Eco-Friendly Hydrogels Loading Polyphenols-Composed Biomimetic Micelles for Topical Administration of Resveratrol and Rutin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Resveratrol- and Rutin-Loaded Biomimetic Micelles
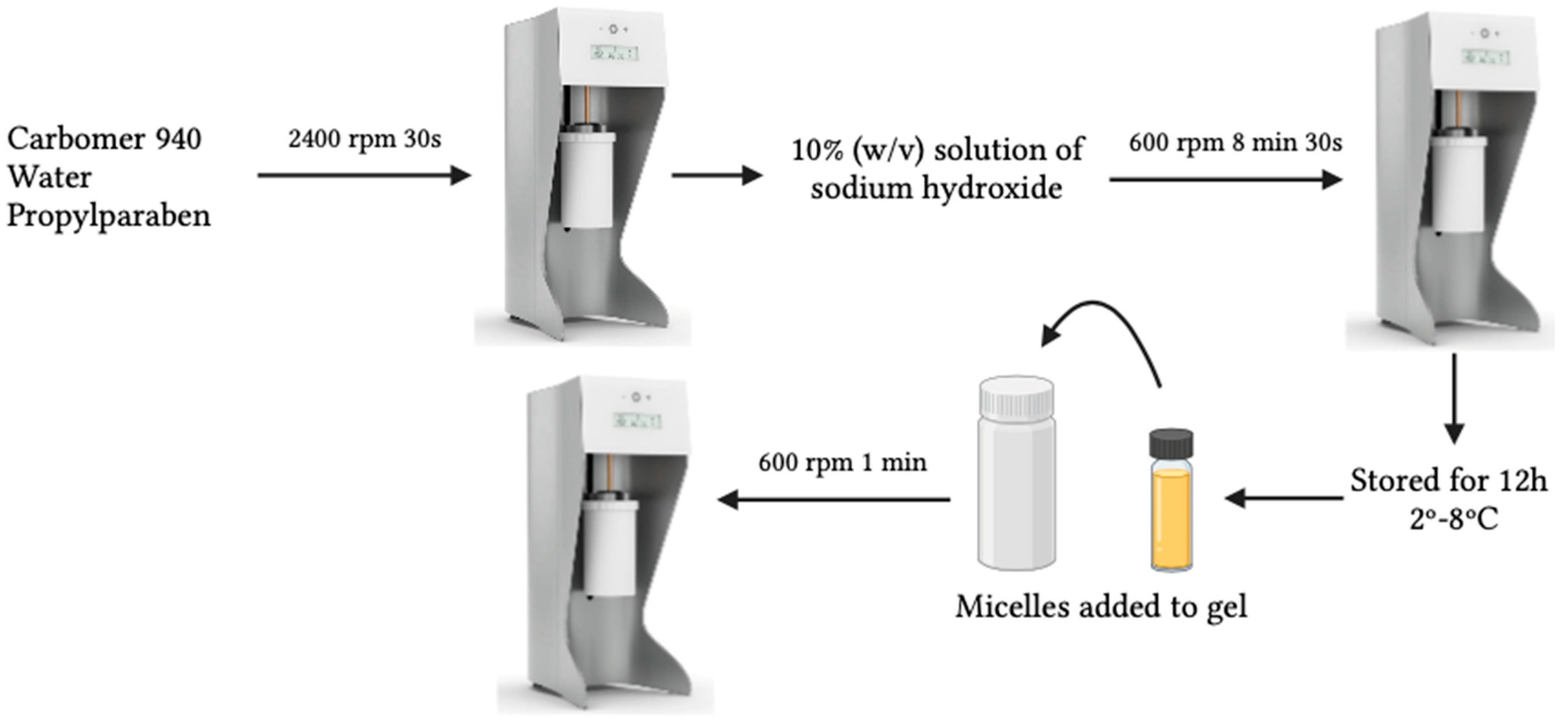
2.3. Production of Resveratrol- and Rutin-Loaded Micelles Composed Hydrogels
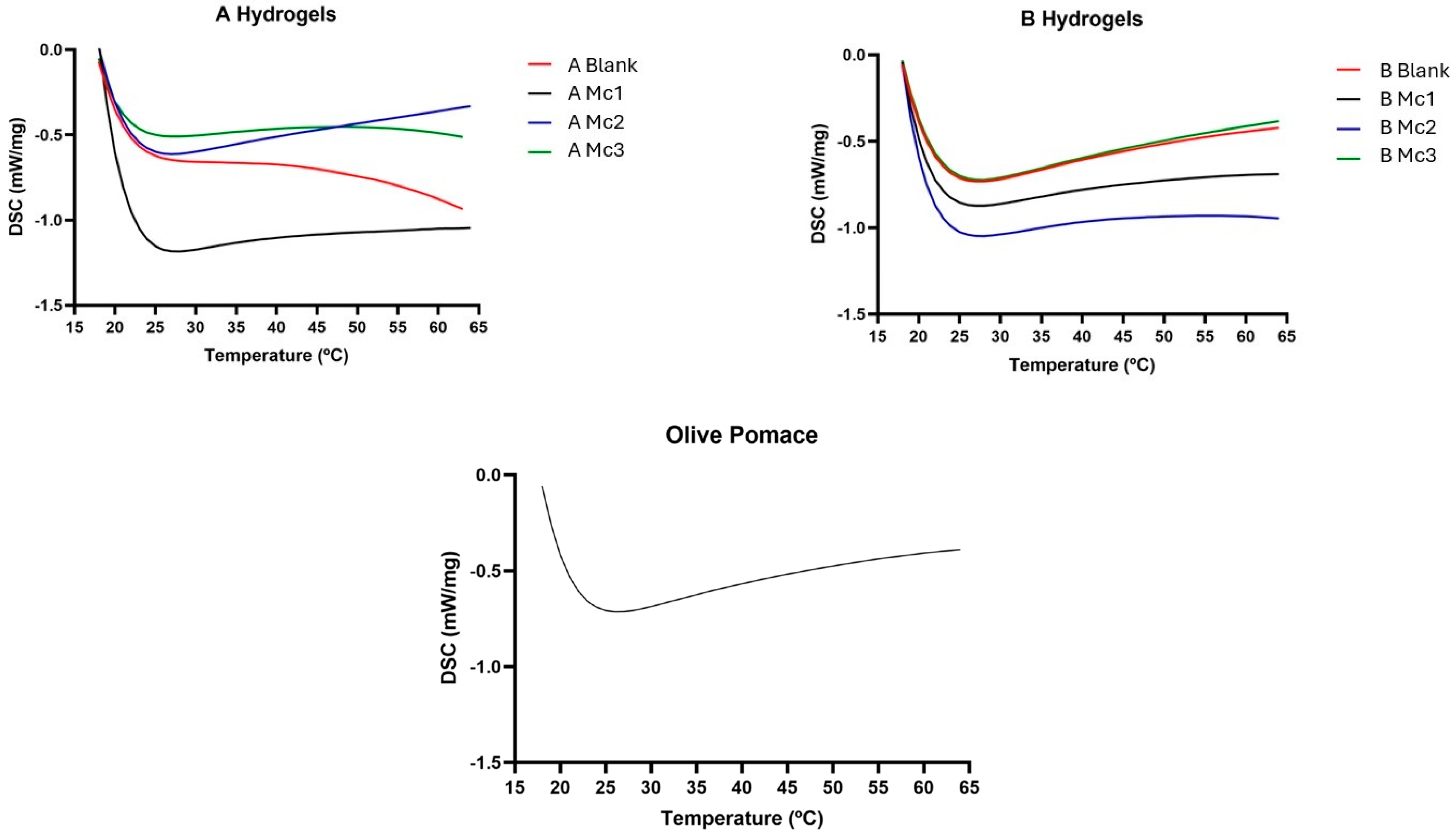
2.4. Differential Scanning Calorimetry
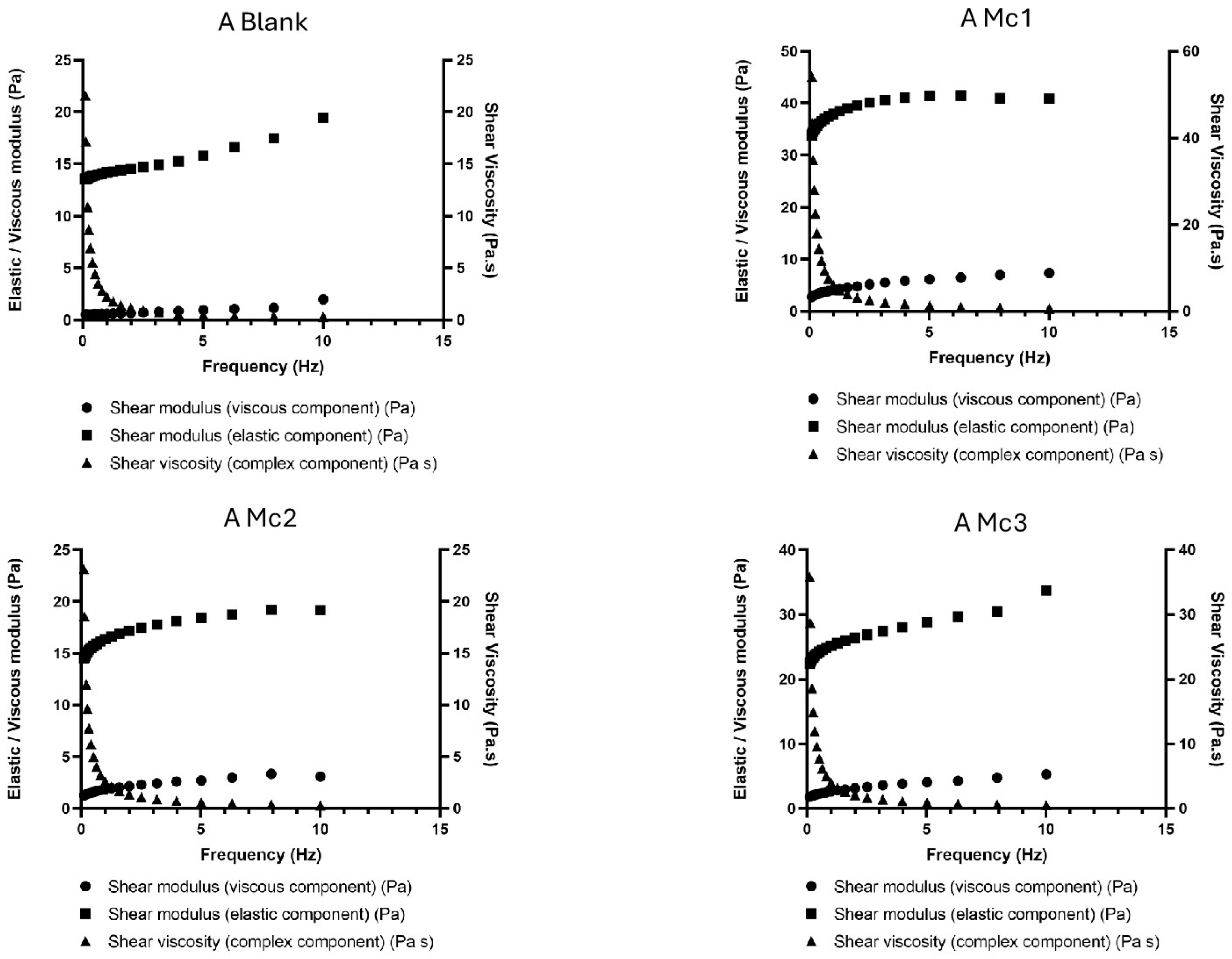
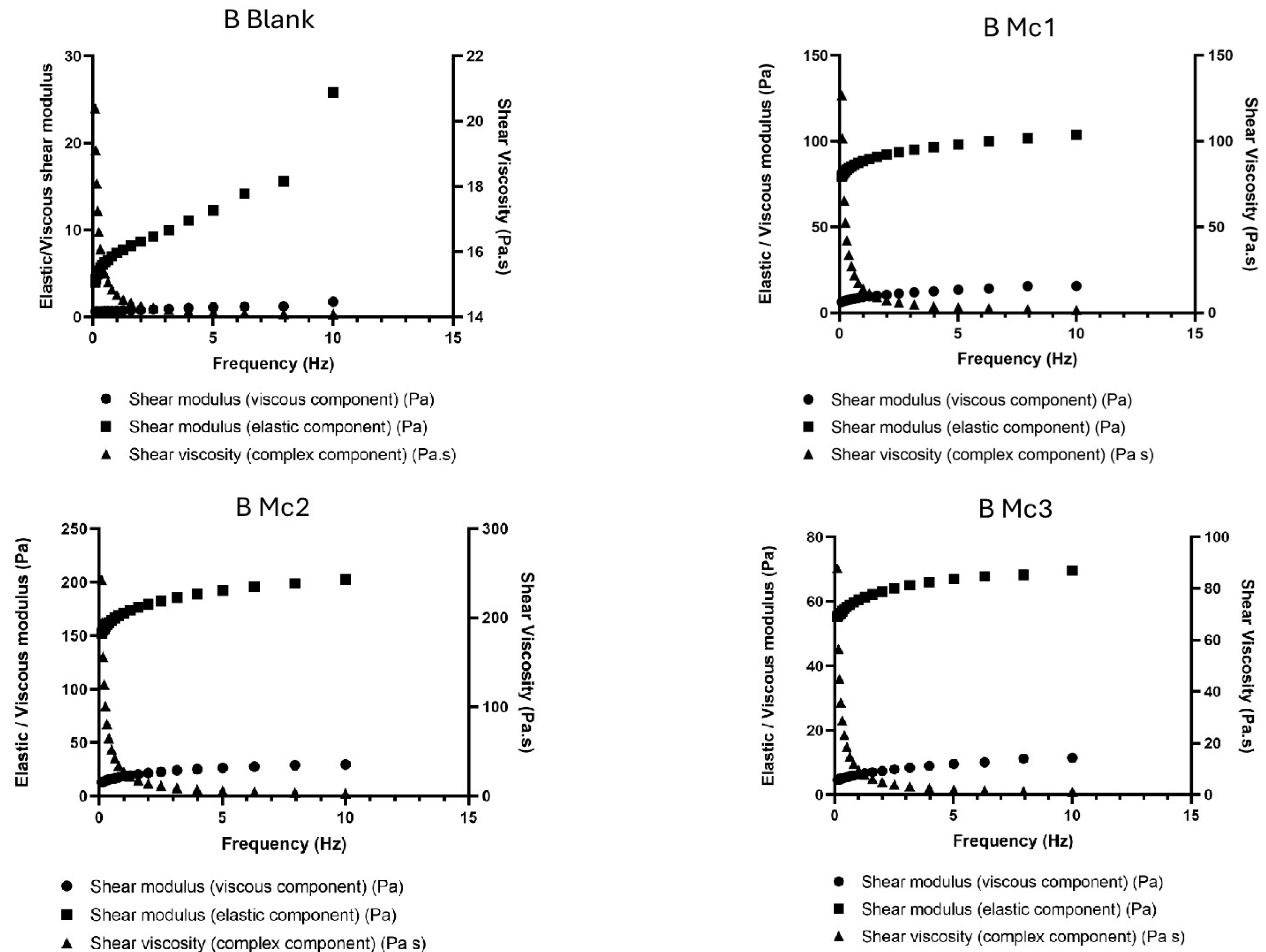
2.5. Rheological Analysis
2.6. Texture Analysis
3. Results and Discussion
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Fan, X.; Domszy, R.; Hu, N.; Wang, N.S.; Yang, A. Hydrogel-based ocular drug delivery systems for hydrophobic drugs. Eur. J. Pharm. Sci. 2020, 154, 105503. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Song, C.; Wang, C.; Hu, Y.; Wu, J. Hydrogel-Based Controlled Drug Delivery for Cancer Treatment: A Review. Mol. Pharm. 2020, 17, 373–391. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. In The Road from Nanomedicine to Precision Medicine; Taylor & Francis Group: Abingdon, UK, 2020; pp. 1117–1150. [Google Scholar]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef] [PubMed]
- Amaral, V.A.; Santana, V.L.; Lisboa, E.S.; Martins, F.S.; Chaud, M.V.; de Albuquerque-Junior, R.L.C.; Santana, W.; Santos, C.; de Jesus Santos, A.; Cardoso, J.C.; et al. Chitosan membranes incorporating Aloe vera glycolic extract with joint synthesis of silver nanoparticles for the treatment of skin lesions. In Drug Delivery and Translational Research; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Francesko, A.; Petkova, P.; Tzanov, T. Hydrogel Dressings for Advanced Wound Management. Curr. Med. Chem. 2018, 25, 5782–5797. [Google Scholar] [CrossRef]
