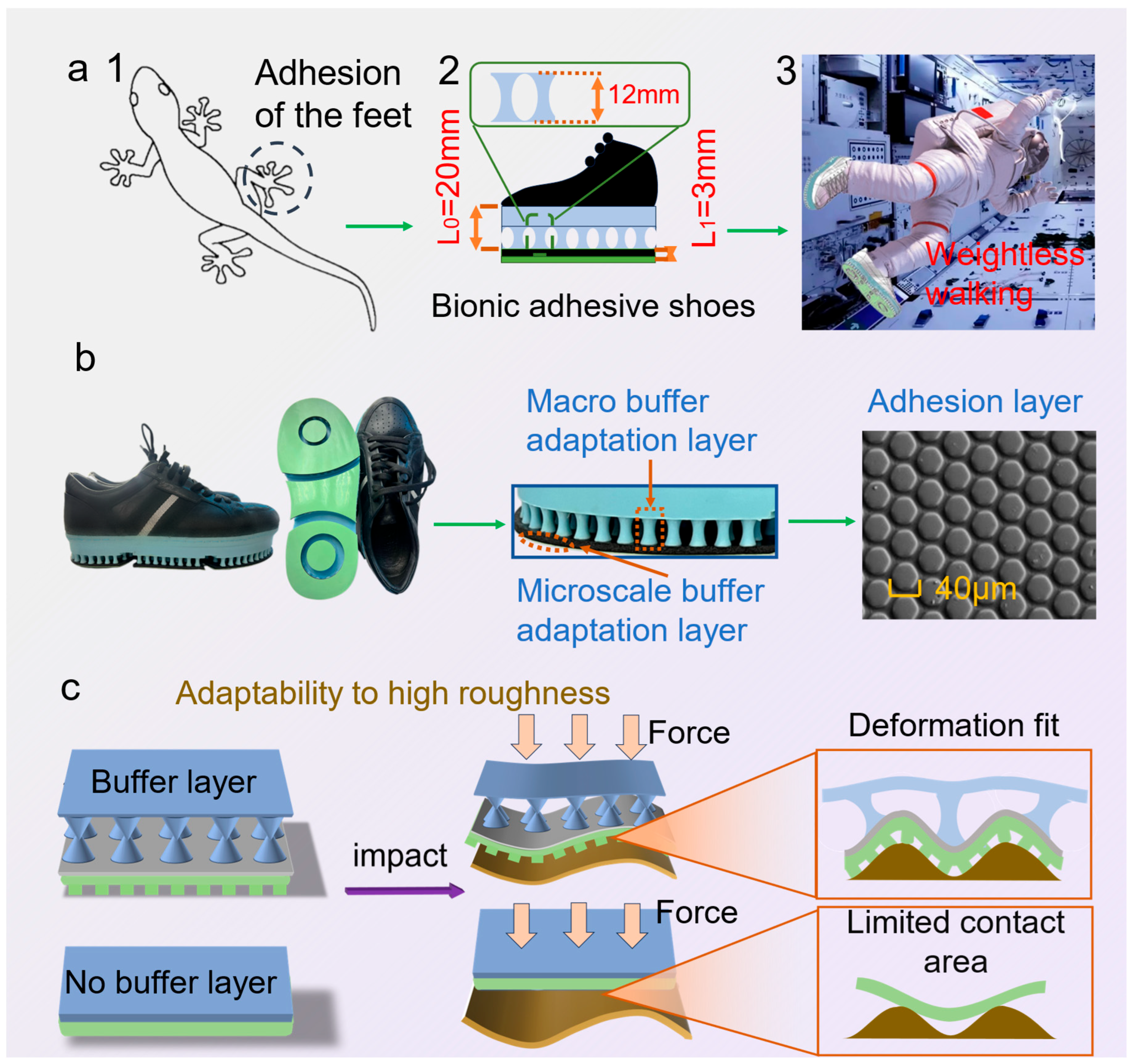
Inspired by the hierarchical setal array of gecko toes, the biomimetic adhesive shoe integrates multi-level, cross-scale cushioning adaptive layers with a microstructured adhesive layer. L
0 denotes the characteristic length/compliance-path scale of the macro-buffer layer, governing energy dissipation in the load–deformation process and the redistribution of interfacial loads; L
1 denotes the characteristic microstructural contact length, primarily controlling the evolution of real contact area and the efficiency of adhesive-energy utilization. This design aims to provide astronauts with an efficient fixation and walking method based on biomimetic adhesion technology under microgravity conditions (
Figure 1a). Following a Buffer→load-shaping→Adhesion (BA) chain, L
0 facilitates pressure equalization and peak attenuation, whereas L
1 affords van der Waals–dominated, direction-selective traction with reversible release under modest preload and a small tangential bias; key geometric/operating parameters (L
0, L
1,
, peeling angle
) are annotated in
Figure 1a to enable direct mapping from biology to engineering.
