Core–Shell Silk Fibroin Hydrogel Microneedles Functionalized with Antibody-Binding Domains for Transdermal Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Construction of Expression Vectors
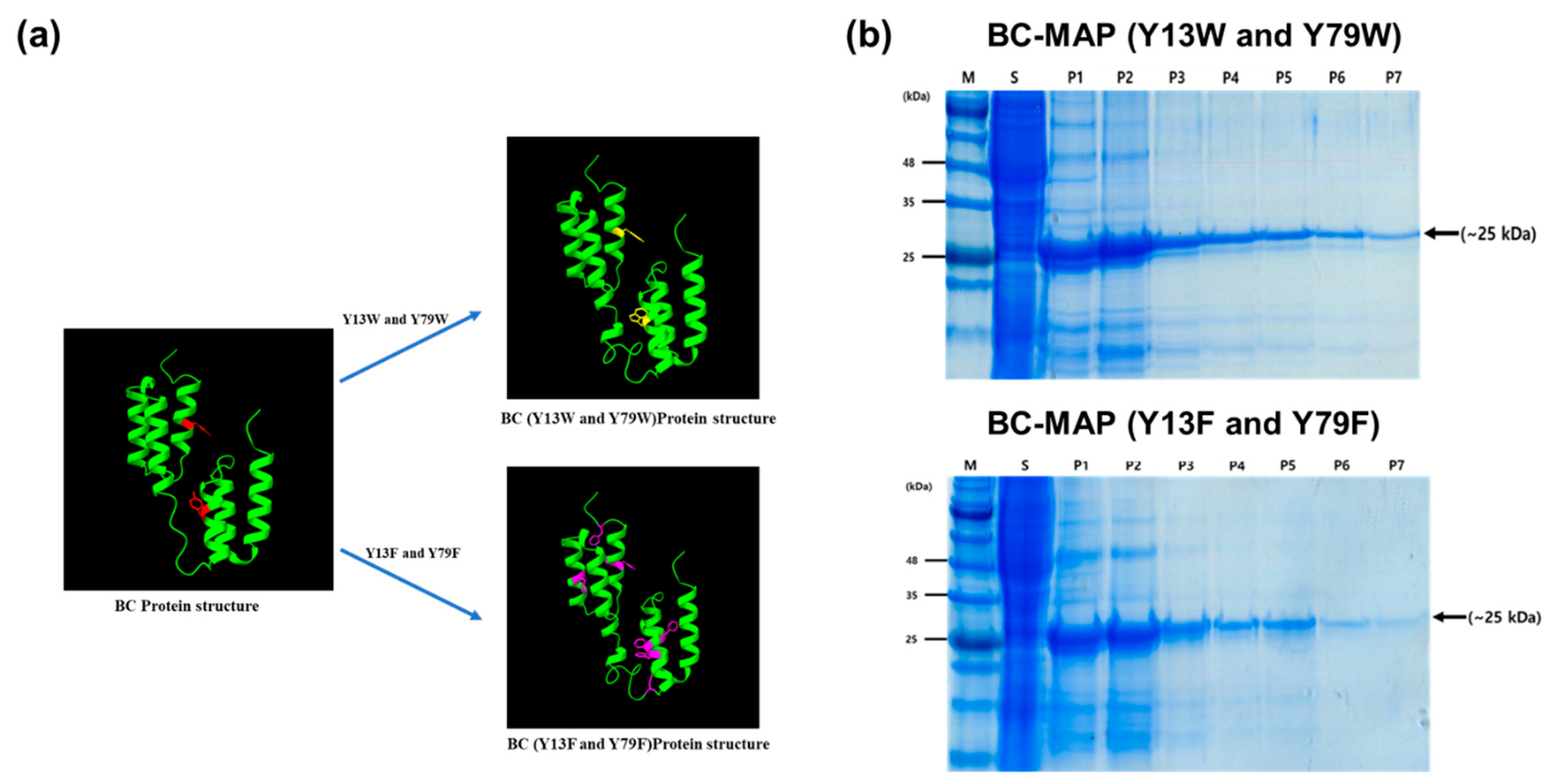
2.3. Expression and Purification of BC-MAP Fusion Protein
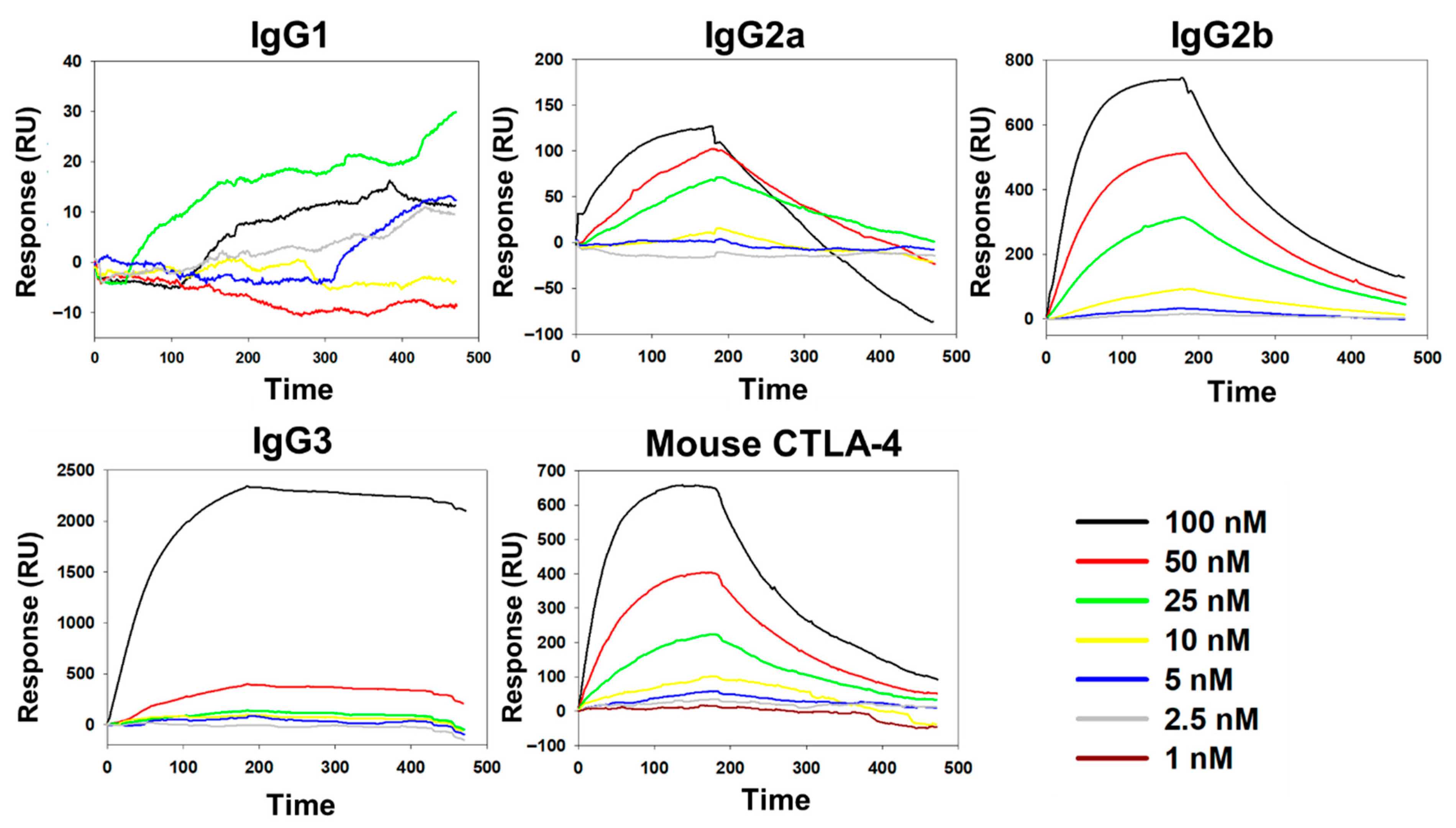
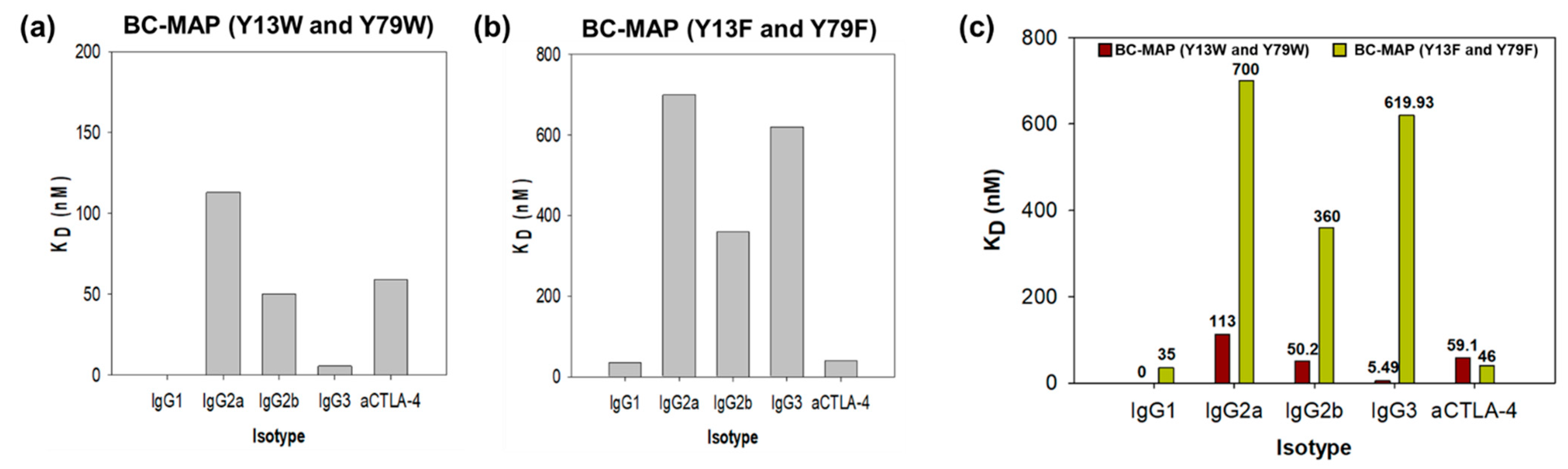
2.4. Antibody Binding Affinity of BC-MAP Proteins
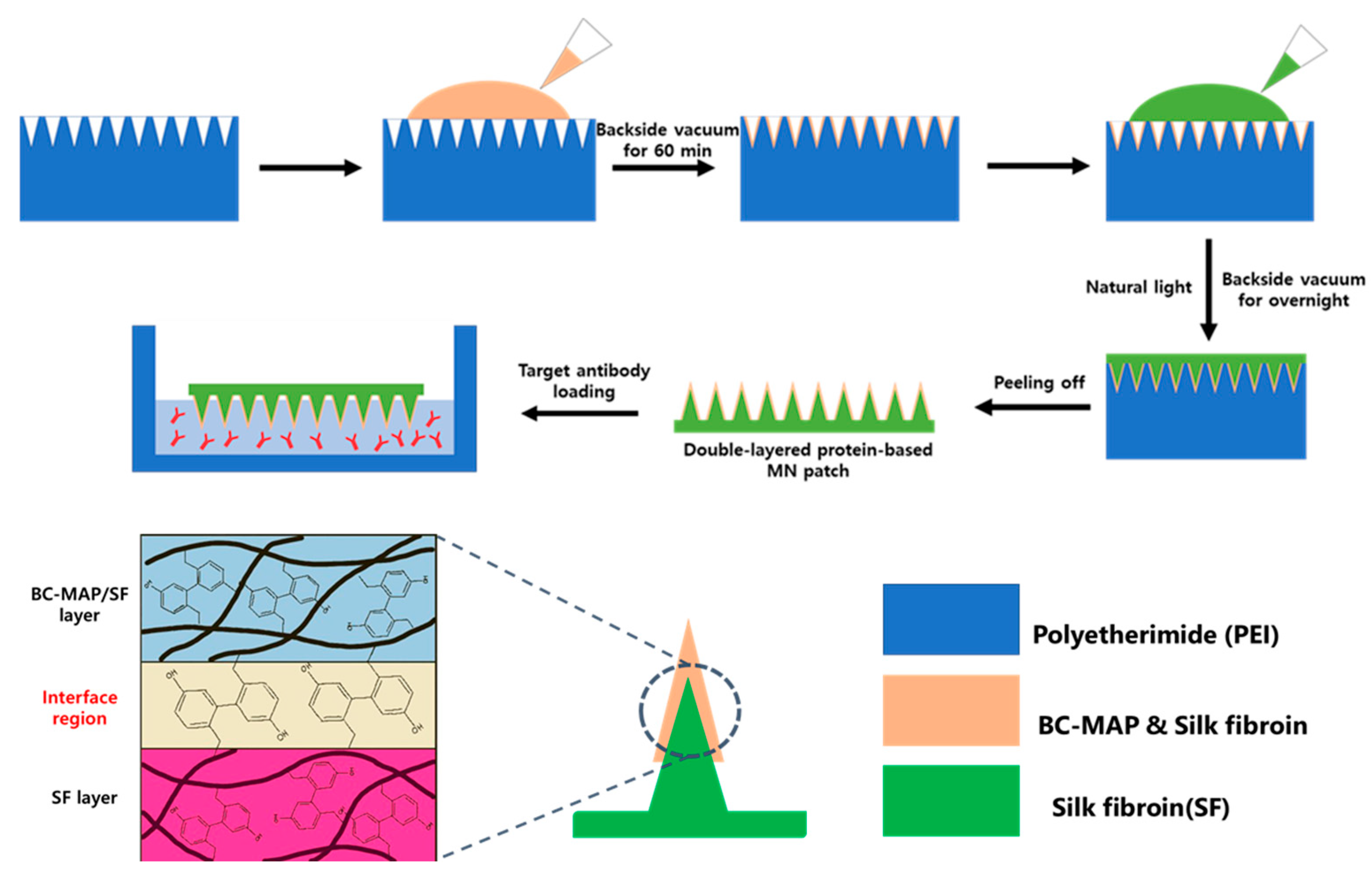
2.5. Fabrication and Characterization of the BC-MAP@SF Core–Shell Hydrogel MN Patch
2.6. In Vitro Cytocompatibility Assay
3. Results and Discussion
3.1. Production and Antibody Binding Affinity of BC-MAP Proteins
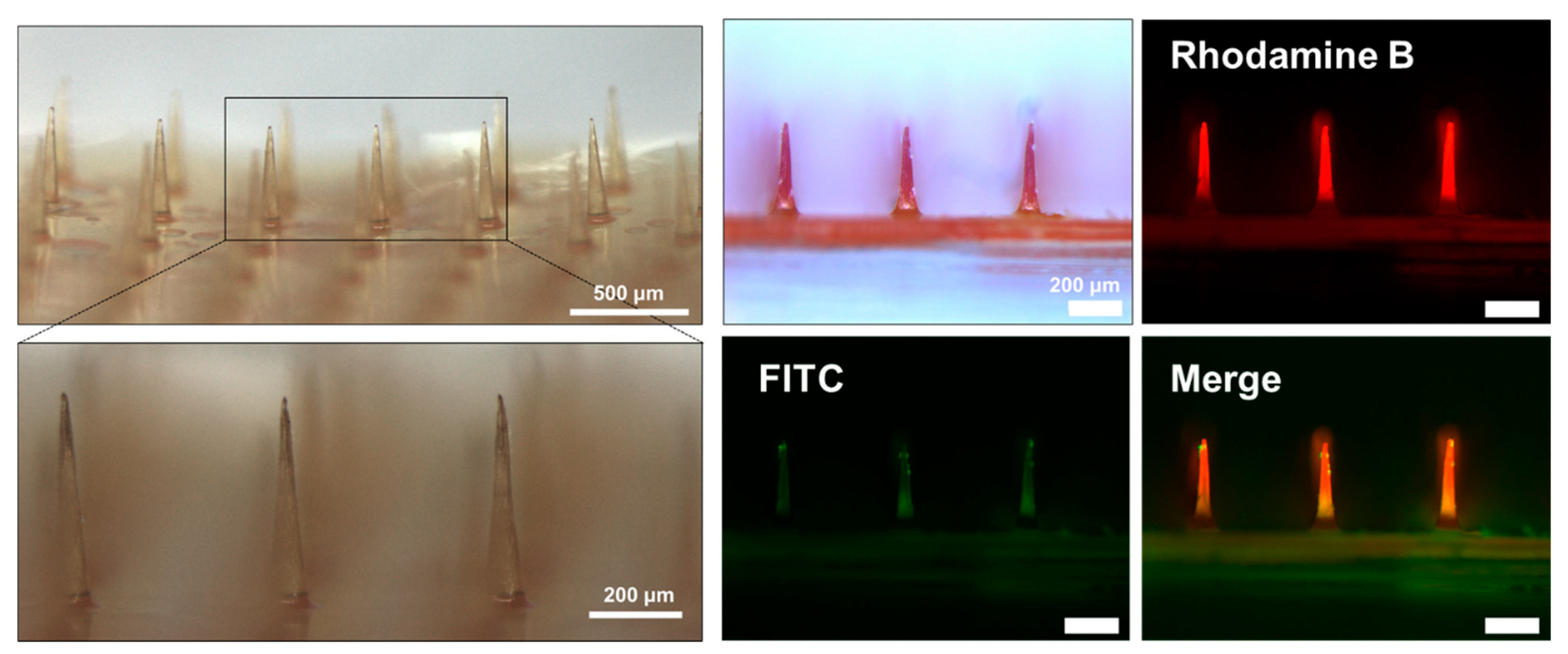
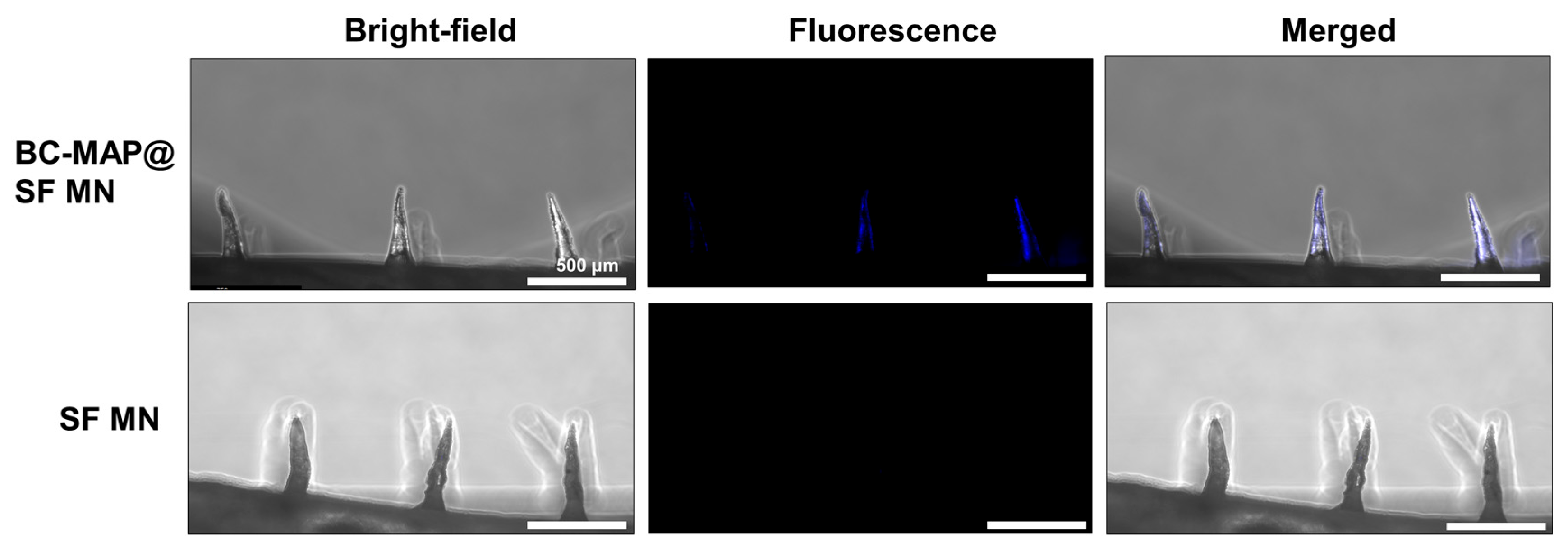
3.2. Fabrication of the BC-MAP@SF Core–Shell Hydrogel MN Patch Comprising
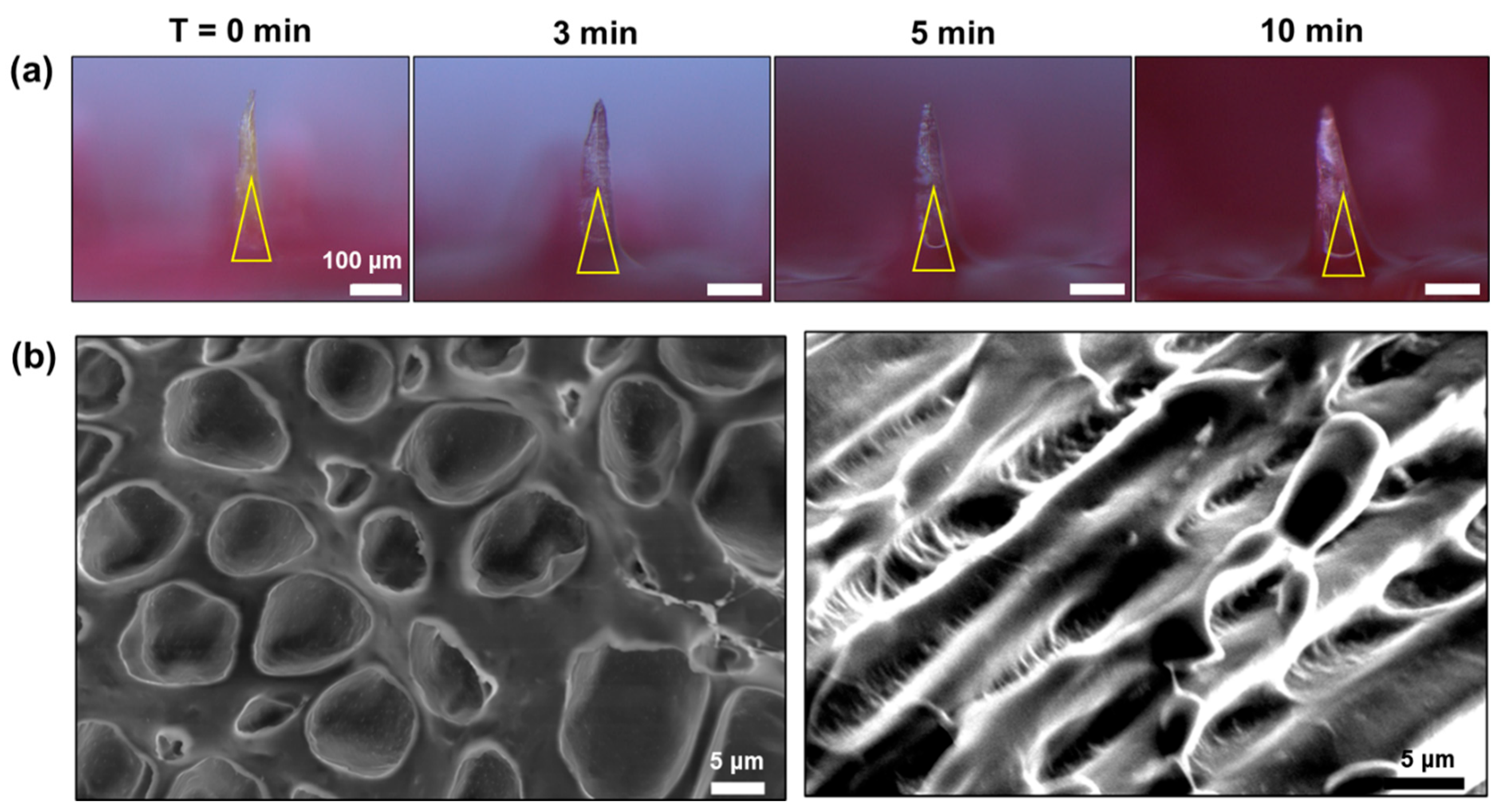
3.3. Swelling and Mechanical Properties of the BC-MAP@SF Core–Shell Hydrogel MN Patch
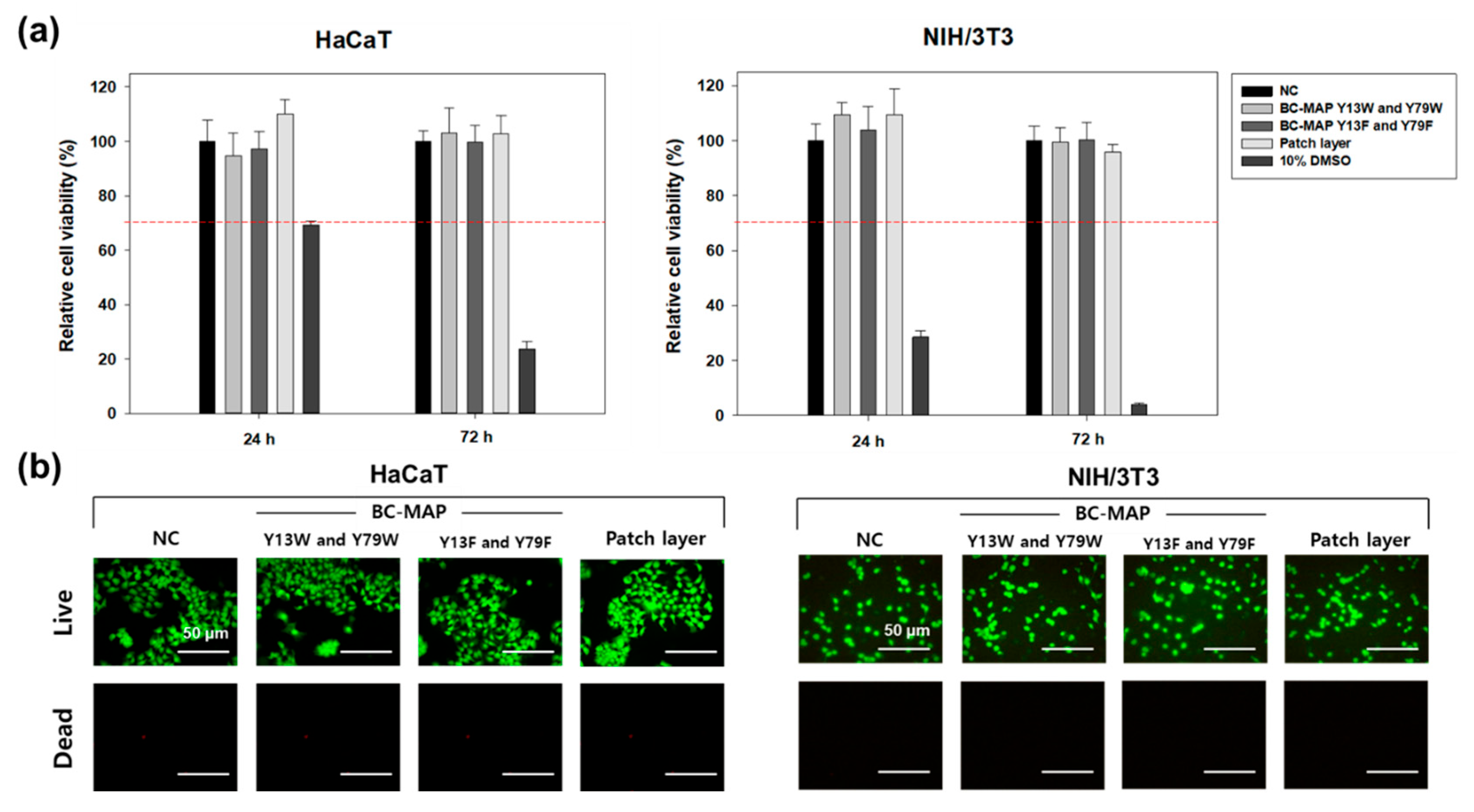
3.4. In Vitro Cytocompatibility of the BC-MAP@SF Core–Shell Hydrogel MN Patch