- Genesi, B.P.; de Melo Barbosa, R.; Severino, P.; Rodas, A.C.D.; Yoshida, C.M.P.; Mathor, M.B.; Lopes, P.S.; Viseras, C.; Souto, E.B.; Ferreira da Silva, C. Aloe vera and copaiba oleoresin-loaded chitosan films for wound dressings: Microbial permeation, cytotoxicity, and in vivo proof of concept. Int. J. Pharm. 2023, 634, 122648. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, B.M.; Dos Santos, I.D.D.; de Carvalho, F.M.A.; Correa, L.C.; Cunha, J.L.S.; Dariva, C.; Severino, P.; Cardoso, J.C.; Souto, E.B.; de Albuquerque-Junior, R.L.C. Himatanthus bracteatus-Composed In Situ Polymerizable Hydrogel for Wound Healing. Int. J. Mol. Sci. 2022, 23, 15176. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Nascimento, M.F.; de Almeida, B.M.; Alves, M.M.A.; Lima-Verde, I.B.; Costa, D.S.; Araujo, D.C.M.; de Paula, M.N.; Mello, J.C.P.; Cano, A.; et al. Hydrophilic Scaffolds Containing Extracts of Stryphnodendron adstringens and Abarema cochliacarpa for Wound Healing: In Vivo Proofs of Concept. Pharmaceutics 2022, 14, 2150. [Google Scholar] [CrossRef]
- do Nascimento, M.F.; Cardoso, J.C.; Santos, T.S.; Tavares, L.A.; Pashirova, T.N.; Severino, P.; Souto, E.B.; Albuquerque-Junior, R.L.C. Development and Characterization of Biointeractive Gelatin Wound Dressing Based on Extract of Punica granatum Linn. Pharmaceutics 2020, 12, 1204. [Google Scholar] [CrossRef]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound dressings. Bmj 2006, 332, 777–780. [Google Scholar] [CrossRef]
- Diniz, F.R.; Maia, R.; de Andrade, L.R.M.; Andrade, L.N.; Vinicius Chaud, M.; da Silva, C.F.; Correa, C.B.; de Albuquerque Junior, R.L.C.; Pereira da Costa, L.; Shin, S.R.; et al. Correction: Diniz et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2020, 12, 4071. [Google Scholar] [CrossRef]
