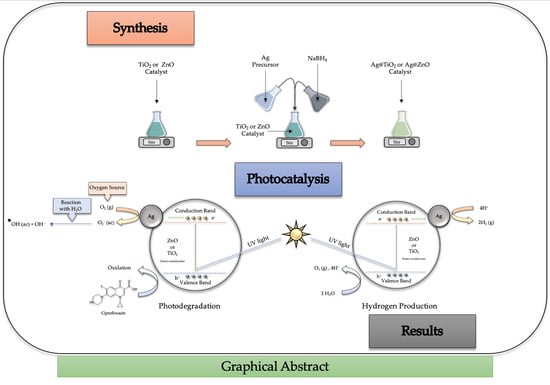
Photocatalytic Activity of Silver-Based Biomimetics Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of TiO2 and ZnO Nanowires
2.3. Incorporation of Silver Nanoparticles (Ag NPs)
2.4. Characterization of the Catalysts
2.5. Photocatalytic Experiments
3. Results and Discussion
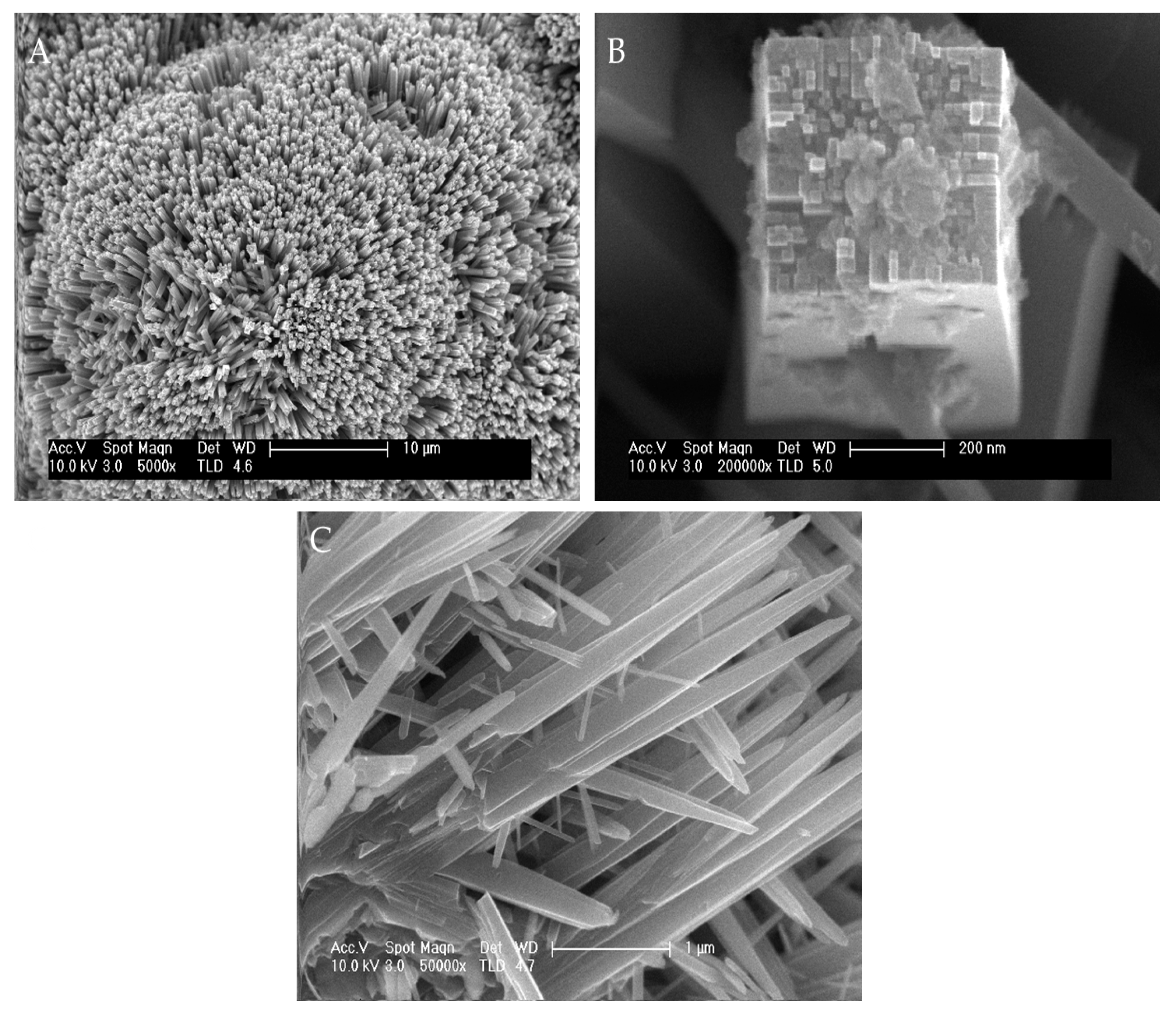
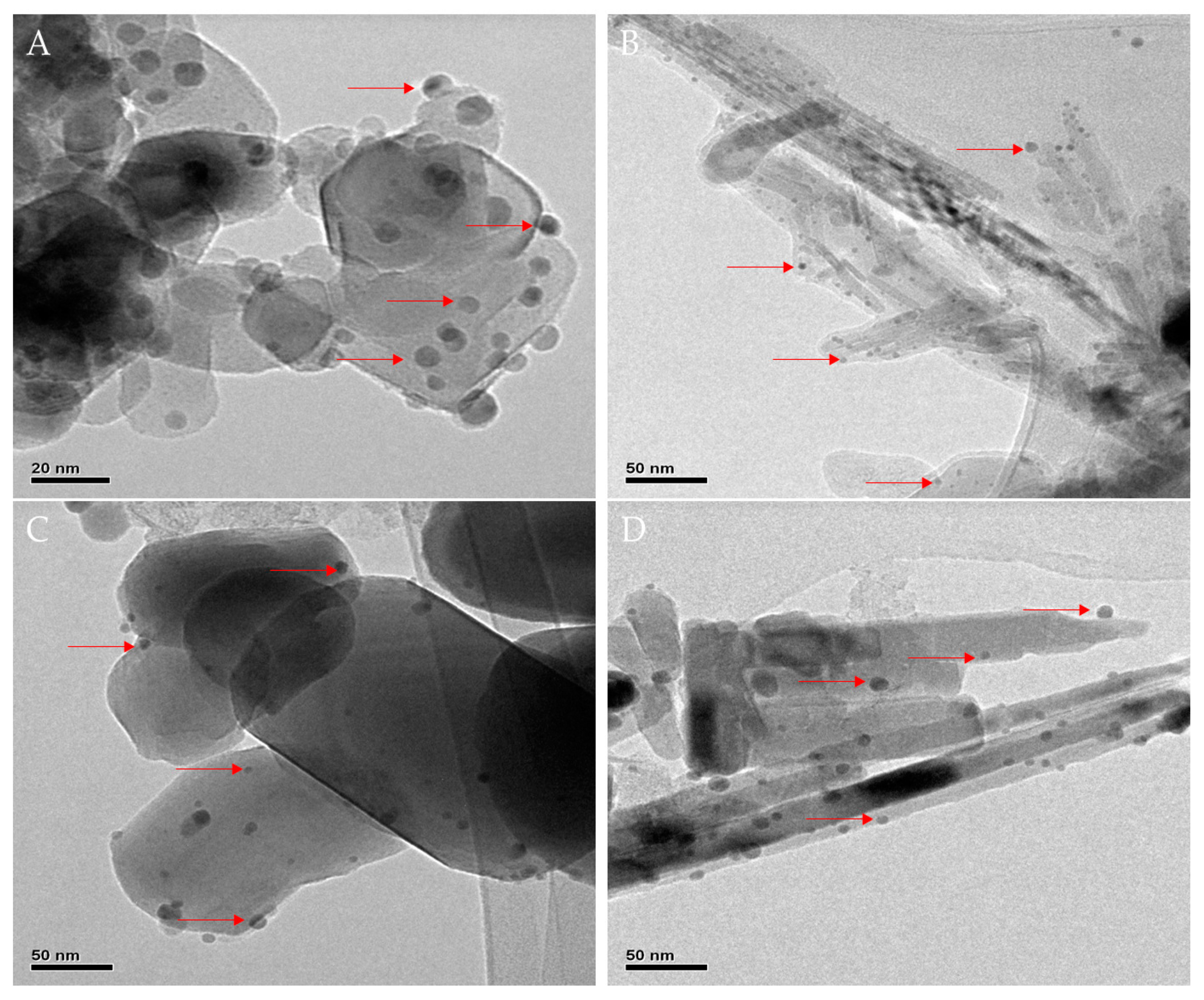
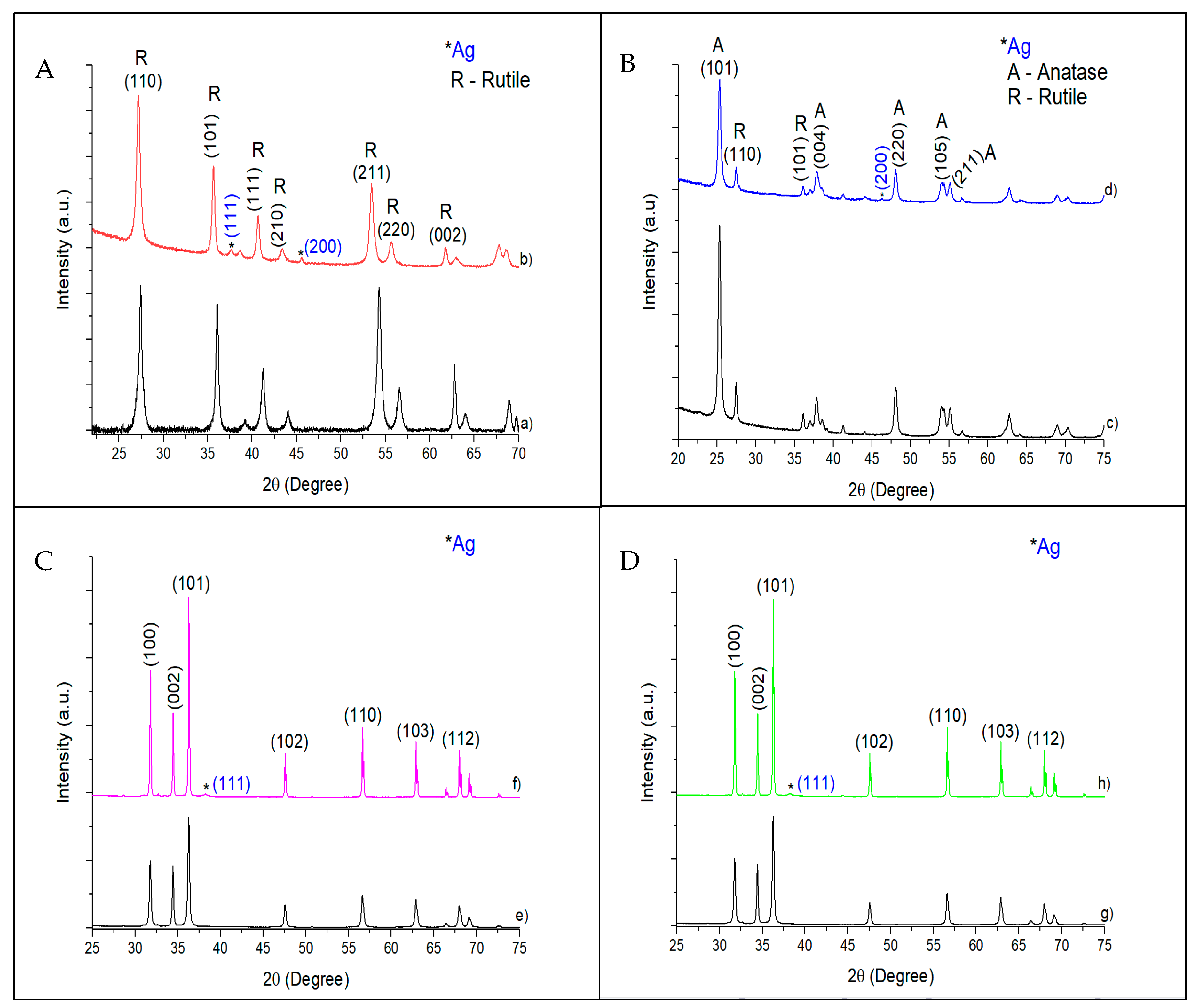
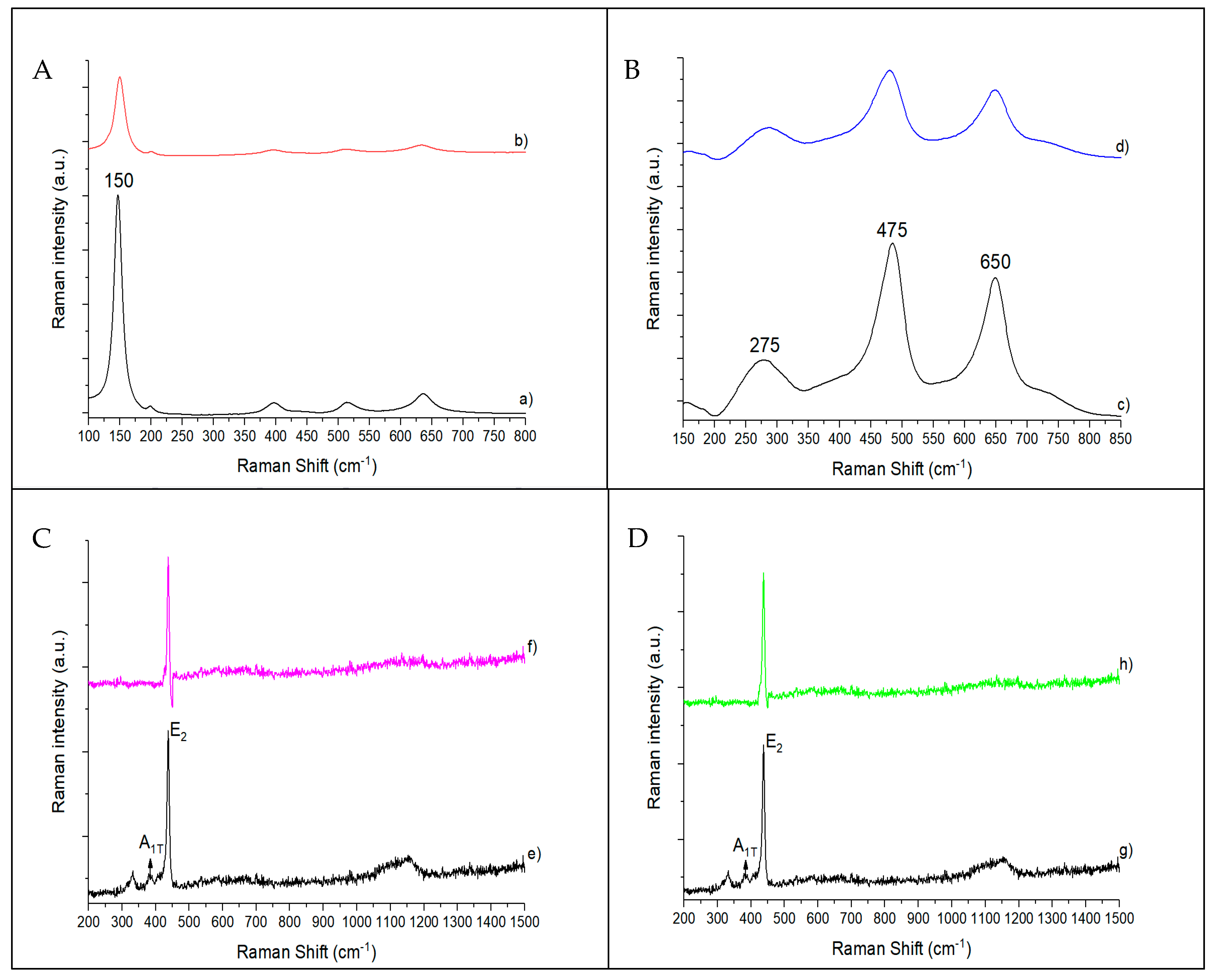
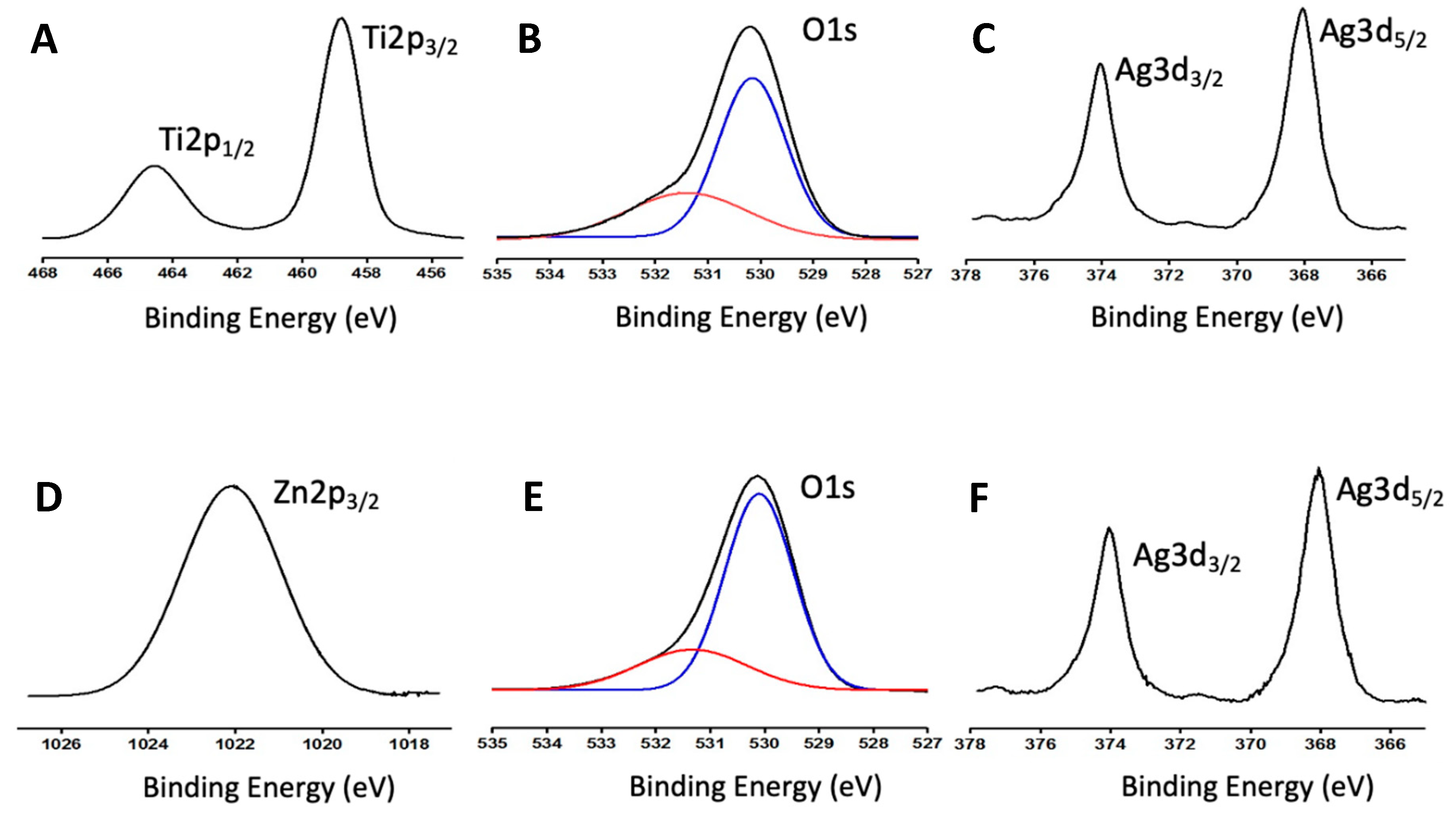
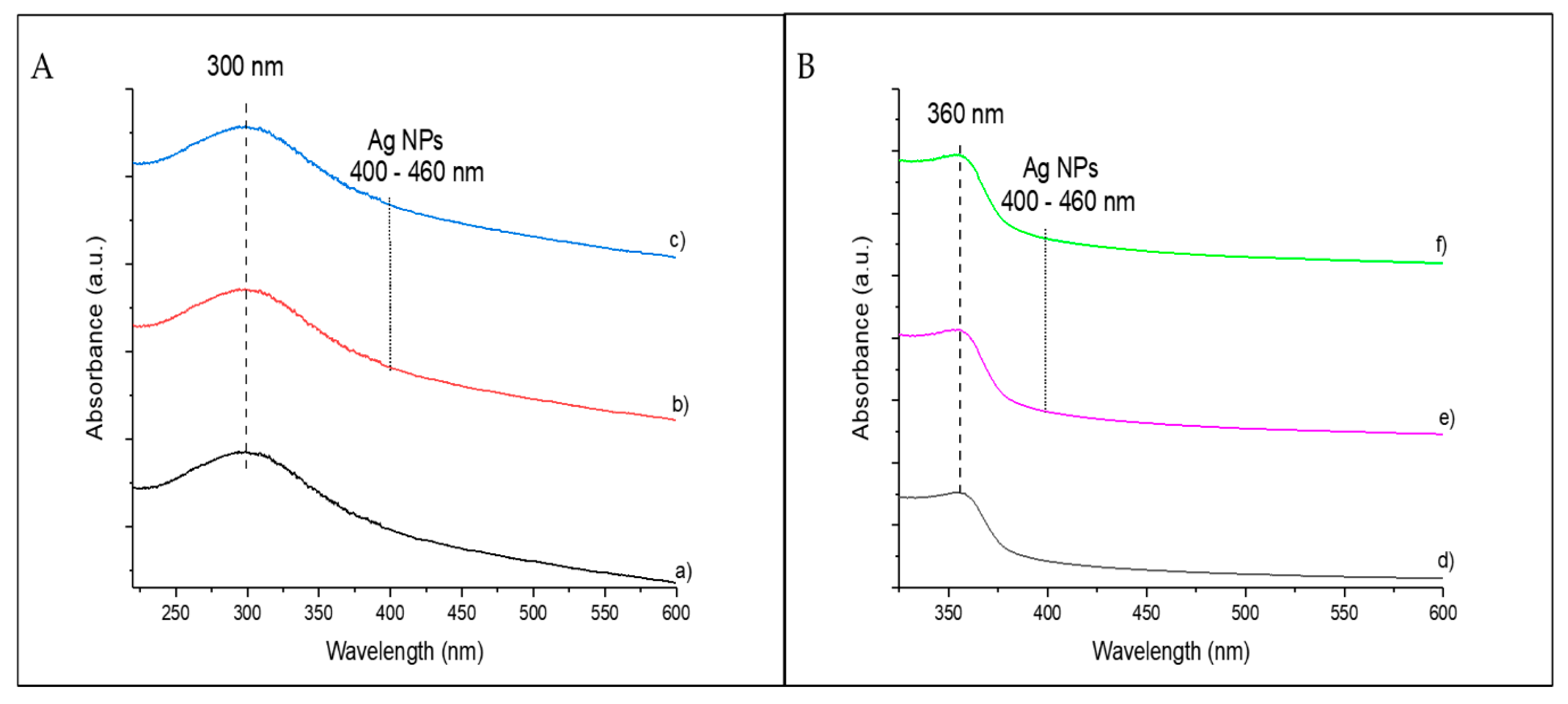
3.1. Characterization of Catalysts
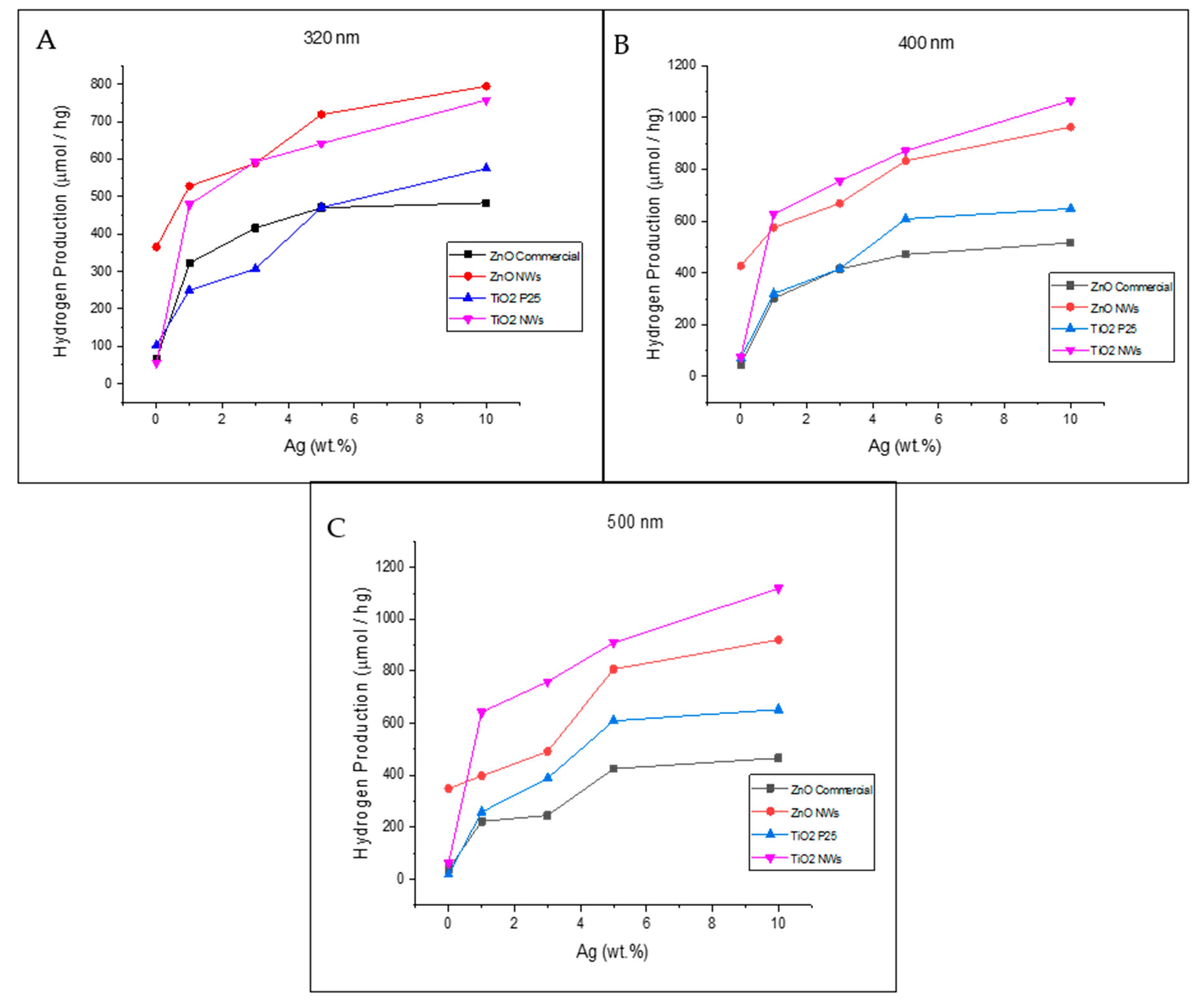
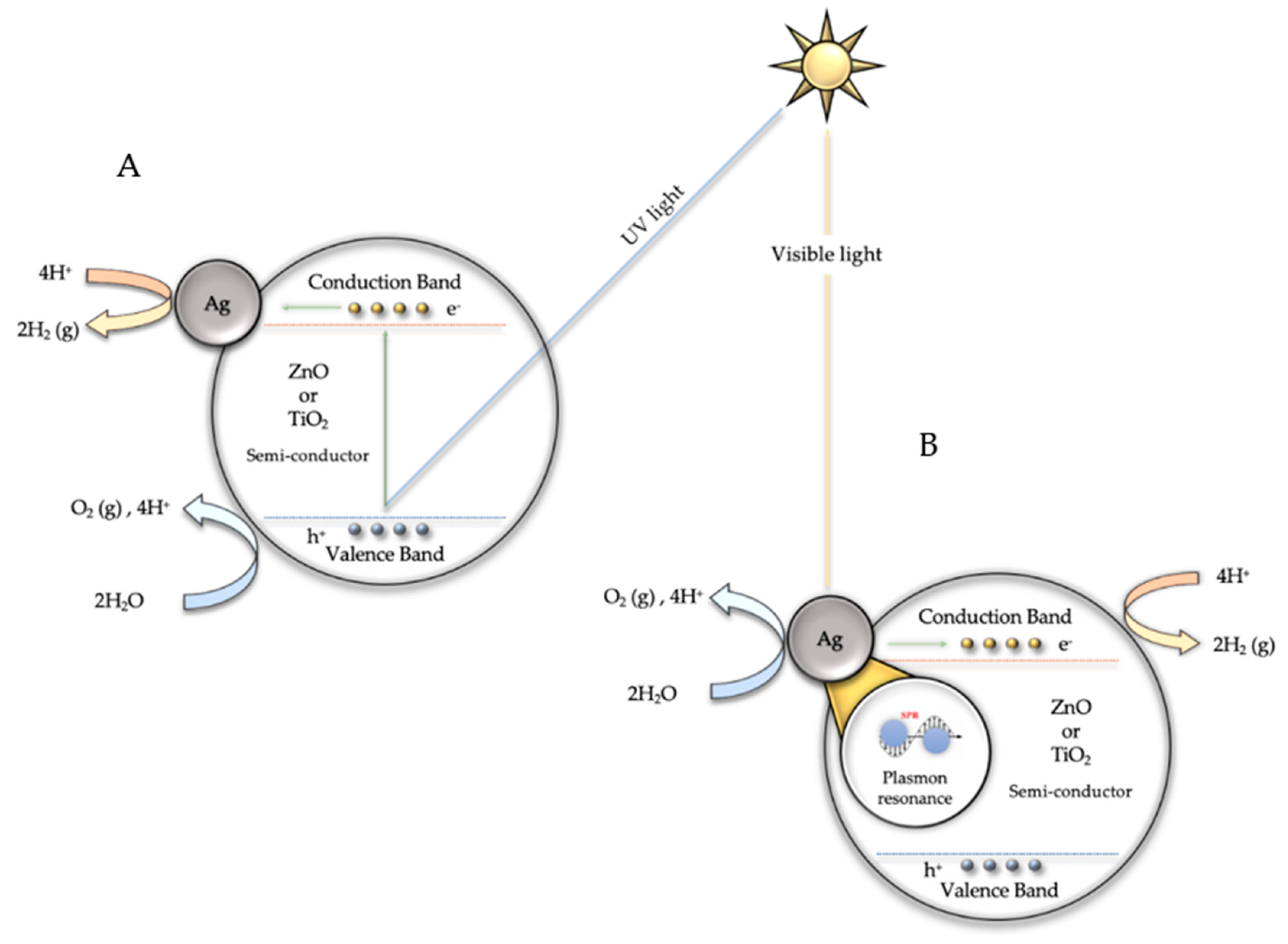
3.2. Photocatalytic Hydrogen Production via Water Splitting
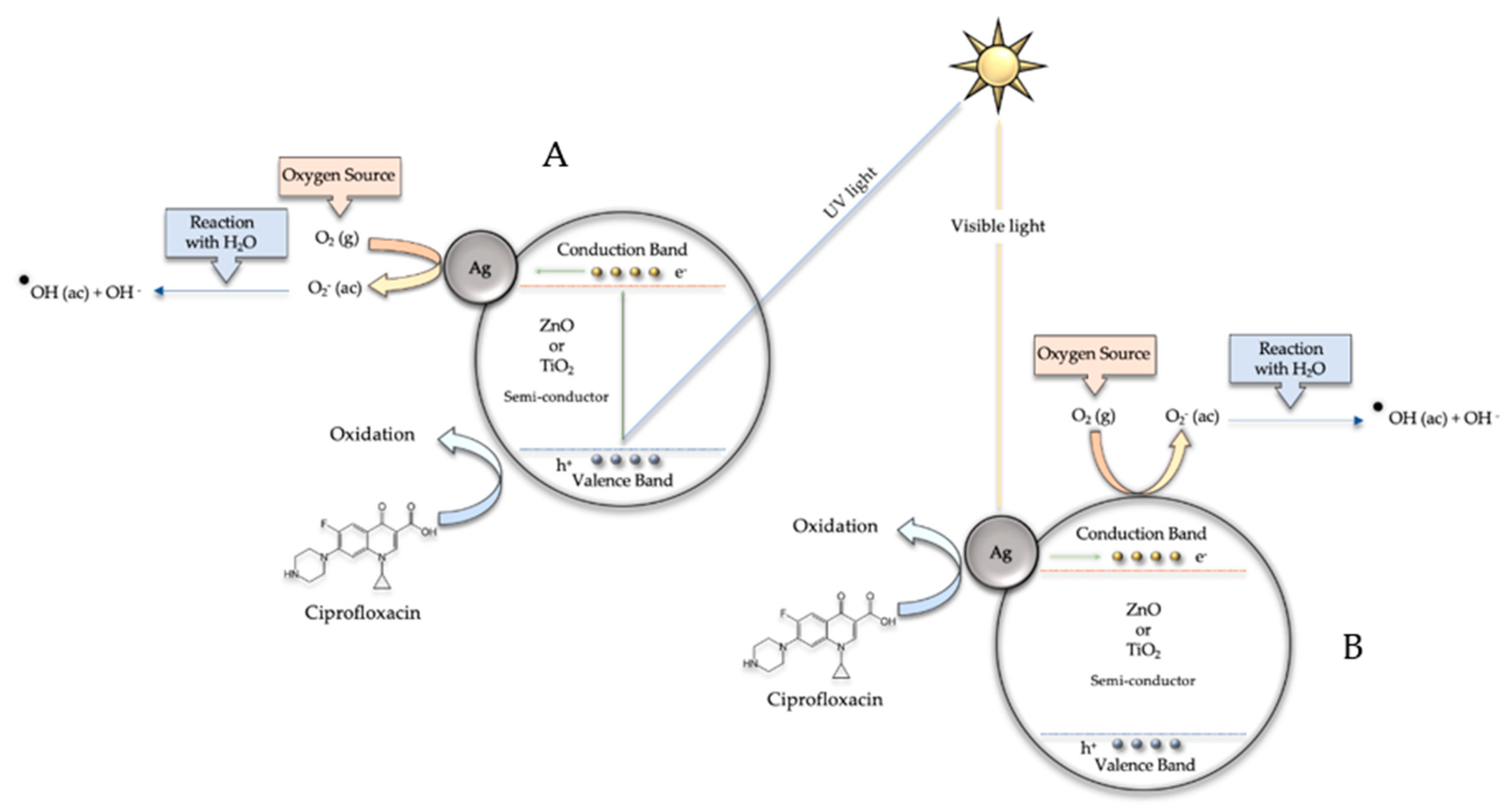
3.3. Photocatalytic Degradation of Ciprofloxacin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zafar, M.W.; Shahbaz, M.; Hou, F.; Sinha, A. From Nonrenewable to Renewable Energy and Its Impact on Economic Growth: The role of Research and Development Expenditures in Asia-Pacific Economic Cooperation Countries. J. Clean. Prod. 2018, 212, 1166–1178. [Google Scholar] [CrossRef]
- Skovgaard, J.; van Asselt, H. The politics of fossil fuel subsidies and their reform: Implications for climate change mitigation. Wiley Interdiscip. Rev. Clim. Chang. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Zhang, H.; Carey, A.M.; Jeon, K.W.; Liu, M.; Murrell, T.D.; Locsin, J.; Lin, S.; Yan, H.; Woodbury, N.; Seo, D.K. Highly Stable and Scalable Photosynthetic Reaction Center-Graphene Hybrids Electrode System for Biomimetic Solar Energy Transduction. J. Mater. Chem. A 2017, 5, 6038–6041. [Google Scholar] [CrossRef]
- Martín-Palma, R.J.; Lakhtakia, A. Progress on bioinspired, biomimetic, and bioreplication routes to harvest solar energy. App. Phys. Rev. 2017, 4, 1–7. [Google Scholar] [CrossRef]
- Ye, S.; Ding, C.; Chen, R.; Fan, F.; Fu, P.; Yin, H.; Wang, X.; Wang, Z.; Du, P.; Li, C. Mimicking the Key functions of Photosystem II in Artificial Photosynthesis for Photoelectrocatalytic Water Splitting. J. Am. Chem. Soc. 2018, 140, 3250–3256. [Google Scholar] [CrossRef]