Figure 1b illustrates the morphology of the biomimetic adhesive shoe sole, highlighting the multi-level cushioning adaptive layers and an outer biomimetic adhesive layer. The adhesive layer comprises a hexagonal prism array with an average circums cribed diameter of approximately 40 μm, an average height of 15 μm, and an average gap of 4 μm. The prisms are arranged in an equidistant pattern, with a center-to-center spacing of 45 μm between adjacent prisms.
3.2. Contact Mechanics Behavior of Biomimetic Adhesive Shoes Under Simulated Microgravity
Participants wore biomimetic adhesive shoes and were suspended using a constant-force suspension system to simulate microgravity conditions (
Figure 3a).
Figure 3b illustrates the variations in plantar force recorded while participants stepped on a force-measurement platform, with stepping frequency controlled at 30 steps/min. At initial foot contact, the support force increased, peaking at approximately 100 N during the late stance phase. Subsequently, as the foot was raised, maximum plantar adhesion occurred before transitioning into the swing phase. Compared with a gravity-based load of approximately 600 N, this represents an approximately 85% reduction in force, confirming the effectiveness of microgravity simulation. The maximum normal adhesion force under simulated microgravity conditions was 75 ± 12 N, reflecting differences due to varying preload conditions during the stepping process.
Under vertical detachment conditions in a gravity environment, the adhesion performance was tested by participants lifting their feet to the same height (10 cm) at detachment speeds ranging from 30 cm/s to 150 cm/s (
Figure 3c). Adhesion force increased with increasing detachment speed, with the peak force at 150 cm/s nearly double that at 30 cm/s. Additionally, the duration of force application decreased as speed increased. The detachment time at 30 cm/s was approximately five times longer than at 150 cm/s. Consequently, the adhesion force impulse decreased with increasing speed up to a certain threshold. Beyond approximately 110 cm/s, additional increases in adhesion force were minimal, indicating a limit in maximum achievable impulse given a fixed contact area.
Stepping exercises under simulated microgravity using different detachment modes (
Figure 3d inset) revealed that the vertical detachment (simultaneous detachment) produced the highest adhesion force compared to the other four modes. The average adhesion force under simultaneous detachment was 67 ± 15 N (
Figure 3d). This result occurs primarily because simultaneous stepping results in the heel and forefoot leaving the ground simultaneously, causing nearly all adhesive micropillars to detach simultaneously. Consequently, adhesion force predominantly acts in the normal direction, minimizing tangential friction and maximizing the observed adhesion.
