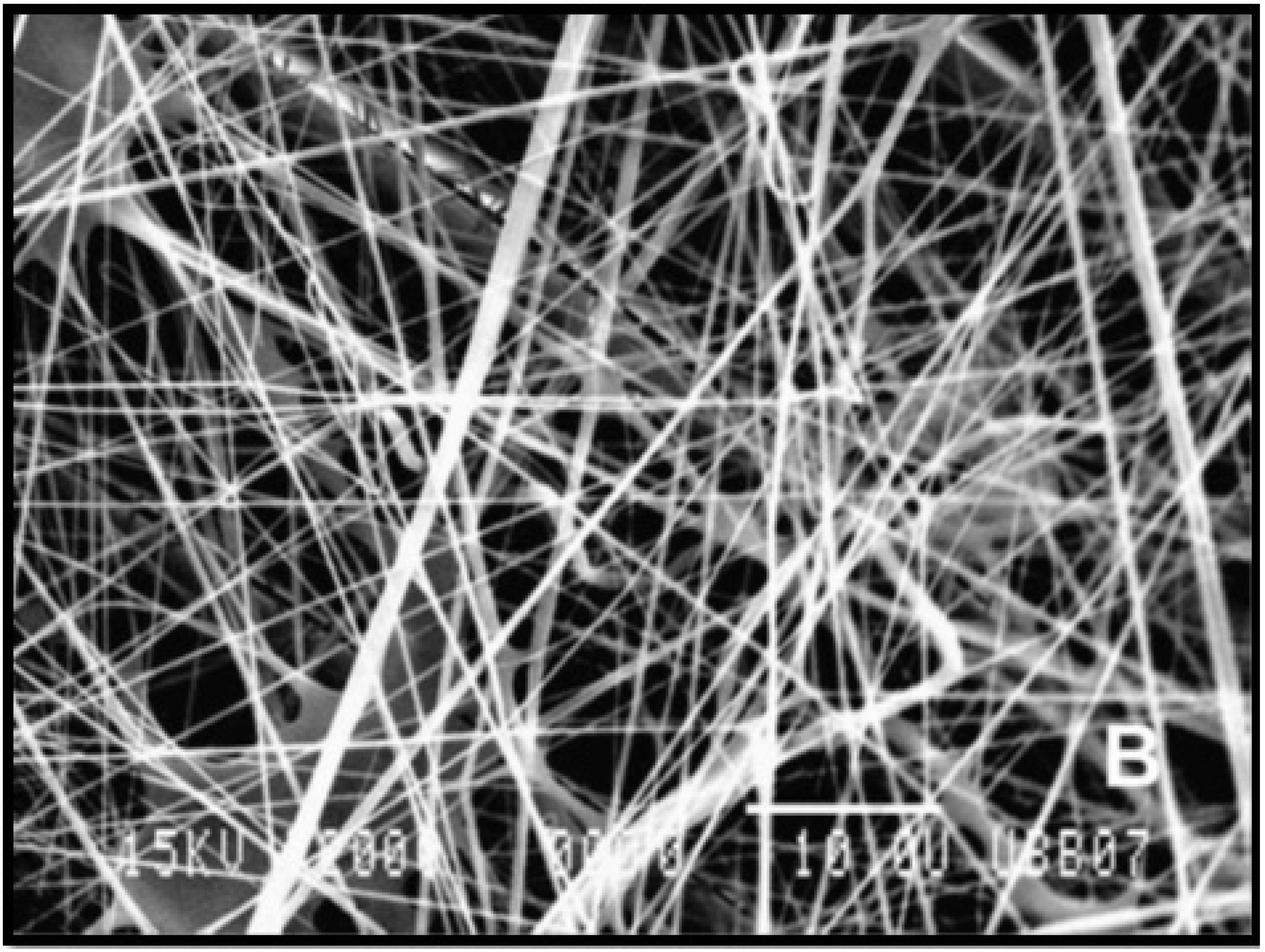
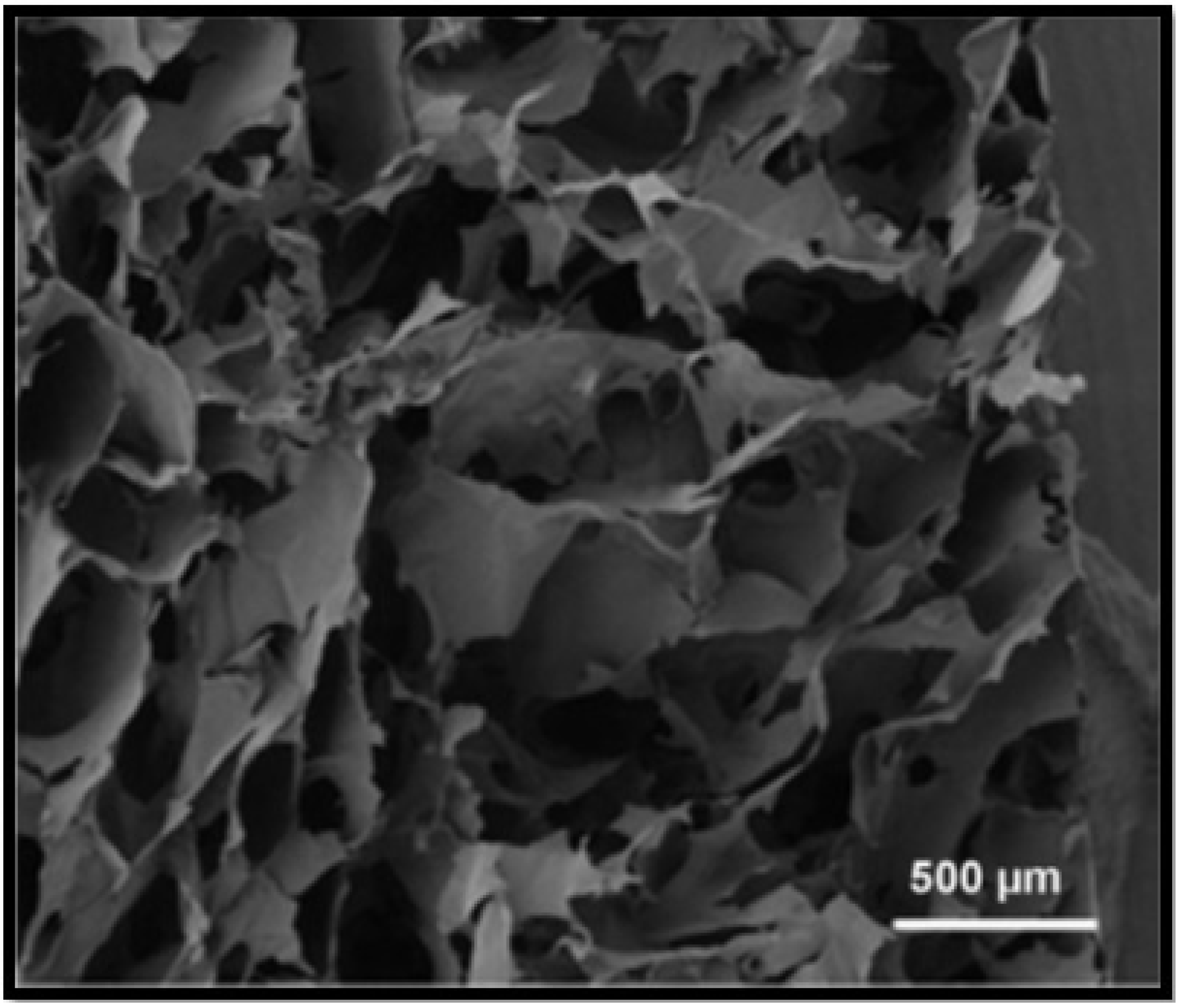
| Biomaterials Utilised for Liver Tissue Engineering Scaffolds | |||
|---|---|---|---|
| Biomaterial | Advantages | Disadvantages | Ref |
| Polylactic Acid (PLA) |
|
| [46,52,53,54] |
| Poly (L-lactide) (PLLA) |
|
| [41,55] |
| Polyglycolide (PGA) |
|
| [41,52,53,55] |
| Polydimethylsiloxane (PDMS) |
|
| [30,40,41] |
| Chitosan |
|
| [47,52,55,56] |
| Alginate |
|
| [47,55,57] |
| Animal extracted ECM |
|
| [44] |
| Derivation of Stem of Stem Cells | ||
|---|---|---|
| Stem Cell Type | Derivation | Key Facts |
| HSC |
|
|
| MSC |
|
|
| ESC |
|
|
| iPSC |
|
|
| EPC |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Haque, N.; Azhar, Z.; Saeinasab, M.; Sefat, F. Regenerative Medicine of Liver: Promises, Advances and Challenges. Biomimetics 2021, 6, 62. https://doi.org/10.3390/biomimetics6040062
Ali S, Haque N, Azhar Z, Saeinasab M, Sefat F. Regenerative Medicine of Liver: Promises, Advances and Challenges. Biomimetics. 2021; 6(4):62. https://doi.org/10.3390/biomimetics6040062
Chicago/Turabian StyleAli, Saiful, Nasira Haque, Zohya Azhar, Morvarid Saeinasab, and Farshid Sefat. 2021. "Regenerative Medicine of Liver: Promises, Advances and Challenges" Biomimetics 6, no. 4: 62. https://doi.org/10.3390/biomimetics6040062
APA StyleAli, S., Haque, N., Azhar, Z., Saeinasab, M., & Sefat, F. (2021). Regenerative Medicine of Liver: Promises, Advances and Challenges. Biomimetics, 6(4), 62. https://doi.org/10.3390/biomimetics6040062