- Ye, S.; Ding, C.; Liu, M.; Wang, A.; Huang, Q.; Li, C. Water Oxidation for Artificial Photosynthesis. Adv. Mater. 2019, 31, 1–33. [Google Scholar] [CrossRef]
- Li, Q.; Xia, Y.; Yang, C.; Lv, K.; Lei, M.; Li, M. Building a direct Z-scheme heterojunction photocatalyst by Znln2S4 nanosheets and TiO2 hollowspheres for highly-efficient artificial photosynthesis. Chem. Eng. J. 2018, 349, 287–296. [Google Scholar] [CrossRef]
- Ji, Y.; Luo, Y. Direct Dontation of Proton from H2O to CO2 in Artificial Photosynhtesis on the Anatase TiO2(101) Surface. J. Phys. Chem. 2019, 123, 3019–3023. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Zhou, C.; Cao, Z.; Zhang, R. ZnO Rod/Reduced Graphene Oxide Sensitized by α-Fe2O3 nanoparticles for effective visible-light photoreduction of CO2. J. Colloid Interface Sci. 2019, 554, 335–343. [Google Scholar] [CrossRef]
- Liang, S.; Han, B.; Liu, X.; Chen, W.; Peng, M.; Guan, G.; Deng, H.; Lin, Z. 3D spatially branched hierarchical Z-scheme CdS-Au nanoclusters-ZnO hybrids with boosted photocatalytic hydrogen evolution. J. Alloys Compd. 2018, 754, 105–113. [Google Scholar] [CrossRef]
- Geng, R.; Yin, J.; Zhou, J.; Jiao, T.; Feng, Y.; Zhang, L.; Chen, Y.; Bai, Z.; Peng, Q. In Situ Construction of Ag/TiO2/g-C3N4 Heterojunction Nanocomposite Based on Hierarchical Co-Assembly with Sustainable Hydrogen Evolution. Nanomaterials 2020, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Manoj, D.; Qin, J.; Naushad, M.; Gracia, F.; Lee, A.F.; Khan, M.M.; Gracia-Pinilla, M. Mechanothermal synthesis of Ag/TiO2 for photocatalytic Methyl Orange degradation and hydrogen production. Process Saf. Environ. Prot. 2018, 120, 339–347. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Y.; Zhang, D.; Chen, Y. One-Step Synthesis of Ag@TiO2 Nanoparticles for Enhanced Photocatalytic Performance. Nanomaterials 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Fei, Z.; Bian, C.; Yu, L.; Chen, S.; Qian, Y. Facile synthesis of high-performance photocatalysts based on Ag/TiO2 composites. Ceram. Int. 2019, 45, 12586–12589. [Google Scholar] [CrossRef]
