Rheological Characterization and Printability of Sodium Alginate–Gelatin Hydrogel for 3D Cultures and Bioprinting
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Hydrogel
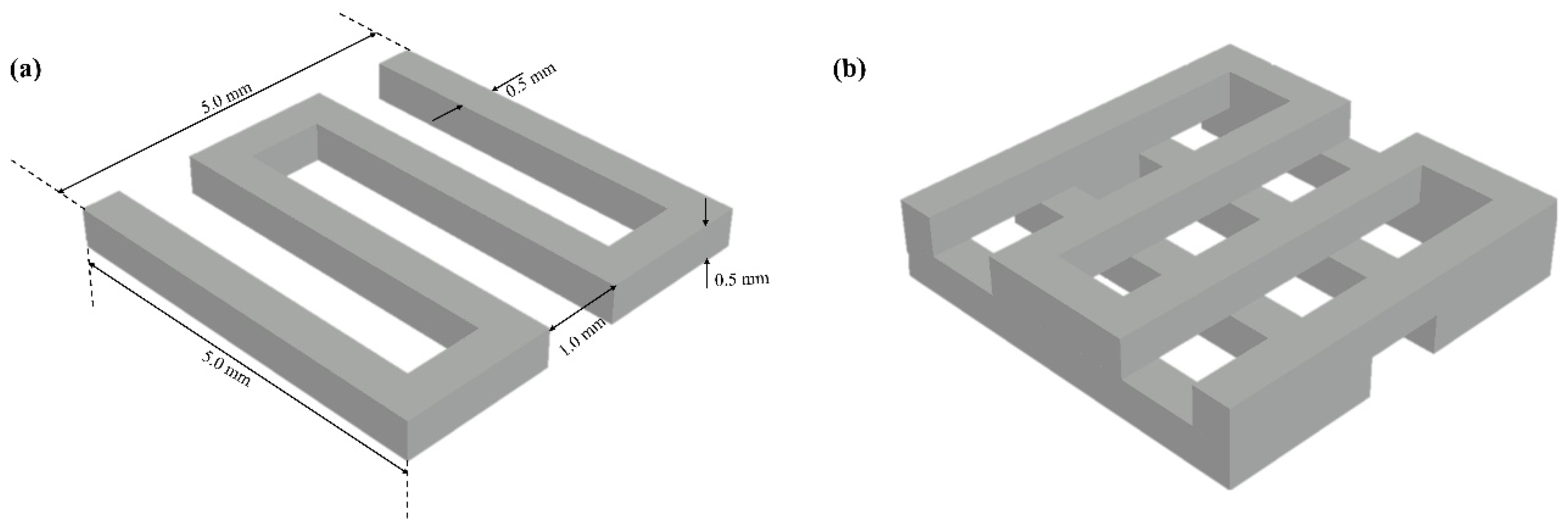
2.2. Scaffold Design
2.3. Rheological Characterization Testing
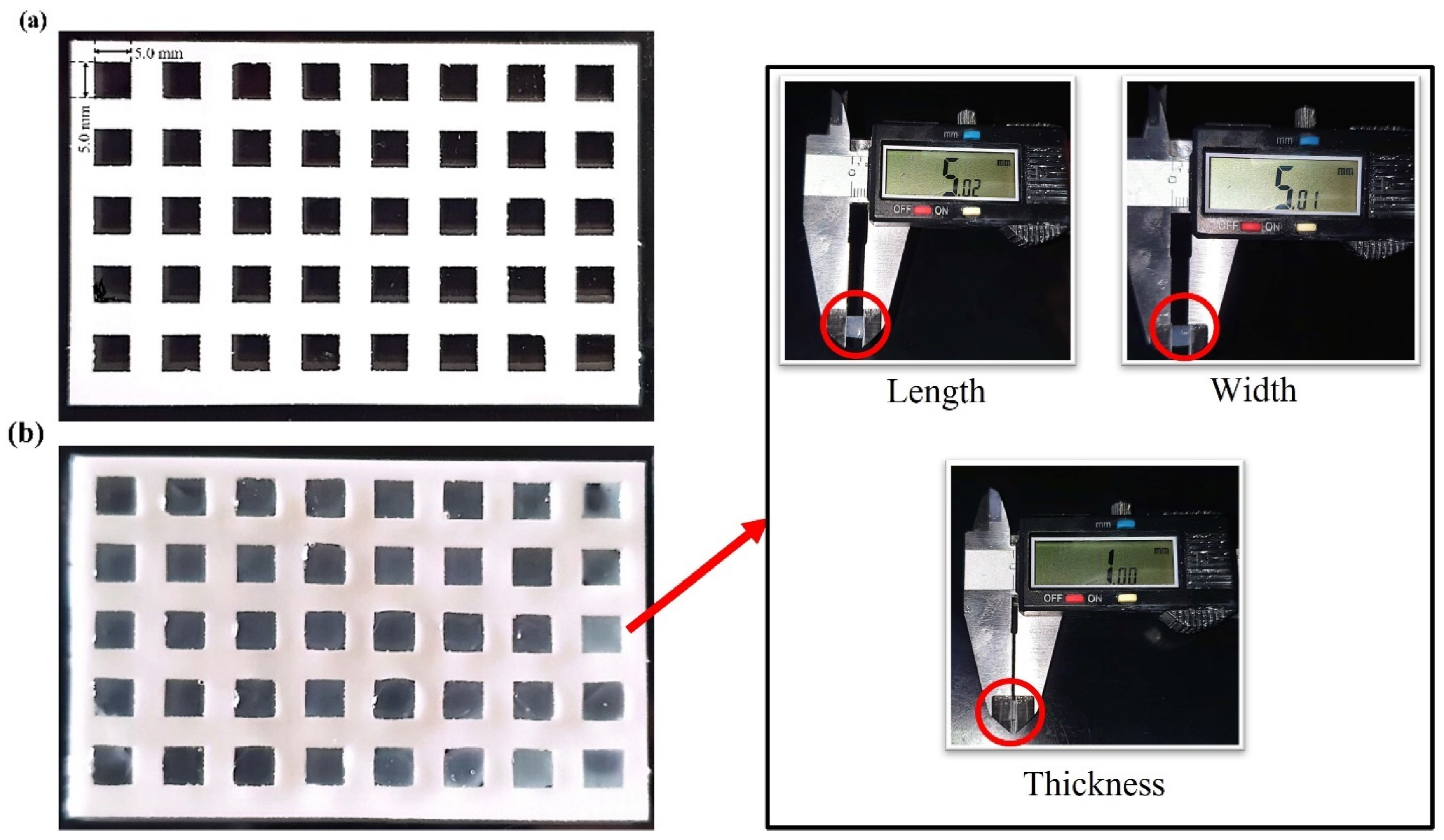
2.4. Bioprinting Fidelity
2.5. Cell Viability and Compatibility with 3D-Bioprinted Scaffolds
3. Results and Discussion
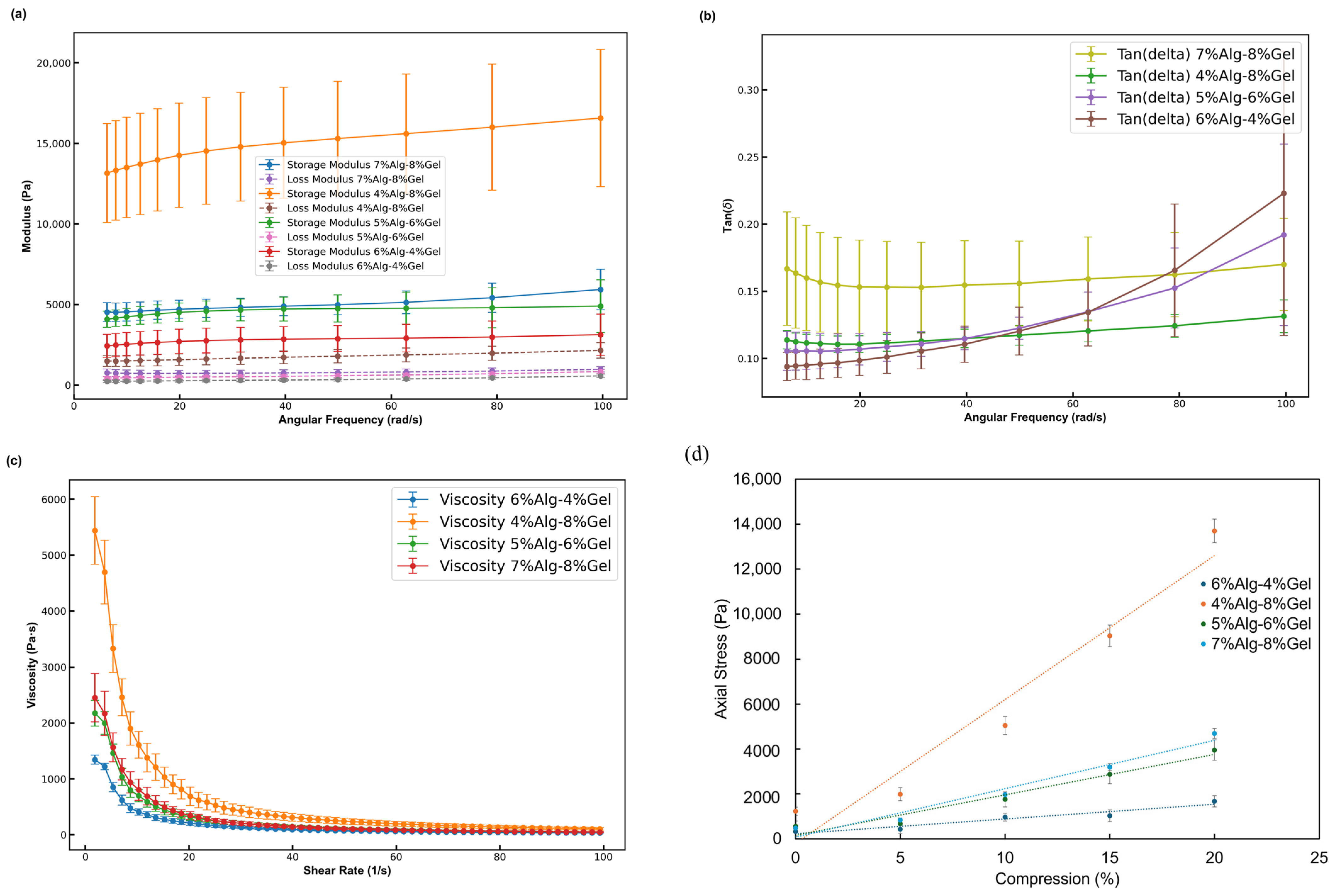
3.1. Rheological Characterization of Alg-Gel Hydrogels
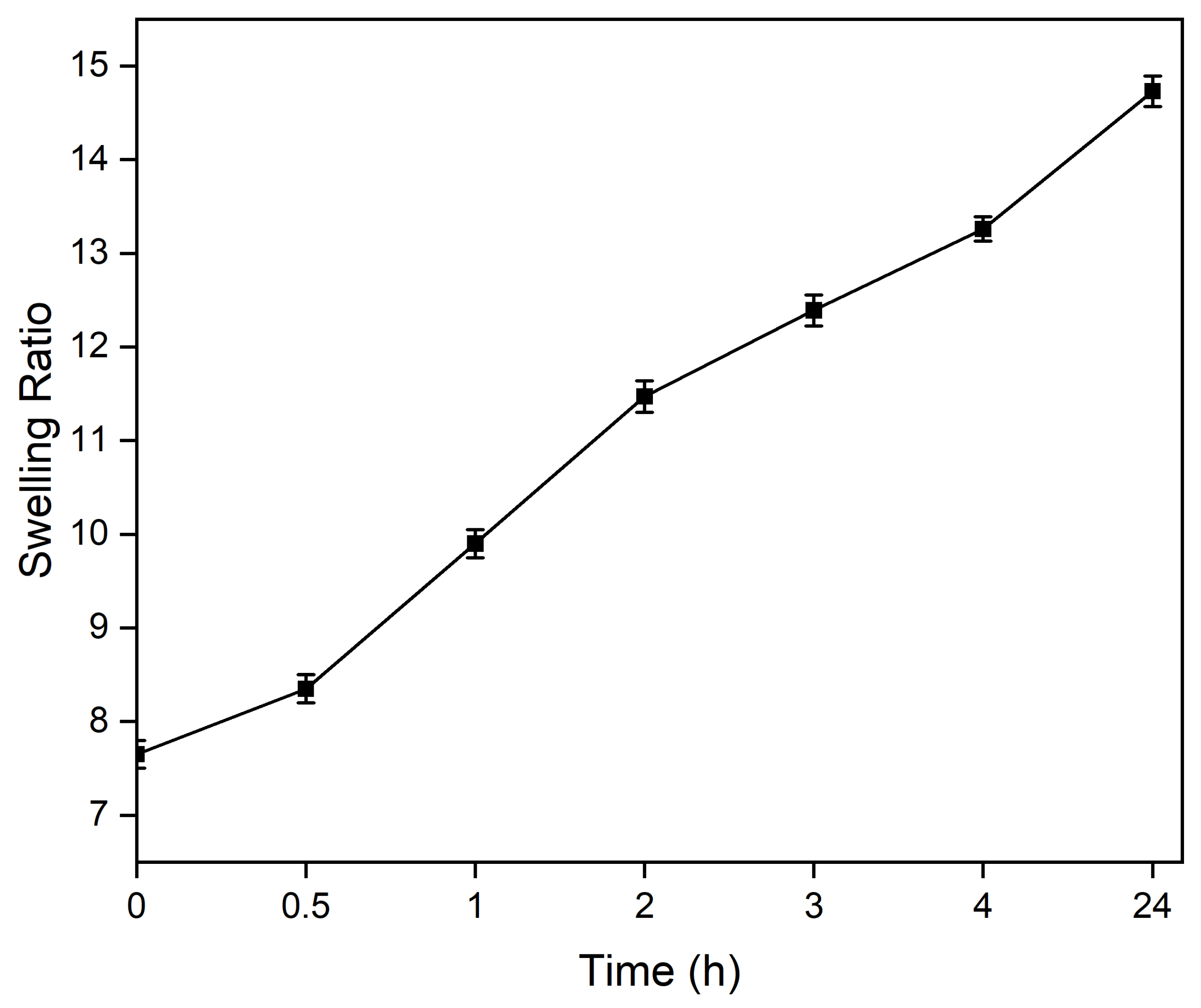
3.2. Swelling of Alg-Gel Hydrogels
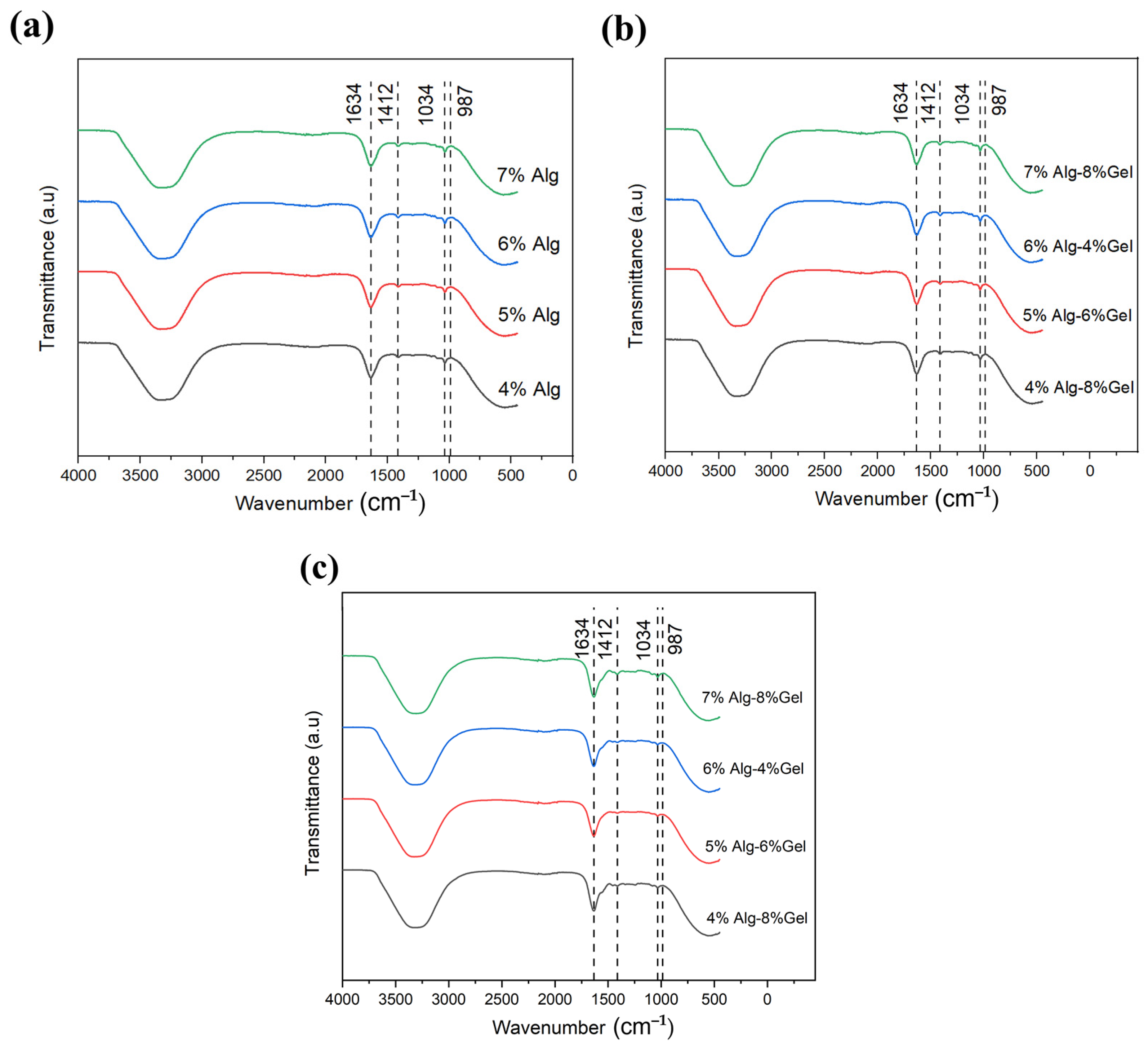
3.3. Chemical Analysis
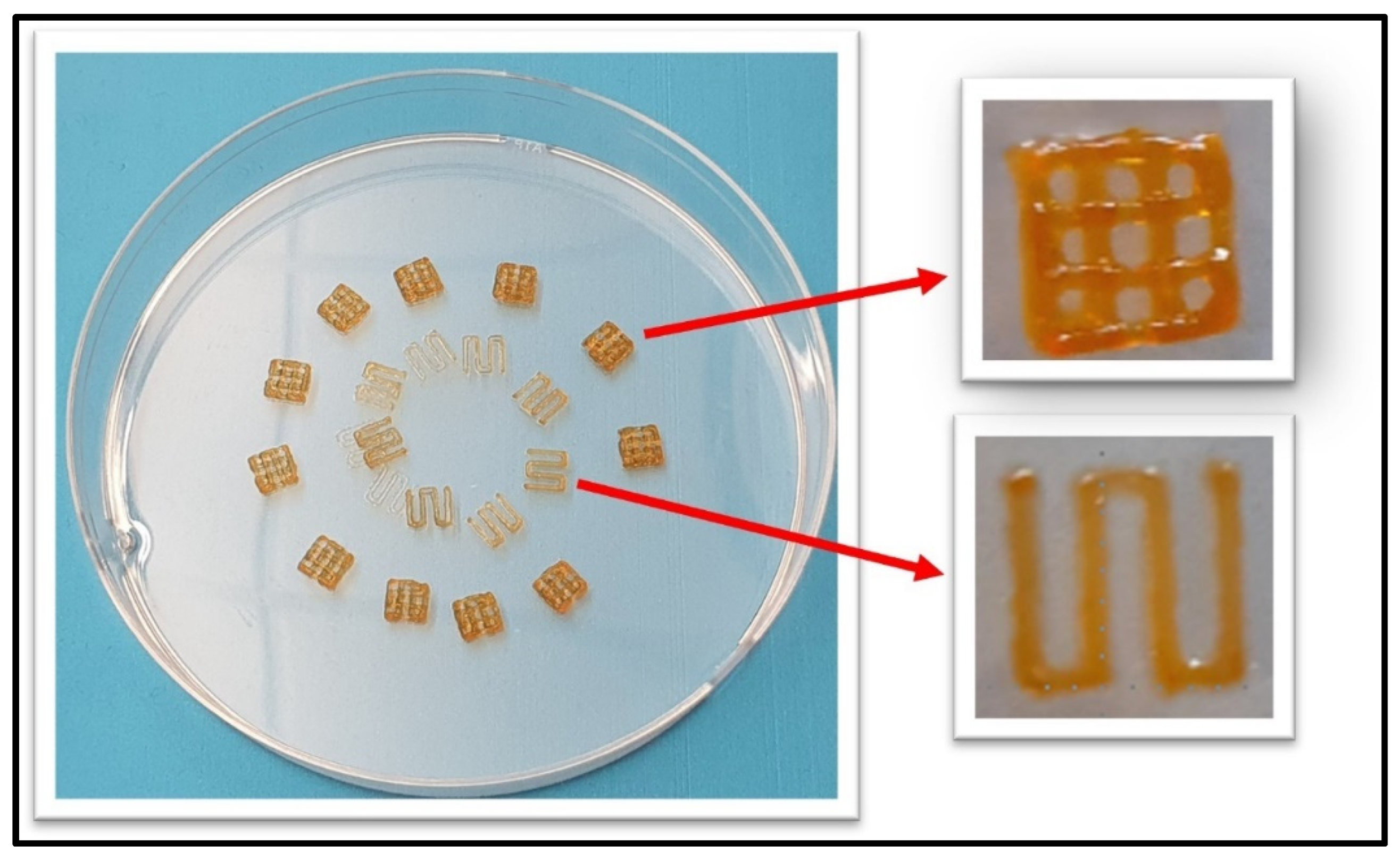
3.4. Printability of 7% Alg–8% Gel Hydrogel
3.5. In Vitro Biocompatibility Assessment
4. Discussion
5. Challenges and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A. Breast cancer statistics: Recent trends. In Breast Cancer Metastasis and Drug Resistance: Challenges and Progress; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–7. [Google Scholar]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone metastases: An overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [PubMed]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- LaBarbera, D.V.; Reid, B.G.; Yoo, B.H. The multicellular tumor spheroid model for high-throughput cancer drug discovery. Expert Opin. Drug Discov. 