- Oliveira, D.M.L.; Rezende, P.S.; Barbosa, T.C.; Andrade, L.N.; Bani, C.; Tavares, D.S.; da Silva, C.F.; Chaud, M.V.; Padilha, F.; Cano, A.; et al. Double membrane based on lidocaine-coated polymyxin-alginate nanoparticles for wound healing: In vitro characterization and in vivo tissue repair. Int. J. Pharm. 2020, 591, 120001. [Google Scholar] [CrossRef] [PubMed]
- Diniz, F.R.; Maia, R.C.A.P.; Andrade, L.R.; Andrade, L.N.; Chaud, M.V.; Da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; Da Costa, L.P.; Shin, S.R.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite Hydrogels: 3D Polymer–Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Karczewski, J.; Eder, P.; Kolanowski, T.; Szalata, M.; Wielgus, K.; Szalata, M.; Kim, D.; Shin, S.R.; Slomski, R.; et al. Scaffolds for drug delivery and tissue engineering: The role of genetics. J. Control. Release 2023, 359, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Martín-Belloso, O.; McClements, D.J. Excipient nanoemulsions for improving oral bioavailability of bioactives. Nanomaterials 2016, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kesharwani, P. Advanced nanomedicine approaches applied for treatment of skin carcinoma. J. Control. Release 2021, 337, 589–611. [Google Scholar] [CrossRef]
- Pool, H.; Mendoza, S.; Xiao, H.; McClements, D.J. Encapsulation and release of hydrophobic bioactive components in nanoemulsion-based delivery systems: Impact of physical form on quercetin bioaccessibility. Food Funct. 2013, 4, 162–174. [Google Scholar] [CrossRef] [PubMed]
- van Hoogevest, P.; Fahr, A. Phospholipids in Cosmetic Carriers. In Nanocosmetics: From Ideas to Products; Cornier, J., Keck, C.M., Van de Voorde, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 95–140. [Google Scholar] [CrossRef]