- Mou, H.; Song, C.; Zhou, Y.; Zhang, B.; Wang, D. Design and synthesis of porous Ag/ZnO nanosheets assemblies as super photocatalysts for enhanced visible-light degradation of 4-nitrophenol and hydrogen evolution. Appl. Catal. B 2018, 221, 565–573. [Google Scholar] [CrossRef]
- Xin, Z.; Li, L.; Zhang, X.; Zhang, W. Microwave-assisted hydrothermal synthesis of chrysanthemum-like Ag/ZnO prismatic nanorods and their photocatalytic properties with multiple modes for dye degradation and hydrogen production. RSC Adv. 2018, 8, 6027–6038. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Yang, J.; Zhai, H.; Liu, X.; Jia, H. Microwave-assisted one-pot synthesis of Ag decorated flower-like ZnO composites photocatalysts for dye degradation and NO removal. Ceram. Int. 2019, 45, 20133–20140. [Google Scholar] [CrossRef]
- Trang, T.N.Q.; Phan, T.B.; Nam, N.D.; Thu, V.T.H. In situ charge transfer at the Ag@ZnO photoelectrochemical interface toward the high photocatalytic performance of H2 evolution and RhB degradation. ACS Appl. Mater. Interfaces 2020, 12, 12195–122206. [Google Scholar] [CrossRef]
- Malakootian, M.; Nasiri, A.; Amiri Gharaghani, M. Photocatalytic degradation of ciprofloxacin antibiotic by TiO2 nanoparticles immobilized on a glass plate. Chem. Eng. Commun. 2019, 207, 1–17. [Google Scholar] [CrossRef]
- Cotto, M.; Campo, T.; Elizalde, E.; Gómez, A.; Morant, C.; Márquez, F. Photocatalytic degradation of rhodamine-B under UV-visible light irradiation using different nanostructured catalyst. Am. Chem. Sci. J. 2013, 3, 178–202. [Google Scholar] [CrossRef]
- Lin, C.C.; Li, Y.Y. Synthesis of ZnO nanowires by thermal decomposition of zinc acetate dihydrate. Mater. Chem. Phys. 2009, 113, 334–337. [Google Scholar] [CrossRef]
- Naldoni, A.; D’Arienzo, M.; Altomare, M.; Marelli, M.; Scotti, R.; Morazzoni, F.; Selli, E.; Dal Santo, V. Pt and Au/TiO2 photocatalysts for methanol reforming: Role of metal nanoparticles in tuning charge trapping properties and photoefficiency. Appl. Catal. B 2013, 130, 239–248. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting-The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Clarizia, L.; Spasiano, D.; Di Somma, I.; Marotta, R.; Andreozzi, R.; Dionysiou, D.D. Copper modified-TiO2 catalysts for hydrogen generation through photoreforming of organics. A short review. Int. J. Hydrogen Energy 2014, 39, 16812. [Google Scholar] [CrossRef]
- Machín, A.; Cotto, M.; Duconge, J.; Arango, J.C.; Morant, C.; Pinilla, S.; Soto-Vázquez, L.; Resto, E.; Márquez, F. Hydrogen production via water splitting using different Au@ZnO catalysts under UV–vis irradiation. J. Photochem. Photobiol. A Chem. 2018, 353, 385–394. [Google Scholar] [CrossRef]
- Pinilla, S.; Machín, A.; Park, S.H.; Arango, J.C.; Nicolosi, V.; Márquez, F.; Morant, C. TiO2-Based Nanomaterials for the Production of Hydrogen and the Development of Lithium-Ion Batteries. J. Phys. Chem. B 2017, 122, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Machín, A.; Arango, J.C.; Fontánez, K.; Cotto, M.; Duconge, J.; Soto-Vázquez, L.; Resto, E.; Petrescu, F.I.T.; Morant, C.; Márquez, F. Biomimetic Catalysts Based on Au@ZnO–Graphene Composites for the Generation of Hydrogen by Water Splitting. Biomimetics 2020, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Cesano, F.; Chowdhury, A.R.; Trad, T.; Cravanzola, S.; Martra, G.; Lorenzo, M.; Zecchina, A.; Scarano, D. Surface Structure and Phase Composition of TiO2 P25 Particles After Thermal Treatments and HF Etching. Front. Mater. 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Kowalska, E.; Rau, S.; Ohtani, B. Plasmonic Titania Photocatalysts Active under UV and Visible-Light Irradiation: Influence of Gold Amount, Size, and Shape. J. Nanotechnol. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Das, J.; Velusamy, P. Biogenic Synthesis of Antifungal Silver Nanoparticles Using Aqueous Stem Extract Banana. Nano. Biomed. Eng. 2013, 5, 34–38. [Google Scholar] [CrossRef]