2012, 7, 819–830. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Z.; Zhou, X.; Khoo, B.L.; Gunawan, R.; Chin, Y.R.; Yang, M. 3D Biomimetic Models to Reconstitute Tumor Microenvironment In Vitro: Spheroids, Organoids, and Tumor-on-a-Chip. Adv. Healthc. Mater. 2023, 12, 2202609. [Google Scholar] [CrossRef] [PubMed]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and animal models: Are 3D cultures the ideal tool to study cancer-microenvironment interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Holen, I.; Speirs, V.; Morrissey, B.; Blyth, K. In vivo models in breast cancer research: Progress, challenges and future directions. Dis. Models Mech. 2017, 10, 359–371. [Google Scholar] [CrossRef]
- Elliott, N.T.; Yuan, F. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J. Pharm. Sci. 2011, 100, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, H.; Eskandari, A.; Poor, A.S.; Zarrabi, A.; Khodadadi, B.; Karimifard, S.; Yaraki, M.T. Nanobiotechnological approaches for breast cancer Management: Drug delivery systems and 3D In-Vitro models. Coord. Chem. Rev. 2024, 508, 215754. [Google Scholar] [CrossRef]
- Di Marzio, N.; Eglin, D.; Serra, T.; Moroni, L. Bio-fabrication: Convergence of 3D bioprinting and nano-biomaterials in tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 2020, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Parodi, I.; Di