Investigation of the Influence of Silicon Oxide Content on Electrolyte Degradation, Gas Evolution, and Thickness Change in Silicon Oxide/Graphite Composite Anodes for Li-Ion Cells Using Operando Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrode Preparation
2.2. Operando Electrochemical Measurements
2.2.1. Test Cells and Evaluation of Recorded Data
2.2.2. Differential Electrochemical Mass Spectrometry
2.2.3. Dilatometry
2.3. Post Mortem Analysis
2.3.1. Chemical and Component Analysis
2.3.2. Gas Chromatography with Coupled Mass Spectrometry (GC-MS)
3. Results and Discussion
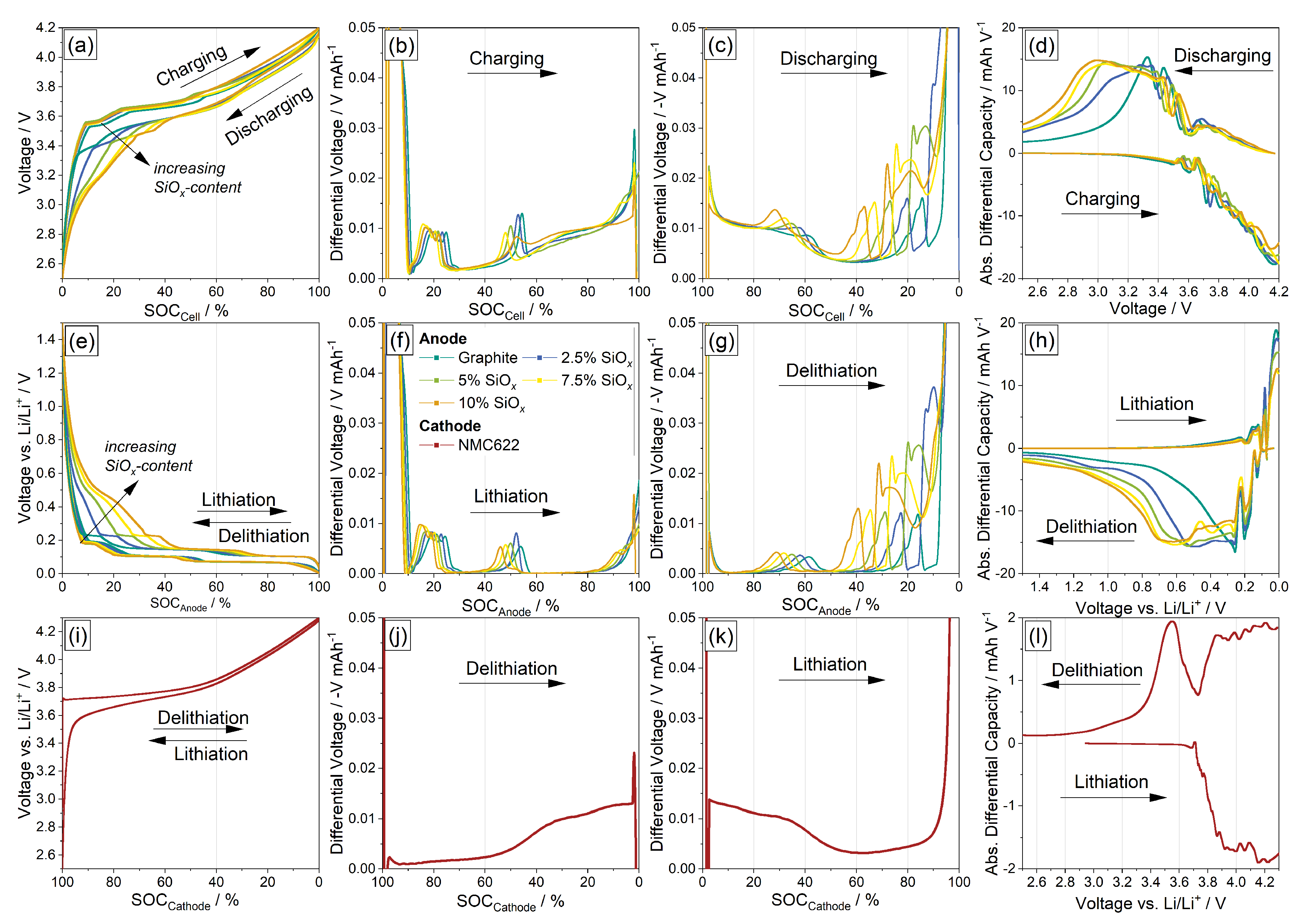
3.1. Electrochemical Measurements
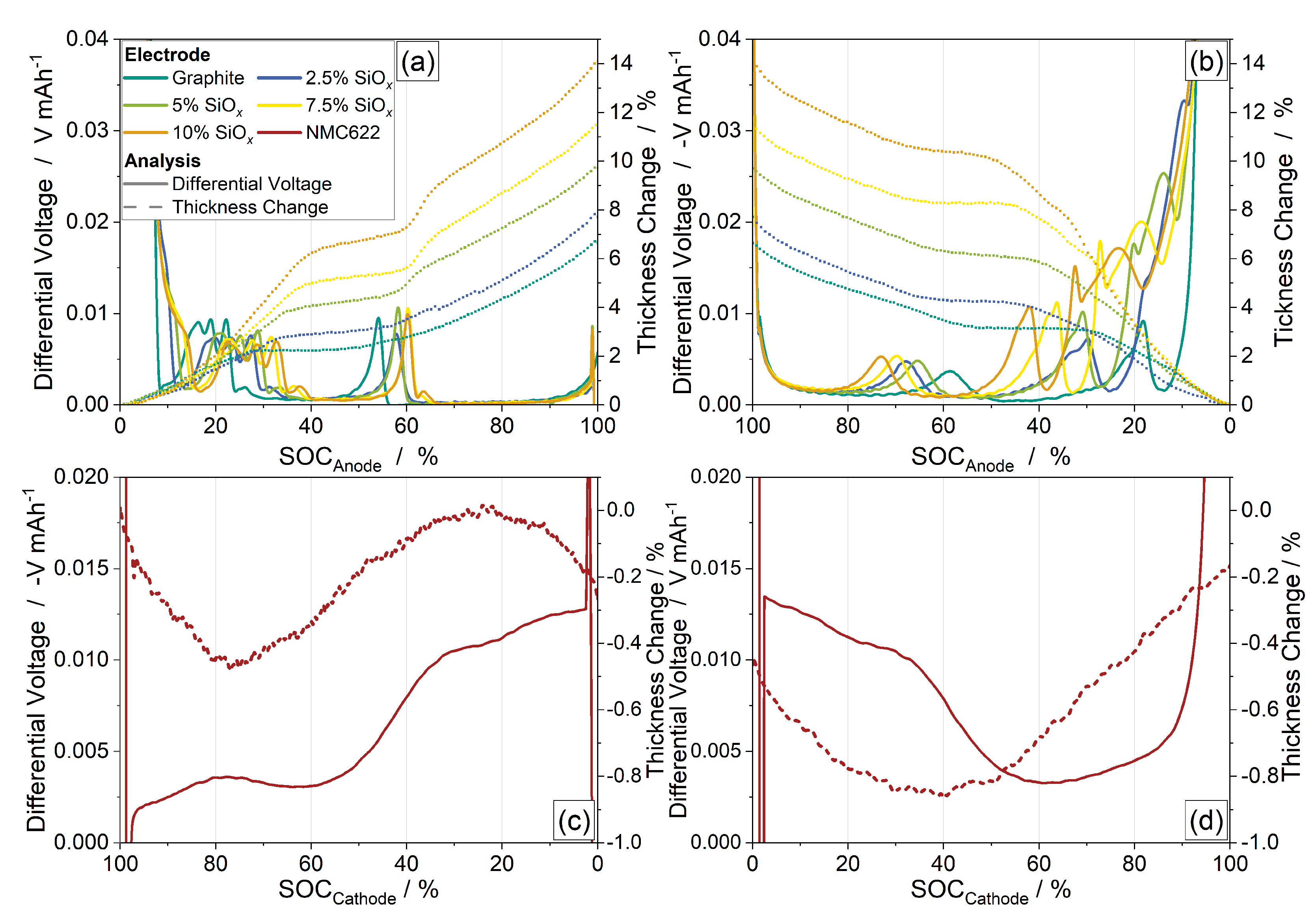
3.2. Thickness Change
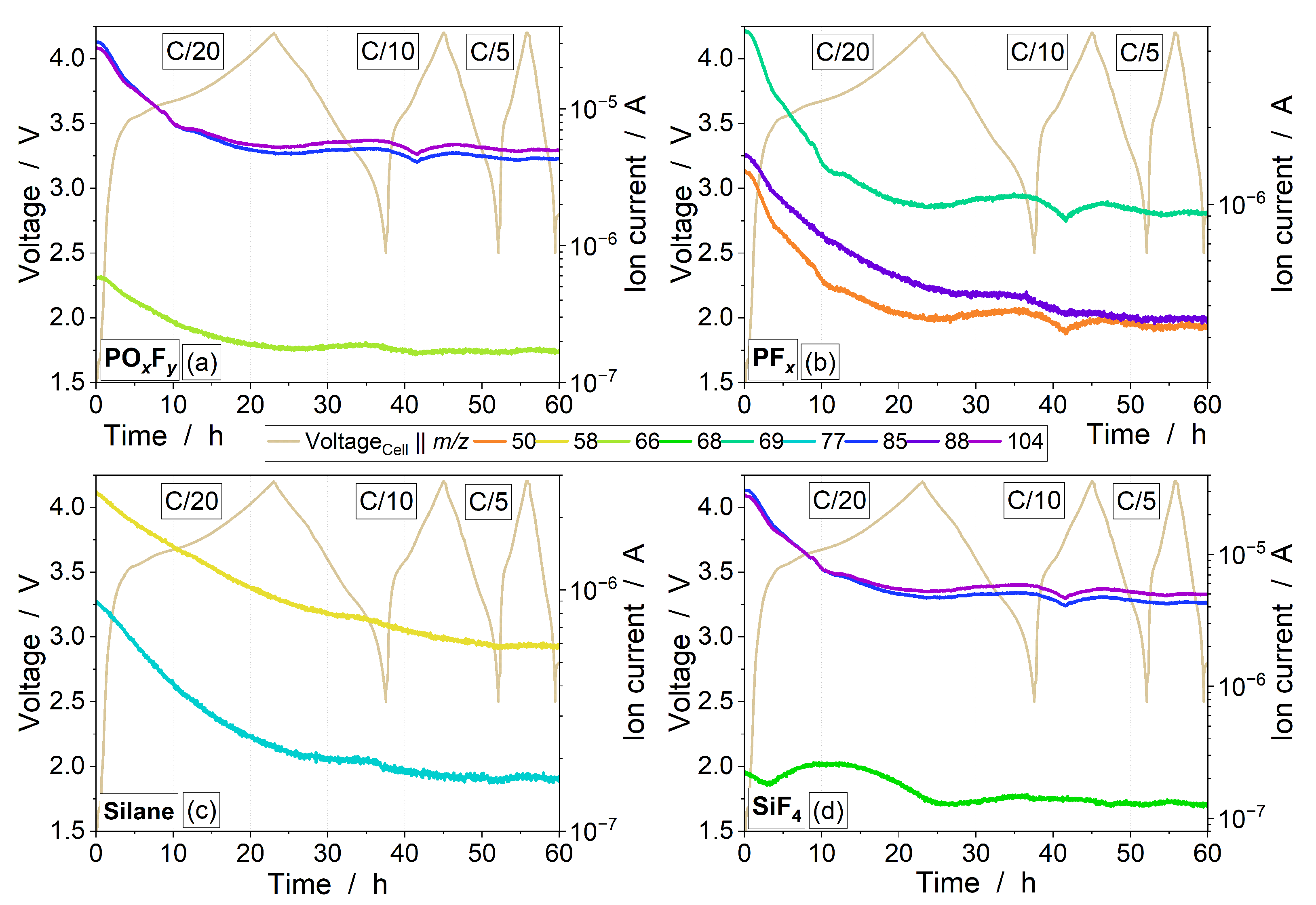
3.3. Electrolyte Degradation
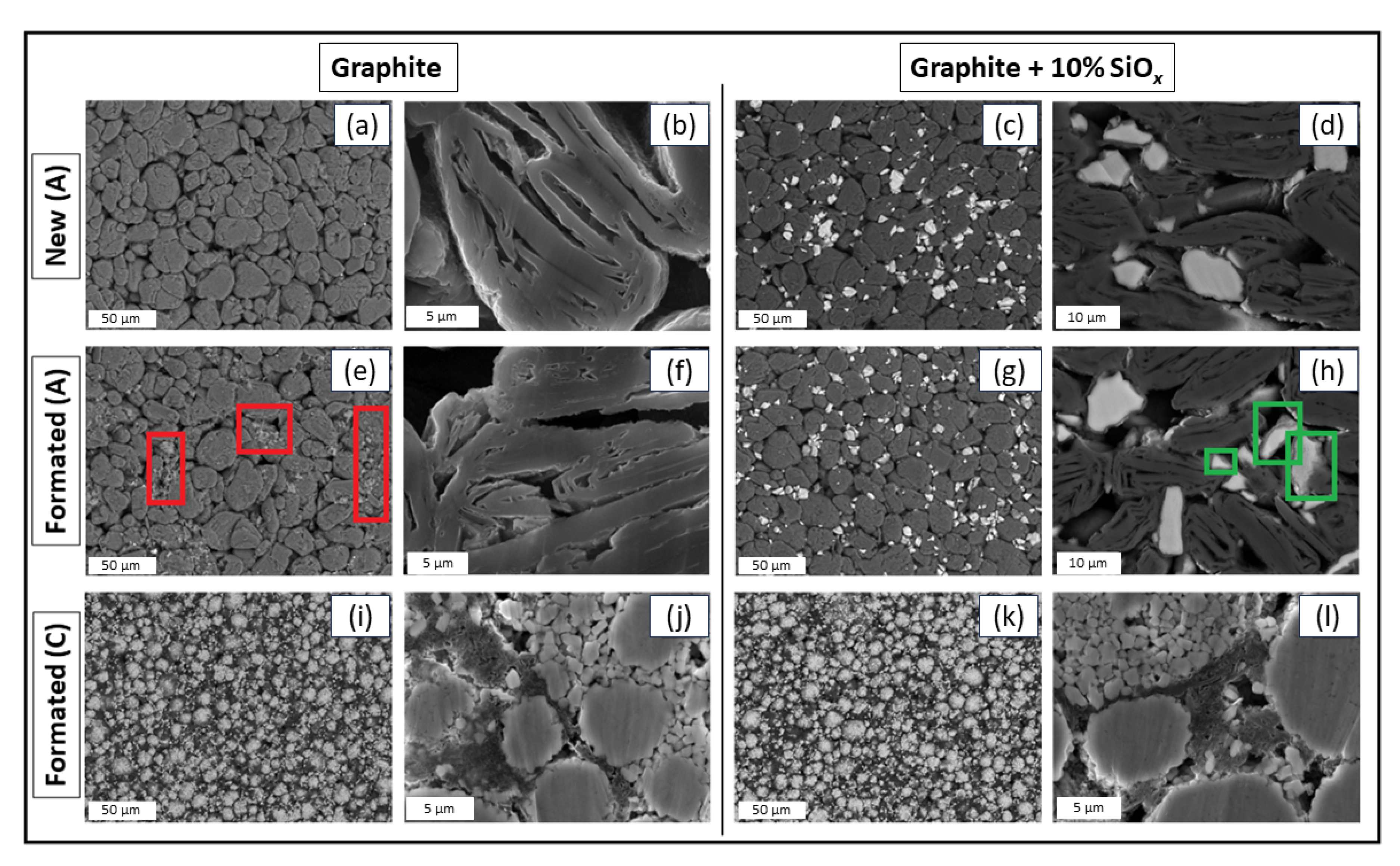
3.4. Chemical and Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CB | Carbon black |
| CC | Constant current |
| CMC | Carboxymethyl cellulose |
| CTS | Cell test system |
| CV | Constant voltage |
| DC | Differential capacity |