- Varadaveenkatesan, T.; Lyubchik, E.; Pai, S.; Pugazhendhi, A.; Vinayagam, R.; Selvaraj, R. Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora. J. Photochem. Photobiol. B 2019, 199, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Vijayarangamuthu, K.; Youn, J.; Park, Y.K.; Jung, S.C.; Jeon, K.J. Degussa P25 TiO2 modified with H2O2 under microwave treatment to enhance photocatalytic properties. Catal. Today 2018, 303, 305–312. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Fang, L.; Yange, H.; Zuo, Y.; Gao, J.; He, G.; Sun, Z. A Multifunctional Ag/TiO2/Reduced Graphene Oxide with Optimal Surface-Enhanced Raman Scattering and Photocatalysis. J. Am. Ceram. Soc. 2019, 102, 4000–4013. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2010, 46, 855–874. [Google Scholar] [CrossRef]
- Korepanov, V.I.; Chan, S.Y.; Hsu, H.C.; Hamaguchi, H. Phonon confinement and size effect in Raman spectra of ZnO nanoparticles. Heliyon 2019, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Piao, C.; Li, S.; Tang, J.; Fang, D.; Zhang, Z.; Wang, J. Construction of fixed Z-scheme Ag|AgBr/Ag/TiO2 photocatalyst composite film for malachite green degradation with simultaneous hydrogen production. J. Power Sources 2020, 469, 228430. [Google Scholar] [CrossRef]
- Lu, L.; Wang, G.; Xiong, Z.; Hu, Z.; Liao, Y.; Wang, J.; Li, J. Enhanced photocatalytic activity under visible light by the synergistic effects of plasmonics and Ti3+-doping at the Ag/TiO2-x heterojunction. Ceram. Int. 2020, 46, 10667–10677. [Google Scholar] [CrossRef]
- Zhu, X.; Liang, X.; Wang, P.; Dai, Y.; Huang, B. Porous Ag-ZnO microspheres as efficient photocatalyst for methane and ethylene oxidation: Insight into the role of Ag particles. Appl. Surf. Sci. 2018, 456, 493–500. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S.; Hashimoto, K.; Kominami, H. Preparation of Au/TiO2 exhibiting strong surface plasmon resonance effective for photoinduced hydrogen formation from organic and inorganic compounds under irradiation of visible light. Catal. Sci. Technol. 2012, 2, 907–909. [Google Scholar] [CrossRef]
- Li, R.; Weng, Y.; Zhou, X.; Wang, X.; Mi, Y.; Chong, R.; Han, H.; Li, C. Achieving overall water splitting using titanium dioxide-based photocatalysts of different phases. Energy Environ. Sci. 2015, 8, 2377–2382. [Google Scholar] [CrossRef]