Compared to other detachment modes, lateral and medial detachment modes are significantly influenced by tangential friction forces. Given that the total adhesion force remains approximately constant, normal adhesion forces under these modes are substantially lower than those observed in vertical and rear-side detachment modes. Specifically, the average normal adhesion force in lateral detachment was only 18.5% and in medial detachment, 28.3% of that observed in vertical detachment. This disparity arises from the differing microstructural interactions during lateral or medial detachment. Assuming the adhesive units’ micropillars contact a locally flat substrate, the adhesive interaction force (
Pf) between the shoe sole and the surface [
24,
25] is given by
where
Pf represents the normal adhesion force (N),
f denotes the effective adhesion energy (mJ/m
2), r is the radius of the micropillars (μm), K is the effective Young’s modulus (MPa), and
ωf is the work of adhesion (W). γL represents liquid surface tension (N), and
θ denotes the adhesion angle (°). According to Equations (1) and (2), the effective adhesion energy of the biomimetic adhesive shoe is influenced by the adhesion angle
θ. During medial-side detachment, the inclination angle of the primary tensile force exerted on the lower leg relative to the
z-axis ranges between 20° and 30°, with lateral forces on the coronal plane dominating the interaction. Consequently, approximately 30% of the adhesion force acts in the tangential direction and approximately 70% in the normal direction under gravitational conditions. As part of the tangential force is offset by interfacial stresses, detachment can be achieved using relatively smaller normal forces.
However, the maximum adhesion force under the forefoot detachment mode is only 8.9% of that observed during vertical detachment under identical conditions, considerably lower than other detachment modes. This significant reduction occurs because lifting the foot from the forefoot side creates a larger peeling angle approaching 90° between the forefoot area and the contact surface. A larger peeling angle facilitates crack propagation at the interface between the adhesive material and the substrate, enabling complete detachment with lower adhesion force. Conversely, the peeling angles in other detachment modes are typically smaller, rendering crack propagation more difficult and thus requiring higher forces to achieve detachment.
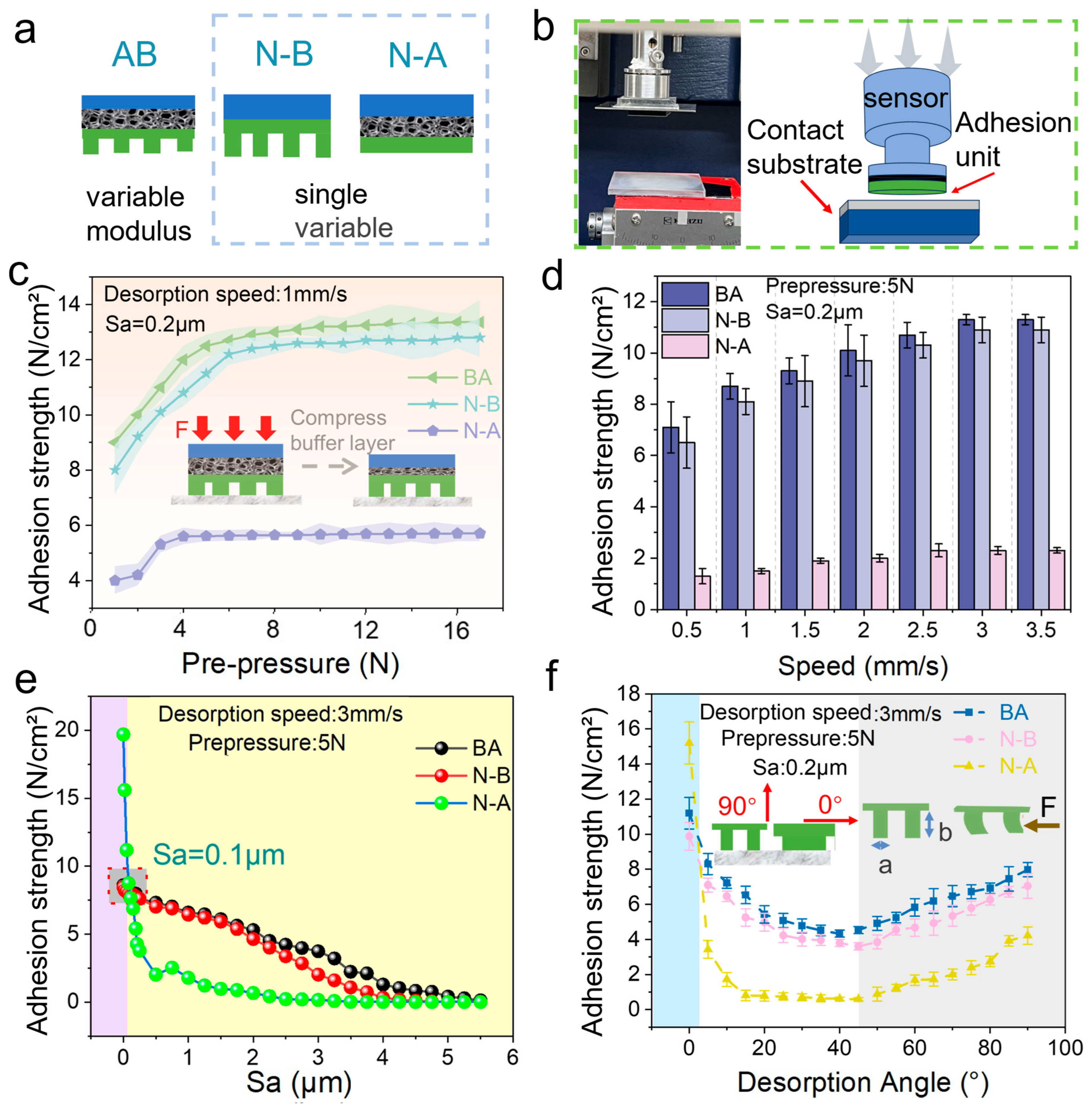
Surface roughness is a critical factor influencing the adhesion performance of the biomimetic adhesive shoes, particularly as contact surfaces in spacecraft exhibit varied roughness levels. Consequently, the biomimetic adhesive shoe must provide sufficient adhesion force while adapting effectively to different surface roughness conditions. The adhesion mechanism of biomimetic adhesive materials is illustrated schematically in the inset of