Lisa, D.; Pastorino, L.; Scaglione, S.; Fato, M.M. 3D bioprinting as a powerful technique for recreating the tumor microenvironment. Gels 2023, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural biomaterials and their use as bioinks for printing tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl–based hydrogels as modular tissue culture platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lyu, W.; Kawakami, K.; Ariga, K. Bio-gel nanoarchitectonics in tissue engineering. Nanoscale 2024, 16, 13230–13246. [Google Scholar] [CrossRef]
- Bupphathong, S.; Quiroz, C.; Huang, W.; Chung, P.F.; Tao, H.Y.; Lin, C.H. Gelatin methacrylate hydrogel for tissue engineering applications—A review on material modifications. Pharmaceuticals 2022, 15, 171. [Google Scholar] [CrossRef]
- Kim, H.D.; Amirthalingam, S.; Kim, S.L.; Lee, S.S.; Rangasamy, J.; Hwang, N.S. Biomimetic materials and fabrication approaches for bone tissue engineering. Adv. Healthc. Mater. 2017, 6, 1700612. [Google Scholar] [CrossRef]
- Chung, J.H.Y.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2013, 101, 1255–1264. [Google Scholar] [CrossRef]
- Di Giuseppe, M.; Law, N.; Webb, B.; Macrae, R.A.; Liew, L.J.; Sercombe, T.B.; Doyle, B.J. Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured Pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 2015, 7, 035006. [Google Scholar] [CrossRef] [PubMed]