- Xu, Y.; Mao, N.; Zhang, C.; Wang, X.; Zeng, J.; Chen, Y.; Wang, F.; Jiang, J.X. Rational design of donor-π-acceptor conjugated microporous polymers for photocatalytic hydrogen production. Appl. Catal. B 2018, 228, 1–9. [Google Scholar] [CrossRef]
- Sachs, M.; Sprick, R.S.; Pearce, D.; Hillman, S.A.J.; Monti, A.; Guilbert, A.A.Y.; Brownbill, N.J.; Dimitrov, S.; Shi, X.; Blanv, F.; et al. Understanding structure-activity relationships in linear polymer photocatalysts for hydrogen evolution. Nat. Commun. 2018, 9, 4968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, Characterization, and Applications of ZnO Nanowires. J. Nanomater. 2012, 2012, 1–22. [Google Scholar] [CrossRef]
- Baruah, S.; Mahmood, M.A.; Myint, M.T.Z.; Bora, T.; Dutta, J. Enhanced visible light photocatalysis through fast crystallization of zinc oxide nanorods. Beilstein J. Nanotechnol. 2010, 1, 14–20. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Effect of seeded substrates on hydrothermally grown ZnO nanorods. J. Sol Gel Sci. Technol. 2009, 50, 456–464. [Google Scholar] [CrossRef]
- Dosado, A.G.; Chen, W.T.; Chan, A.; Sun-Waterhouse, D.; Waterhouse, G.I.N. Novel Au/TiO2 photocatalysts for hydrogen production in alcohol-water mixtures based on hydrogen titanate nanotube precursors. J. Catal. 2015, 330, 238–254. [Google Scholar] [CrossRef]
- Rather, R.A.; Singh, S.; Pal, B. Visible and direct sunlight induced H2 production from water by plasmonic Ag-TiO2 nanorods hybrid interface. Sol. Energy Mater. Sol. Cells 2017, 160, 463–469. [Google Scholar] [CrossRef]
- Imam, S.S.; Adnan, R.; Kaus, N.H.M. Photocatalytic degradation of ciprofloxacin in aqueous media: A short review. Toxicol. Environ. Chem. 2018, 100, 1–30. [Google Scholar] [CrossRef]
- Soto-Vázquez, L.; Cotto, M.; Ducongé, J.; Morant, C.; Márquez, F. Synthesis and photocatalytic activity of TiO2 nanowires in the degradation of p-aminobenzoic acid: A comparative study with a commercial catalyst. J. Environ. Manag. 2016, 167, 23–28. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.; Peng, Q.; Zhou, L.; Tan, X.; Jiang, L.; Tang, C.; Wang, H.; Liu, S.; Wang, Y.; et al. Mechanisms underlying the photocatalytic degradation pathway of ciprofloxacin with heterogeneous TiO2. Chem. Eng. J. 2020, 380, 1–11. [Google Scholar] [CrossRef]