Figure 3e. When micropillars are angled and pulled, both tangential friction forces and normal adhesion forces are generated, collectively ensuring adequate adhesion at certain inclination angles. For the biomimetic adhesive shoe with a multi-level hierarchical design under normal force, the frictional mechanism relies on dual effects: mechanical interlocking and van der Waals forces. Mechanical interlocking refers to the tangential resistance generated as the elastomer deforms and conforms to the microstructure arrays of the acrylic surface under normal force. As surface roughness increases, the effective interlocking area initially increases but decreases at higher roughness. Tangential adhesion forces resulting from van der Waals interactions can be expressed by Equation (3) [
26,
27,
28]:
where
Gc represents the critical strain energy for interface separation, a constant for specific material interfaces.
A denotes the actual contact area between the elastomer and the acrylic substrate, while
C is the compliance of the elastomer sample in the direction of the frictional force. Increasing the interfacial contact area and decreasing the elastomer’s compliance enhances the tangential adhesion force. The variable-modulus biomimetic adhesive units address the interface properties required for high adhesion forces. The high-modulus backing layer of the adhesive unit reduces the elastomer’s tangential compliance, while the low-modulus contact layer increases compliance with surface roughness, thereby improving the actual contact area between the adhesive unit and the acrylic substrate. This results in a higher tangential adhesion force for the variable-modulus biomimetic adhesive unit.
As the surface roughness of the acrylic substrate increases from Sa = 0.02 μm to Sa = 3.3 μm, the adhesion force of the biomimetic adhesive shoes, tested under vertical detachment in a gravity environment, gradually decreases. A metronome was used to control the stepping frequency to 30 steps/min.
Figure 3e shows that as the roughness increases, the adhesion force of the shoe decreases. At the microscale, higher roughness leads to fewer contact points between the biomimetic adhesive protrusions and the substrate, resulting in variations in the total adhesion force due to differences in the number of protrusions in contact with surfaces of varying roughness. Therefore, the performance of the biomimetic adhesive shoes can be estimated based on different levels of frictional forces.
In conclusion, increasing the contact area and reducing the compliance of the elastomer in the direction of the frictional force can significantly enhance the tangential adhesion force. The variable-modulus biomimetic adhesive unit optimizes this mechanism for effective performance across various surface conditions.