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez-Ramirez, M.A.; Soto, F.; Wang, C.; Rueda, R.; Shukla, S.; Silva-Lopez, C.; Kupor, D.; McBride, D.A.; Pokorski, J.K.; Nourhani, A.; et al. Built-in active microneedle patch with enhanced autonomous drug delivery. Adv. Mat. 2020, 32, 1905740. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.; Cao, J.; Luo, R.; Zhu, B.; Miao, R.; Yu, L.; Wang, X.; Li, W.; Fu, Y.; Zhang, J.; et al. Drug-loaded microneedle patches containing regulatory T-cell derived exosomes for psoriasis treatment. Acta Biomater. 2025, 198, 452–466. [Google Scholar] [CrossRef]
- Tang, Q.; Song, C.; Wu, X.; Chen, H.; Yu, C.; Zhao, Y.; Qian, X. Dual-functional core-shell microneedle patches for oral ulcers treatment. Chem. Eng. J. 2024, 500, 157041. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.T.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef]
- Wei, H.; Liu, S.; Tong, Z.; Chen, T.; Yang, M.; Guo, Y.; Sun, H.; Wu, Y.; Chu, Y.; Fan, L. Hydrogel-based microneedles of chitosan derivatives for drug delivery. React. Funct. Polym. 2022, 172, 105200. [Google Scholar] [CrossRef]