- Saarai, A.; Kasparkova, V.; Sedlacek, T.; Saha, P. On the development and characterisation of crosslinked sodium alginate/gelatine hydrogels. J. Mech. Behav. Biomed. Mater. 2013, 18, 152–166. [Google Scholar] [CrossRef]
- Serafin, A.; Murphy, C.; Rubio, M.C.; Collins, M.N. Printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. Mater. Sci. Eng. C 2021, 122, 111927. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Pielesz, A.; Bąk, M.K.K. Raman spectroscopy and WAXS method as a tool for analysing ion-exchange properties of alginate hydrogels. Int. J. Biol. Macromol. 2008, 43, 438–443. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, S.A.; da Silva, B.C.; Riegel-Vidotti, I.C.; Urbano, A.; de Sousa Faria-Tischer, P.C.; Tischer, C.A. Production and characterization of bacterial cellulose membranes with hyaluronic acid from chicken comb. Int. J. Biol. Macromol. 2017, 97, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Rubio, M.C.; Carsi, M.; Ortiz-Serna, P.; Sanchis, M.J.; Garg, A.K.; Oliveira, J.M.; Koffler, J.; Collins, M.N. Electroconductive PEDOT nanoparticle integrated scaffolds for spinal cord tissue repair. Biomater. Res. 2022, 26, 63. [Google Scholar] [CrossRef]