| TiO2-P25 (m2·g−1) | ZnO Commercial (m2·g−1) | TiO2 NWs (m2·g−1) | ZnO NWs (m2·g−1) | |
|---|---|---|---|---|
| 0% (Bare) | 53 | 18 | 403 | 160 |
| 1%Ag | 63 | 78 | 417 | 167 |
| 3%Ag | 72 | 81 | 426 | 179 |
| 5%Ag | 81 | 99 | 438 | 194 |
| 10%Ag | 89 | 112 | 443 | 202 |
| Catalyst | Maximum H2 Production; Silver-Based Catalysts (µmol/gh) | Maximum H2 Production; Unmodified Catalyst (µmol/gh) | Difference in H2 Production (µmol/gh) | Times Greater Than Unmodified Catalyst |
|---|---|---|---|---|
| 10%AgTiO2 NWs | 758 | TiO2 NWs; 56 | 702 | 13.53 |
| 10%AgTiO2-P25 | 575 | TiO2-P25; 103 | 389 | 5.58 |
| 10%AgZnO NWs | 795 | ZnO NWs; 365 | 430 | 2.18 |
| 10%AgZnO comm ** | 483 | ZnO Comm * 66 | 417 | 7.32 |
| Catalyst | Maximum H2 Production; Silver-Based Catalysts (µmol/gh) | Maximum H2 Production Unmodified Catalyst (µmol/gh) | Difference in H2 Production (µmol/gh) | Times Greater Than Unmodified Catalyst |
|---|---|---|---|---|
| 10%AgTiO2 NWs | 1065 | TiO2 NWs; 75 | 990 | 14.20 |
| 10%AgTiO2-P25 | 648 | TiO2-P25; 71 | 577 | 9.13 |
| 10%AgZnO NWs | 963 | ZnO NWs; 427 | 430 | 2.26 |
| 10%AgZnO comm. ** | 516 | ZnO Comm * 45 | 471 | 11.47 |
| Catalyst | Maximum H2 Production Silver-Based Catalysts (µmol/gh) | Maximum H2 Production Unmodified Catalyst (µmol/gh) | Difference in H2 Production (µmol/gh) | Times Greater Than Unmodified Catalyst |
|---|---|---|---|---|
| 10%AgTiO2 NWs | 1119 | TiO2 NWs; 62 | 1057 | 18.05 |
| 10%AgTiO2-P25 | 653 | TiO2-P25; 18 | 635 | 36.28 |
| 10%AgZnO NWs | 921 | ZnO NWs; 349 | 572 | 2.64 |
| 10%AgZnO comm. ** | 466 | ZnO Comm *; 38 | 428 | 12.26 |
| Reference | H2 Production (µmol) | Source (nm) | Irradiation Time (h) | TiO2 or ZnO Crystal Structure * | Reaction Mixture | Ag (wt.%) |
|---|---|---|---|---|---|---|
| [12] | 910 | λ > 400 | 2 | TiO2; A | Water: Methanol | 14 |
| [47] | 90 | λ = 457 | 8 | TiO2; A | Water: 0.1 M Na2S | 2 |