- Miao, M.; Wu, Q.; Zhou, X.; Wang, L.; Chen, L.; Zhu, J. Interfacing hydrogel microneedle patch for diagnosis. Surf. Interfaces 2024, 55, 105474. [Google Scholar] [CrossRef]
- Chi, Y.; Zheng, Y.; Pan, X.; Huang, Y.; Kang, Y.; Zhong, W.; Xu, K. Enzyme-mediated fabrication of nanocomposite hydrogel microneedles for tunable mechanical strength and controllable transdermal efficiency. Acta Biomater. 2024, 174, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Wei, C.; Feng, L.; Guo, R. Silk fibroin-based hydrogel microneedles delivery α-MSH to promote melanosome delivery for vitiligo treatment. ACS Biomater. Sci. Eng. 2023, 9, 3368–3378. [Google Scholar] [CrossRef]
- Tan, G.; Jiang, F.; Jia, T.; Qi, Z.; Xing, T.; Kundu, S.C.; Lu, S. Glucose-responsive silk fibroin microneedles for transdermal delivery of insulin. Biomimetics 2023, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.Y.; Lee, J.; Kim, B.J.; Joo, K.I.; Kim, K.H.; Lim, G.; Cha, H.J. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials 2019, 222, 119439. [Google Scholar] [CrossRef]