- Webb, B.; Doyle, B.J. Parameter optimization for 3D bioprinting of hydrogels. Bioprinting 2017, 8, 8–12. [Google Scholar] [CrossRef]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Hauser, S.; Neffe, A.T.; Liu, Y.; Lendlein, A.; Pietzsch, J.; Jung, F. Response of endothelial cells to gelatin-based hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 527–540. [Google Scholar] [CrossRef]
- Kim, A.Y.; Kim, Y.; Lee, S.H.; Yoon, Y.; Kim, W.-H.; Kweon, O.-K. Effect of gelatin on osteogenic cell sheet formation using canine adipose-derived mesenchymal stem cells. Cell Transplant. 2017, 26, 115–123. [Google Scholar] [CrossRef]
- Panwar, A.; Tan, L.P. Current status of bioinks for micro-extrusion-based 3D bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Wilson, S.A.; Cross, L.M.; Peak, C.W.; Gaharwar, A.K. Shear-thinning and thermo-reversible nanoengineered inks for 3D bioprinting. ACS Appl. Mater. Interfaces 2017, 9, 43449–43458. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Liu, W.; Heinrich, M.A.; Zhou, Y.; Akpek, A.; Hu, N.; Liu, X.; Guan, X.; Zhong, Z.; Jin, X.; Khademhosseini, A.; et al. Extrusion bioprinting of shear-thinning gelatin methacryloyl bioinks. Adv. Healthc. Mater. 2017, 6, 1601451. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Zhu, W.; Tang, M.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv. Drug Deliv. Rev. 2018, 132, 235–251. [Google Scholar] [CrossRef]