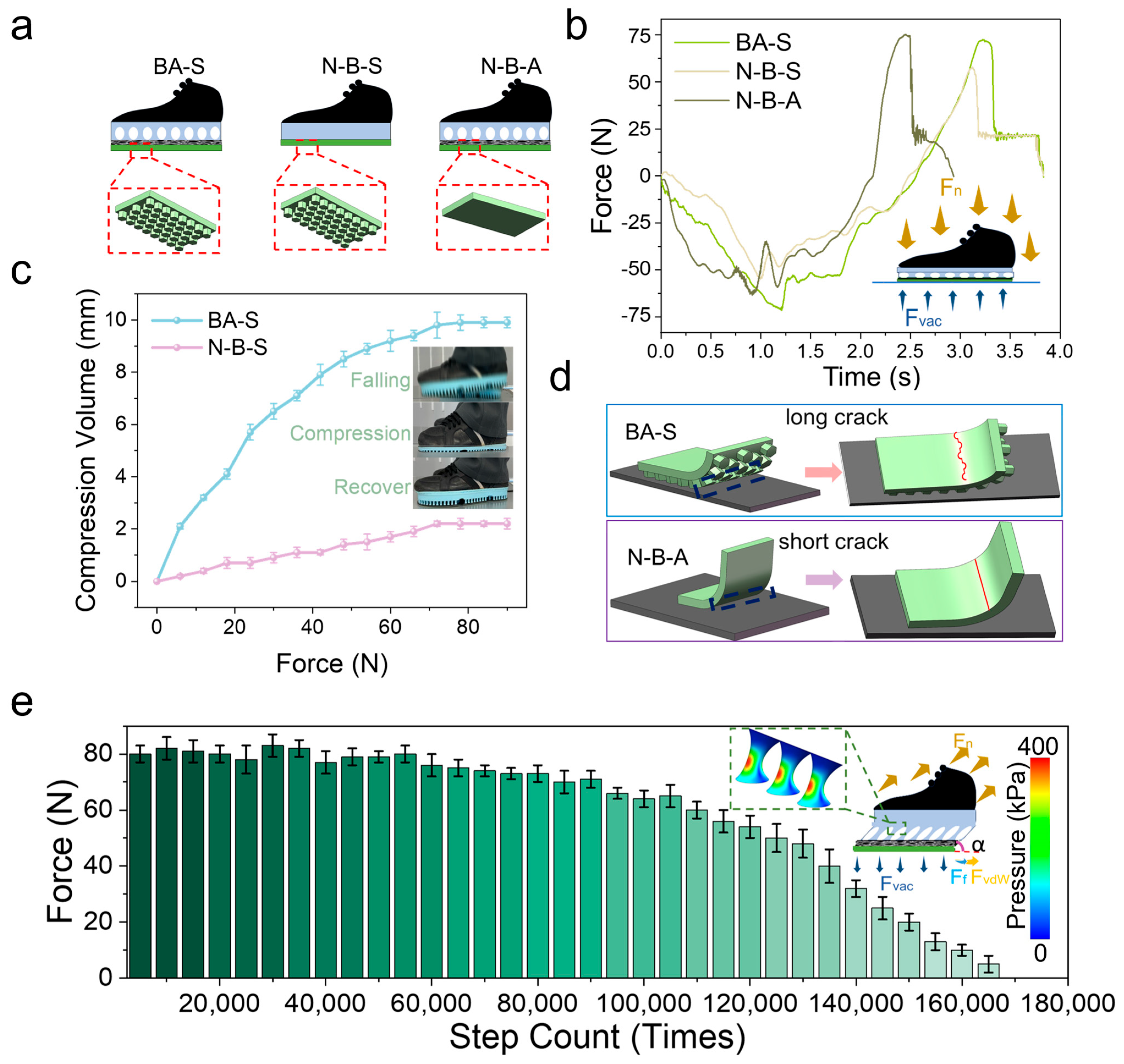
Three variants were considered to analyze the multi-level, cross-scale performance of the biomimetic adhesive shoes. The biomimetic adhesive shoe with a buffering layer and a biomimetic adhesive layer is designated as BA-S (buffer and adhesion shoes). The control group, comprising a shoe with an adhesive layer without a buffering layer, is labeled as N-B-S (no-buffer and adhesion shoes). The N-B-A (no-adhesion and buffer shoes) group refers to the biomimetic adhesive shoe with a buffering layer but without an adhesive layer (
Figure 4a). The experiment was conducted with a constant impact speed of 50 cm/s, a preload of 70 N, and an impact applied to the aluminum plate substrate connected to sensors. The buffering time for the N-B-S group is approximately 2 s, compared to the BA-S and N-B-A groups (~3 s) (
Figure 4b). This reduced buffering time in the N-B-S configuration results from the absence of a buffering layer, which shortens the compression phase during impact and accelerates step completion.