- Niu, L.; Chen, S.; Guo, X.; Feng, Y.; Wang, R. Physically cross-linked silk fibroin hydrogel with rapid sol-gel transition and enhanced mechanical performance. Macromol. Rapid Commun. 2025, 46, e2401016. [Google Scholar] [CrossRef]
- Lee, A.S.; Kim, S.M.; Kim, K.R.; Park, C.; Lee, D.-G.; Heo, H.R.; Cha, H.J.; Kim, C.S. A colorimetric lateral flow immunoassay based on oriented antibody immobilization for sensitive detection of SARS-CoV-2. Sens. Actuators B Chem. 2023, 379, 133245. [Google Scholar] [CrossRef]
- Yang, J.M.; Kim, K.R.; Jeon, S.; Cha, H.J.; Kim, C.S. A sensitive paper-based lateral flow immunoassay platform using engineered cellulose-binding protein linker fused with antibody-binding domains. Sens. Actuators B Chem. 2021, 329, 129099. [Google Scholar] [CrossRef]
- Makvandi, P.; Maleki, A.; Shabani, M.; Hutton, A.R.J.; Kirkby, M.; Jamaledin, R.; Fang, T.; He, J.; Lee, J.; Mazzolai, B.; et al. Bioinspired microneedle patches: Biomimetic designs, fabrication, and biomedical applications. Matter 2022, 5, 390–429. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Partlow, B.P.; Applegate, M.B.; Omenetto, F.G.; Kaplan, D.L. Dityrosine cross-linking in designing biomaterials. ACS Biomater. Sci. Eng. 2016, 2, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.; Korner, S.; Zintel, L.; Hubbuch, J. Changing mechanical properties of photopolymerized, dityrosine-crosslinked protein-based hydrogles. Front. Bioeng. Biotechnol. 2022, 10, 1006438. [Google Scholar] [CrossRef]
- Zhao, X.; Ming, H.; Wang, Y.; Luo, F.; Li, Z.; Li, J.; Tan, H.; Fu, Q. Mussel-inspired, injectable polyurethane tissue adhesives demonstrate in situ gel formation under mild conditions. ACS Appl. Bio Mater. 2021, 4, 5352–5361. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef]
- Dong, J.; Kojima, T.; Ohashi, H.; Ueda, H. Optimal fusion of antibody binding domains resulted in higher affinity and wider specificity. J. Biosci. Bioeng. 2015, 120, 504–509. [Google Scholar] [CrossRef]
- Makvandi, P.; Kirkby, M.; Hutton, A.R.J.; Shabani, M.; Yiu, C.K.Y.; Baghbantaraghdari, Z.; Jamaledin, R.; Carlotti, M.; Mazzolai, B.; Mattoli, V.; et al. Engineering