- Francioso, A.; Mastromarino, P.; Masci, A.; d’Erme, M.; Mosca, L. Chemistry, stability and bioavailability of resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Gullon, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, C.; Chen, X.; Yang, Z. Zinc oxide nanoparticles induce renal toxicity through reactive oxygen species. Food Chem. Toxicol. 2016, 90, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Tomoiagǎ, L.L.; Macovei, Ş.O.; Mǎgureanu, D.C.; Iliescu, M.L.; Bocsan, I.C.; Buzoianu, A.D.; Voşloban, C.M.; Pop, R.M. Antioxidant/Pro-Oxidant Actions of Polyphenols from Grapevine and Wine By-Products-Base for Complementary Therapy in Ischemic Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 750508. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxidative Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Rodrigues, F.; Pimentel, F.B.; Oliveira, M.B.P. Olive by-products: Challenge application in cosmetic industry. Ind. Crops Prod. 2015, 70, 116–124. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Thielmann, J.; Kohnen, S.; Hauser, C. Antimicrobial activity of Olea europaea Linné extracts and their applicability as natural food preservative agents. Int. J. Food Microbiol. 2017, 251, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Dahdouh, A.; Khay, I.; Le Brech, Y.; El Maakoul, A.; Bakhouya, M. Olive oil industry: A review of waste stream composition, environmental impacts, and energy valorization paths. Environ. Sci. Pollut. Res. Int. 2023, 30, 45473–45497. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Nunes, M.A.; Costa, A.S.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid-and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.; Alves, R.C.; Oliveira, M.B.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Miralles, P.; Chisvert, A.; Salvador, A. Determination of hydroxytyrosol and tyrosol by liquid chromatography for the quality control of cosmetic products based on olive extracts. J. Pharm. Biomed. Anal. 2015, 102, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Soto, M.; Redhu, D.; Sánchez-Hidalgo, M.