Comparing the BA-S and N-B-S groups, evidently, the lack of biomimetic microstructures in the N-B-S group results in lower adhesion force and shorter adhesion time than in the BA-S group. Force analysis during compression and tension of the biomimetic adhesive shoe (
Figure 4b inset) shows that during compression, pressure forces are the primary forces at play, concentrating the compression in the cushioning layer through force transmission.
In the microgravity environment, comparison of the compression behavior of BAS and N-B-S under the same stepping pressure (
Figure 4c) shows that the maximum compression of BAS during a single step can reach 9.9 mm, which is significantly greater than that of N-B-S (2.2 mm). This indicates that the cushioning layer of the biomimetic adhesive shoes can effectively reduce shocks and impacts during stepping and walking. As illustrated in the inset of
Figure 4c, during rapid descent and ground contact, the cushioning layer of BAS first undergoes compression, thereby prolonging the force application time, attenuating the impact, and subsequently recovering.
The maximum adhesion force for BA-S (75 N) is higher than that for N-B-A (50 N). This difference can be explained by the schematic in
Figure 4d, which illustrates the detachment process from the edge of the biomimetic adhesive shoe. As the BA-S shoe features micropillars on its contact surface, cracks that form during detachment must overcome additional obstacles during their expansion. When encountering protrusions, the cracks are forced to bypass these protrusions, increasing the crack propagation path length and energy consumption. This phenomenon is described by Equation (4) [
29]:
where
denotes the adhesion enhancement factor,
Es is the elastic modulus of the stiff region,
Is is the area moment of inertia of the stiff region, and
ws is the actual contact width of the stiff region. Similarly,
Ec,
Ic, and
wc represent the elastic modulus, area moment of inertia, and actual contact width of the compliant region, respectively.
The relationship reveals that increasing the contact width of the compliant region (wc) effectively enhances the overall adhesion performance. Conversely, the N-B-S group lacks surface microstructures and presents a relatively smooth contact interface, enabling cracks to propagate more easily, thereby reducing adhesion strength.
Furthermore, protrusions on the contact surface induce stress concentration under loading. These stress concentration zones hinder crack propagation because greater energy is required for a crack to continue growing through these localized high-stress regions. This effect is particularly pronounced on surfaces with microstructural protrusions, improving overall adhesion strength due to increased crack resistance.