microneedle patches for improved penetration: Analysis, skin models and factors affecting needle insertion. Nanomicro Lett. 2021, 13, 93. [Google Scholar] [CrossRef]
- Liu, C.; Hua, J.; Ng, P.F.; Wang, Y.; Fei, B.; Shao, Z. Bioinspired photo-cross-linking of stretched solid silks for enhanced strength. ACS Biomater. Sci. Eng. 2022, 8, 484–492. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Tailoring swelling-shrinkable behavior of hydrogels for biomedical applications. Adv. Sci. 2023, 10, 2303326. [Google Scholar] [CrossRef]
- Yadav, P.R.; Nasiri, M.I.; Vora, L.K.; Larraneta, E.; Donnelly, R.F.; Pattanayek, S.K.; Das, D.B. Super-swelling hydrogel-forming microneedle based transdermal drug delivery: Mathmatical modelling, simulation and experimental validation. Int. J. Pharm. 2022, 622, 121835. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, S.I.; Nguyen, L.Q.; Bøgh, K.L.; Keller, S.S. Dermal tissue penetration of in-plane silicon microneedles evaluated in skin-simulating hydrogel. Biomater. Adv. 2023, 155, 213659. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004, 37, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; O’Cearbhaill, E.D.; Sisk, G.C.; Park, K.M.; Cho, W.K.; Villiger, M.; Bouma, B.E.; Pomahac, B.; Karp, J.M. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Commun. 2013, 4, 1702. [Google Scholar] [CrossRef]
- Rojas, J.E.U.; de Oliveira, V.L.; de Araujo, D.R.; Tofoli, G.R.; de Oliveira, M.M.; Carastan, D.J.; Palaci, M.; Giuntini, F.; Alves, W.A. Silk fibroin/poly(vinyl alcohol) microneedle as carriers for the delivery of singlet oxygen photosensitizers. ACS Biomater. Sci. Eng. 2022, 8, 128–139. [Google Scholar] [CrossRef]
- Contardi, M.; Ayyoub, A.M.M.; Summa, M.; Kossyvaki, D.; Fadda, M.; Liessi, N.; Armirotti, A.; Fragouli, D.; Bertorelli, R.; Athanassiou, A. Self-adhesive and antioxidant poly(vinylpyrrolidone)/alginate-based bilayer films loaded with Malva sylvestris extracts as potential skin dressings. ACS Appl. Bio Mater. 2022, 5, 2880–2893. [Google Scholar] [CrossRef]