- Nair, K.; Gandhi, M.; Khalil, S.; Yan, K.C.; Marcolongo, M.; Barbee, K.; Sun, W. Characterization of cell viability during bioprinting processes. Biotechnol. J. Healthc. Nutr. Technol. 2009, 4, 1168–1177. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Mao, S.; Sun, W.; Yao, R. The influence of printing parameters on cell survival rate and printability in microextrusion-based 3D cell printing technology. Biofabrication 2015, 7, 045002. [Google Scholar] [CrossRef]
- Amorim, P.; D’ávila, M.; Anand, R.; Moldenaers, P.; Van Puyvelde, P.; Bloemen, V. Insights on shear rheology of inks for extrusion-based 3D bioprinting. Bioprinting 2021, 22, e00129. [Google Scholar] [CrossRef]
- Karvinen, J.; Kellomäki, M. Design aspects and characterization of hydrogel-based bioinks for extrusion-based bioprinting. Bioprinting 2023, 32, e00274. [Google Scholar] [CrossRef]
- Patel, A.; Taufik, M. Extrusion-based technology in additive manufacturing: A comprehensive review. Arab. J. Sci. Eng. 2024, 49, 1309–1342. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, S.; Liu, Y.; Yao, B.; Hu, T.; Shi, H.; Xie, J.; Fu, X. Tuning alginate-gelatin bioink properties by varying solvent and their impact on stem cell behavior. Sci. Rep. 2018, 8, 8020. [Google Scholar] [CrossRef]
- Edward, S.; Golecki, H.M. Gelatin Soft Actuators: Benefits and Opportunities. Actuators 2023, 12, 63. [Google Scholar] [CrossRef]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 042001. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Z.; Chen, H.; Yan, W. 3D printing of short fiber reinforced composites via material extrusion: Fiber breakage. Addit. Manuf. 2022, 58, 103067. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Ahmadi, M. Degradable hydrogels: Design mechanisms and versatile applications. Mater. Today Sustain. 2023, 23, 100468. [Google Scholar] [CrossRef]
- Liu, J.; Song, Q.; Yin, W.; Li, C.; An, N.; Le, Y.; Wang, Q.; Feng, Y.; Hu, Y.; Wang, Y. Bioactive scaffolds for tissue engineering: A review of decellularized extracellular matrix applications and innovations. In Exploration; Wiley Online Library: Minneapolis, MN, USA, 2024. [Google Scholar]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking cell-matrix adhesions to the third dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Alemany-Ribes, M.; Semino, C.E. Bioengineering 3D environments for cancer models. Adv. Drug Deliv. Rev. 2014, 79, 40–49. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Gebeyehu, A.; Miranda, M.; Bahadur, D.; Patel, N.; Ramakrishnan, S.; Rishi, A.K.; Singh, M. Characterization and printability of Sodium alginate-Gelatin hydrogel for bioprinting NSCLC co-culture. Sci. Rep. 2019, 9, 19914. [Google Scholar] [CrossRef]

| Hydrogel Composition | Needle Gauge (G) | Printing Temperature (°C) | Printing Pressure (psi) |
|---|---|---|---|
| 4% Alg–8% Gel | 30 R, 30 T, 27 R, and 27 T | 25–35 | 30–80 |