Figure 4e illustrates the relationship between the adhesion force of biomimetic adhesive shoes and the number of steps under microgravity conditions. The experiment was conducted at a stepping frequency of 30 steps/min on an aluminum alloy substrate. As shown in
Figure 4e, the adhesion force of BAS decreased by approximately 12.5% within the first 90,000 steps, followed by a rapid decline thereafter. This degradation is attributed to the repeated action of normal preload, tensile force, and tangential friction, which damaged the micropillar structures of the adhesive layer, thereby reducing the intermolecular contact area between the adhesive layer and the substrate and ultimately lowering the adhesion force. The inset of
Figure 4e illustrates the forces acting on BAS when stretched at an angle
α, including the tensile force (
), ground reaction force (
Fvac), adhesive resistance force (
FvdW), and tangential frictional force (
Ff). Mechanical analysis of the cushioning layer indicates that, during tensile detachment at angle
α, tangential frictional and normal adhesion forces are mitigated by the buffering effect of the micropillars, which also facilitates uniform stress distribution and concentrates the load within the micropillars. Within a two-dimensional plane-strain framework consisting of a rigid loading plate, an elastic buffer layer, an effective adhesion/friction interface, and a rigid substrate, a normal preload was applied to establish contact, followed by a small rotational perturbation to realize angled peeling. The computed interfacial normal contact pressure
and the von Mises stress field within the buffer layer conform to the colormap (0–400 kPa) in
Figure 4e. With increasing peeling angle
, pressure magnitude and shear–tension coupling at the trailing edge are markedly amplified; the peak
increases and migrates toward the trailing edge, indicating an edge-dominated detachment process. Concurrently, the buffer layer exhibits a through-thickness “stress-diffusion cone,” characterized by smoother iso-stress contours and a reduced peak-to-mean ratio, evidencing redistribution and diffusion of interfacial loads and the suppression of local stress concentrations. These trends suggest that a moderate buffer thickness promotes pressure homogenization and peak attenuation without compromising controllability, whereas an overly thin layer is insufficient for diffusion, and an overly thick layer introduces excessive compliance. The numerical results are consistent with the spatial distribution of the pressure scale in
Figure 4e and provide a mechanistic rationale for the enhanced stability and comfort of the biomimetic adhesive footwear under angled loading
.
Under microgravity conditions, the biomimetic adhesive shoe demonstrates considerable application potential owing to its strong adhesive capabilities. One such application is in astronaut exercise, aiming to mitigate the risk of muscle atrophy. In this study, volunteers wore the biomimetic adhesive shoes while suspended by a constant-force suspension system to simulate microgravity, with EMG sensors attached to relevant lower limb muscle groups.
During leg-raising movements, the adhesion force provided by the biomimetic adhesive shoes simulated ground reaction forces to stimulate the muscular system. A control experiment was conducted with participants wearing shoes that lacked the biomimetic adhesive layer. An aluminum alloy plate with a surface roughness of Sa = 0.2 μm was used as the contact substrate. The stepping frequency was set to 30 steps/min, corresponding to a detachment speed of 75 cm/s for the adhesive shoe. The average detachment force recorded was approximately 80 N.
Figure 5a–e show EMG signals and corresponding RMS values from the main force-generating muscle groups during stepping, both with and without the biomimetic adhesive shoes. The multiple peaks in each EMG trace indicate repeated activation of the respective muscle groups during the stepping cycles.
EMG signals (RMS) were consistently weaker when participants did not wear the adhesive shoes. The biceps femoris exhibited the highest RMS values among the recorded muscle groups, indicating its dominant role in leg lifting regardless of shoe condition. However, for muscles such as the tibialis anterior, peroneus longus, medial gastrocnemius, and vastus lateralis, EMG values were significantly higher when participants wore the biomimetic adhesive shoes, suggesting that detachment motions relied more heavily on calf muscle contractions. An engineering threshold was defined from the ΔEMG%–Fn curve as the knee point of a piecewise linear fit with two lines; this knee point represents the minimum Fn that yields a clear increase in ΔEMG%. As a stability cross-check, a ≥95% gait success rate (absence of premature detachment or major slip) was additionally required. Group-level recommendations derived from these knee points are provided in Dataset S2; per-subject thresholds with fit diagnostics and 95% confidence intervals are listed in Dataset S3; the paired raw cells used for estimation (ΔEMG% and Fn) are provided in Dataset S4.
Figure 5f directly compares EMG amplitudes for each muscle group with and without the adhesive shoes. The results show significant increases in EMG signal amplitudes when using the biomimetic adhesive shoes, with improvements ranging from 34.5% to 79.2%. Statistical details (normality checks; paired
t/Wilcoxon; one-/two-way ANOVA for shoe × speed/roughness;
p-values, effect sizes, and 95% CIs) are summarized in Dataset S5. This demonstrates the effectiveness of the biomimetic adhesive shoes in stimulating lower limb muscle activity.
Compared to traditional exercise devices, these shoes stimulate key flexor groups such as the biceps femoris and gastrocnemius during microgravity stepping tasks, supporting their application in astronaut musculoskeletal conditioning.