| DCM | Methylene chloride |
| DEC | Diethyl carbonate |
| DEMS | Differential electrochemical mass spectrometry |
| DMC | Dimethyl carbonate |
| DV | Differential voltage |
| EDX | Energy-dispersive X-ray spectroscopy |
| FCE | First-cycle efficiency |
| FID | Flame ionisation detector |
| GC | Gas chromatography |
| Gr | Graphite |
| ICP | Inductively coupled plasma |
| LIB | Lithium-ion battery |
| MID | Multiple ion detection |
| MS | Mass spectrometry |
| NIST | National Institute of Standards and Technology |
| NMC | Nickel–manganese–cobalt |
| OES | Optical emission spectrometry |
| PVDF | Polyvinylidene fluoride |
| SBR | Styrene butadiene rubber |
| SEI | Solid electrolyte interface |
| SEM | Surface electron microscopy |
| SiO | Silicon oxide |
| SOC | State of charge |
| TCD | Thermal conductivity detector |
| TGA | Thermogravimetric analysis |
| TIC | Total ion current |
References
- Ziegler, M.S.; Trancik, J.E. Re-examining rates of lithium-ion battery technology improvement and cost decline. Energy Environ. Sci. 2021, 14, 1635–1651. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and Recent Progress in the Development of Si Anodes for Lithium-Ion Battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Moyassari, E.; Roth, T.; Kücher, S.; Chang, C.C.; Hou, S.C.; Spingler, F.B.; Jossen, A. The Role of Silicon in Silicon-Graphite Composite Electrodes Regarding Specific Capacity, Cycle Stability, and Expansion. J. Electrochem. Soc. 2022, 169, 010504. [Google Scholar] [CrossRef]
- Edge, J.S.; O’Kane, S.; Prosser, R.; Kirkaldy, N.D.; Patel, A.N.; Hales, A.; Ghosh, A.; Ai, W.; Chen, J.; Yang, J.; et al. Lithium ion battery degradation: What you need to know. Phys. Chem. Chem. Phys. 2021, 23, 8200–8221. [Google Scholar] [CrossRef] [PubMed]
- Obrovac, M.N.; Christensen, L. Structural Changes in Silicon Anodes during Lithium Insertion/Extraction. Electrochem. Solid-State Lett. 2004, 7, A93. [Google Scholar] [CrossRef]
- Chen, T.; Wu, J.; Zhang, Q.; Su, X. Recent advancement of SiOx based anodes for lithium-ion batteries. J. Power Sources 2017, 363, 126–144. [Google Scholar] [CrossRef]