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C.; Worm, M.; Babina, M. Olive-Oil-Derived Polyphenols Effectively Attenuate Inflammatory Responses of Human Keratinocytes by Interfering with the NF-κB Pathway. Mol. Nutr. Food Res. 2019, 63, 1900019. [Google Scholar] [CrossRef] [PubMed]
- Carito, V.; Ciafrh, S.; Tarani, L.; Ceccanti, M.; Natella, F.; Iannitelli, A.; Tirassa, P.; Chaldakov, G.N.; Ceccanti, M.; Boccardo, C. TNF-α and IL-10 modulation induced by polyphenols extracted by olive pomace in a mouse model of paw inflammation. Ann. Dell’Istituto Super. Sanità 2015, 51, 382–386. [Google Scholar]
- Yonezawa, Y.; Miyashita, T.; Nejishima, H.; Takeda, Y.; Imai, K.; Ogawa, H. Anti-inflammatory effects of olive-derived hydroxytyrosol on lipopolysaccharide-induced inflammation in RAW264. 7 cells. J. Vet. Med. Sci. 2018, 80, 1801–1807. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tsatalas, P.; Galanakis, I.M. Phenols from olive mill wastewater and other natural antioxidants as UV filters in sunscreens. Environ. Technol. Innov. 2018, 9, 160–168. [Google Scholar] [CrossRef]
- Salucci, S.; Burattini, S.; Curzi, D.; Buontempo, F.; Martelli, A.M.; Zappia, G.; Falcieri, E.; Battistelli, M. Antioxidants in the prevention of UVB-induced keratynocyte apoptosis. J. Photochem. Photobiol. B Biol. 2014, 141, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol exerts anti-inflammatory and anti-oxidant activities in a mouse model of systemic inflammation. Molecules 2018, 23, 3212. [Google Scholar] [CrossRef]