- Kim, T.; Kim, M.; Kim, N.; Linh, N.V.; Doan, H.V.; Kim, Y.; Park, S.; Jung, W. Multifunctional microneedle patch with diphlorethohydroxycarmalol for potential wound dressing. Tissue Eng. Regen. Med. 2024, 21, 1007–1019. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.K.; Lee, A.S.; Kim, C.S. Core–Shell Silk Fibroin Hydrogel Microneedles Functionalized with Antibody-Binding Domains for Transdermal Delivery. Biomimetics 2025, 10, 798. https://doi.org/10.3390/biomimetics10120798
Lee MK, Lee AS, Kim CS. Core–Shell Silk Fibroin Hydrogel Microneedles Functionalized with Antibody-Binding Domains for Transdermal Delivery. Biomimetics. 2025; 10(12):798. https://doi.org/10.3390/biomimetics10120798
Chicago/Turabian StyleLee, Min Ki, Ae Sol Lee, and Chang Sup Kim. 2025. "Core–Shell Silk Fibroin Hydrogel Microneedles Functionalized with Antibody-Binding Domains for Transdermal Delivery" Biomimetics 10, no. 12: 798. https://doi.org/10.3390/biomimetics10120798
APA StyleLee, M. K., Lee, A. S., & Kim, C. S. (2025). Core–Shell Silk Fibroin Hydrogel Microneedles Functionalized with Antibody-Binding Domains for Transdermal Delivery. Biomimetics, 10(12), 798. https://doi.org/10.3390/biomimetics10120798