- Louli, A.J.; Li, J.; Trussler, S.; Fell, C.R.; Dahn, J.R. Volume, Pressure and Thickness Evolution of Li-Ion Pouch Cells with Silicon-Composite Negative Electrodes. J. Electrochem. Soc. 2017, 164, A2689–A2696. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Dai, H.; Yu, C.; Wei, X. Impedance Investigation of Silicon/Graphite Anode during Cycling. Batteries 2023, 9, 242. [Google Scholar] [CrossRef]
- Heugel, P.; Märkle, W.; Deich, T.; von Kessel, O.; Tübke, J. Thickness change and jelly roll deformation and its impact on the aging and lifetime of commercial 18650 cylindrical Li-ion cells with silicon containing anodes and nickel-rich cathodes. J. Energy Storage 2022, 53, 105101. [Google Scholar] [CrossRef]
- Zhang, L.; Tsolakidou, C.; Mariyappan, S.; Tarascon, J.M.; Trabesinger, S. Unraveling gas evolution in sodium batteries by online electrochemical mass spectrometry. Energy Storage Mater. 2021, 42, 12–21. [Google Scholar] [CrossRef]
- Holzapfel, M.; Würsig, A.; Scheifele, W.; Vetter, J.; Novák, P. Oxygen, hydrogen, ethylene and CO2 development in lithium-ion batteries. J. Power Sources 2007, 174, 1156–1160. [Google Scholar] [CrossRef]