- Jeon, S.; Choi, M. Anti-inflammatory and anti-aging effects of hydroxytyrosol on human dermal fibroblasts (HDFs). Biomed. Dermatol. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Guo, W.; An, Y.; Jiang, L.; Geng, C.; Zhong, L. The protective effects of hydroxytyrosol against UVB-induced DNA damage in HaCaT cells. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 352–359. [Google Scholar] [CrossRef]
- Salucci, S.; Burattini, S.; Battistelli, M.; Buontempo, F.; Canonico, B.; Martelli, A.M.; Papa, S.; Falcieri, E. Tyrosol prevents apoptosis in irradiated keratinocytes. J. Dermatol. Sci. 2015, 80, 61–68. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tsatalas, P.; Galanakis, I.M. Implementation of phenols recovered from olive mill wastewater as UV booster in cosmetics. Ind. Crops Prod. 2018, 111, 30–37. [Google Scholar] [CrossRef]
- Siddique, M.I.; Katas, H.; Jamil, A.; Mohd Amin, M.C.I.; Ng, S.-F.; Zulfakar, M.H.; Nadeem, S.M. Potential treatment of atopic dermatitis: Tolerability and safety of cream containing nanoparticles loaded with hydrocortisone and hydroxytyrosol in human subjects. Drug Deliv. Transl. Res. 2019, 9, 469–481. [Google Scholar] [CrossRef]
- Guedes, B.N.; Bahu, J.O.; Concha, V.O.C.; Andreani, T.; Oliveira, M.B.P.P.; Fathi, F.; Souto, E.B. Rutin-loaded micelles: Design and characterization of a new hybrid delivery system for pharmaceutical applications. Chem. Eng. Res. Des. 2024. under review. [Google Scholar]
- Souto, E.; Almeida, A.; Müller, R. Lipid nanoparticles (SLN®, NLC®) for cutaneous drug delivery: Structure, protection and skin effects. J. Biomed. Nanotechnol. 2007, 3, 317–331. [Google Scholar] [CrossRef]
- Souto, E.; Wissing, S.; Barbosa, C.; Müller, R. Evaluation of the physical stability of SLN and NLC before and after incorporation into hydrogel formulations. Eur. J. Pharm. Biopharm. 2004, 58, 83–90. [Google Scholar] [CrossRef]