| 5% Alg–6% Gel | |||
| 6% Alg–4% Gel | |||
| 7% Alg–8% Gel |
| Needle Gauge (G) | Inner Diameter (mm) | Printing Pressure (psi) | Strand Width (mm) | Printing Accuracy (%) | POInormalized |
|---|---|---|---|---|---|
| 30 R | 0.152 | 80 | 0.70 ± 0.01 | 88.8 | 0.274 |
| 30 T | 0.150 | 35 | 0.63 ± 0.01 | 96.0 | 0.758 |
| 27 R | 0.200 | 50 | 0.57 ± 0.02 | 92.0 | 0.558 |
| 27 T | 0.203 | 30 | 0.56 ± 0.02 | 97.2 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, M.K.; Devireddy, R.V. Rheological Characterization and Printability of Sodium Alginate–Gelatin Hydrogel for 3D Cultures and Bioprinting. Biomimetics 2025, 10, 28. https://doi.org/10.3390/biomimetics10010028
Dey MK, Devireddy RV. Rheological Characterization and Printability of Sodium Alginate–Gelatin Hydrogel for 3D Cultures and Bioprinting. Biomimetics. 2025; 10(1):28. https://doi.org/10.3390/biomimetics10010028
Chicago/Turabian StyleDey, Mohan Kumar, and Ram V. Devireddy. 2025. "Rheological Characterization and Printability of Sodium Alginate–Gelatin Hydrogel for 3D Cultures and Bioprinting" Biomimetics 10, no. 1: 28. https://doi.org/10.3390/biomimetics10010028
APA StyleDey, M. K., & Devireddy, R. V. (2025). Rheological Characterization and Printability of Sodium Alginate–Gelatin Hydrogel for 3D Cultures and Bioprinting. Biomimetics, 10(1), 28. https://doi.org/10.3390/biomimetics10010028