| [15] | 443.6 | λ > 400 | 4 | ZnO; W | Water: 0.25 M Na2S, 0.35 M Na2SO3 | 3.12 |
| [16] | 55 | λ > 400 | 8 | ZnO; W | Water: 3.0 g Na2S, 2.2 g Na2SO3 | 0.5 |
| This work | 1119 | λ = 500 | 2 | TiO2 NWs; R | Water: 0.5 M Na2S, 0.03 M Na2SO3 | 10 |
| This work | 653 | λ = 500 | 2 | TiO2-P25; A, R | Water: 0.5 M Na2S, 0.03 M Na2SO3 | 10 |
| This work | 963 | λ = 400 | 2 | ZnO NWs; W | Water: 0.5 M Na2S, 0.03 M Na2SO3 | 10 |
| This work | 516 | λ = 400 | 2 | ZnO comm. ** W | Water: 0.5 M Na2S, 0.03 M Na2SO3 | 10 |
| Ag Loading (wt.%) | TiO2 NWs (%) | TiO2-P25 (%) | ZnO NWs (%) | ZnO Commercial (%) |
|---|---|---|---|---|
| 0 | 69.12 | 71.36 | 68.26 | 94.83 |
| 1 | 76.11 | 80.11 | 73.37 | 95.60 |
| 3 | 83.09 | 87.09 | 78.26 | 99.72 |
| 5 | 85.92 | 89.92 | 71.11 | 98.39 |
| 10 | 81.26 | 82.26 | 70.65 | 92.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machín, A.; Soto-Vázquez, L.; Colón-Cruz, C.; Valentín-Cruz, C.A.; Claudio-Serrano, G.J.; Fontánez, K.; Resto, E.; Petrescu, F.I.; Morant, C.; Márquez, F. Photocatalytic Activity of Silver-Based Biomimetics Composites. Biomimetics 2021, 6, 4. https://doi.org/10.3390/biomimetics6010004
Machín A, Soto-Vázquez L, Colón-Cruz C, Valentín-Cruz CA, Claudio-Serrano GJ, Fontánez K, Resto E, Petrescu FI, Morant C, Márquez F. Photocatalytic Activity of Silver-Based Biomimetics Composites. Biomimetics. 2021; 6(1):4. https://doi.org/10.3390/biomimetics6010004
Chicago/Turabian StyleMachín, Abniel, Loraine Soto-Vázquez, Carla Colón-Cruz, Carlos A. Valentín-Cruz, Gerardo J. Claudio-Serrano, Kenneth Fontánez, Edgard Resto, Florian I. Petrescu, Carmen Morant, and Francisco Márquez. 2021. "Photocatalytic Activity of Silver-Based Biomimetics Composites" Biomimetics 6, no. 1: 4. https://doi.org/10.3390/biomimetics6010004
APA StyleMachín, A., Soto-Vázquez, L., Colón-Cruz, C., Valentín-Cruz, C. A., Claudio-Serrano, G. J., Fontánez, K., Resto, E., Petrescu, F. I., Morant, C., & Márquez, F. (2021). Photocatalytic Activity of Silver-Based Biomimetics Composites. Biomimetics, 6(1), 4. https://doi.org/10.3390/biomimetics6010004