- Varges, P.R.; Costa, C.M.; Fonseca, B.S.; Naccache, M.F.; De Souza Mendes, P.R. Rheological Characterization of Carbopol® Dispersions in Water and in Water/Glycerol Solutions. Fluids 2019, 4, 3. [Google Scholar] [CrossRef]
- Souto, E.B.; Gohla, S.H.; Müller, R.H. Rheology of nanostructured lipid carriers (NLC) suspended in a viscoelastic medium. Die Pharm. 2005, 60, 671–673. [Google Scholar]
- Lippacher, A.; Müller, R.; Mäder, K. Preparation of semisolid drug carriers for topical application based on solid lipid nanoparticles. Int. J. Pharm. 2001, 214, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Raposo, S.; Salgado, A.; Eccleston, G.; Urbano, M.; Ribeiro, H.M. Cold processed oil-in-water emulsions for dermatological purpose: Formulation design and structure analysis. Pharm. Dev. Technol. 2014, 19, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.B.D.S.; Da Silva, J.B.; Borghi-Pangoni, F.B.; Junqueira, M.V.; Bruschi, M.L. Linear correlation between rheological, mechanical and mucoadhesive properties of polycarbophil polymer blends for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 68, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Dawre, S.; Waghela, S.; Saraogi, G. Statistically designed vitamin D3 Encapsulated PLGA microspheres dispersed in thermoresponsive in-situ gel for nasal delivery. J. Drug Deliv. Sci. Technol. 2022, 75, 103688. [Google Scholar] [CrossRef]
- Teng, L.; Chin, N.; Yusof, Y. Rheological and textural studies of fresh and freeze-thawed native sago starch–sugar gels. I. Optimisation using response surface methodology. Food Hydrocoll. 2011, 25, 1530–1537. [Google Scholar] [CrossRef]
- Rodrigues, R.; Alves, R.C.; Oliveira, M.B.P.P. Exploring Olive Pomace for Skincare Applications: A Review. Cosmetics 2023, 10, 35. [Google Scholar] [CrossRef]


| Sample |
Resveratrol
(%, w/w) |
Rutin
(%, w/w) | Soy Lecithin (%, w/w) |
Polysorbate 80
(%, w/w) |
Span 80
(%, w/w) |
Water ad.
(%, w/w) |
|---|---|---|---|---|---|---|
| Mc1 | 0.10 | - | 4.90 | 1.00 | - | 100 |
| Mc2 | - | 0.10 | 4.90 | 0.60 | 0.400 | 100 |
| Mc3 | 0.05 | 0.05 | 4.90 | 1.00 | - | 100 |
| Carbomer 940 (g) | 10% Sodium Hydroxide 0.1 M (mL; w/v) | Propylparaben (g) | Purified Water ad (g) |
|---|---|---|---|
| 2 | 3.2 | 1.5 | 100 |
| Materials | A Blank Hydrogel | A Mc1 | A Mc2 | A Mc3 | B Blank Hydrogel | B Mc1 | B Mc2 | B Mc3 |
|---|---|---|---|---|---|---|---|---|
| 2% Carbomer 940 (g) | 29.85 | 15 | 15 | 15 | 30 | 15 | 15 | 15 |
| Olive Pomace (g) | 0.15 | 0.15 | 0.15 | 0.15 | - | - | - | - |
| Resveratrol-loaded micelles (g) | - | 14.85 | - | - | - | 15 | - | - |
| Rutin-loaded micelles (g) | - | 14.85 | - | - | - | 15 | - | |
| Resveratrol + Rutin-loaded micelles (g) | - | - | - | 14.85 | - | - | - | 15 |
| Parameters | A Blank | A Mc1 | A Mc2 | A Mc3 | B Blank | B Mc1 | B Mc2 | B Mc3 |
|---|---|---|---|---|---|---|---|---|
| Firmness (N) | 0.3305 | 0.2628 | 0.2470 | 0.2323 | 0.3049 | 0.2592 | 0.2745 | 0.2886 |
| Cohesiveness (N) | −0.1328 | −0.1536 | −0.1391 | −0.1302 | −0.1427 | −0.1416 | −0.1538 | −0.1341 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guedes, B.N.; Andreani, T.; Oliveira, M.B.P.P.; Fathi, F.; Souto, E.B. Eco-Friendly Hydrogels Loading Polyphenols-Composed Biomimetic Micelles for Topical Administration of Resveratrol and Rutin. Biomimetics 2025, 10, 8. https://doi.org/10.3390/biomimetics10010008
Guedes BN, Andreani T, Oliveira MBPP, Fathi F, Souto EB. Eco-Friendly Hydrogels Loading Polyphenols-Composed Biomimetic Micelles for Topical Administration of Resveratrol and Rutin. Biomimetics. 2025; 10(1):8. https://doi.org/10.3390/biomimetics10010008
Chicago/Turabian StyleGuedes, Beatriz N., Tatiana Andreani, M. Beatriz P. P. Oliveira, Faezeh Fathi, and Eliana B. Souto. 2025. "Eco-Friendly Hydrogels Loading Polyphenols-Composed Biomimetic Micelles for Topical Administration of Resveratrol and Rutin" Biomimetics 10, no. 1: 8. https://doi.org/10.3390/biomimetics10010008
APA StyleGuedes, B. N., Andreani, T., Oliveira, M. B. P. P., Fathi, F., & Souto, E. B. (2025). Eco-Friendly Hydrogels Loading Polyphenols-Composed Biomimetic Micelles for Topical Administration of Resveratrol and Rutin. Biomimetics, 10(1), 8. https://doi.org/10.3390/biomimetics10010008