- McBrayer, J.D.; Rodrigues, M.T.F.; Schulze, M.C.; Abraham, D.P.; Apblett, C.A.; Bloom, I.; Carroll, G.M.; Colclasure, A.M.; Fang, C.; Harrison, K.L.; et al. Calendar aging of silicon-containing batteries. Nat. Energy 2021, 6, 866–872. [Google Scholar] [CrossRef]
- Seitzinger, C.L.; Sacci, R.L.; Coyle, J.E.; Apblett, C.A.; Hays, K.A.; Armstrong, R.R.; Rogers, A.M.; Armstrong, B.L.; Bennet, T.H.; Neale, N.R.; et al. Intrinsic Chemical Reactivity of Silicon Electrode Materials: Gas Evolution. Chem. Mater. 2020, 32, 3199–3210. [Google Scholar] [CrossRef]
- Yao, K.P.C.; Okasinski, J.S.; Kalaga, K.; Almer, J.D.; Abraham, D.P. Operando Quantification of (De)Lithiation Behavior of Silicon–Graphite Blended Electrodes for Lithium–Ion Batteries. Adv. Energy Mater. 2019, 9, 1803380. [Google Scholar] [CrossRef]
- Berhaut, C.L.; Dominguez, D.Z.; Kumar, P.; Jouneau, P.H.; Porcher, W.; Aradilla, D.; Tardif, S.; Pouget, S.; Lyonnard, S. Multiscale Multiphase Lithiation and Delithiation Mechanisms in a Composite Electrode Unraveled by Simultaneous Operando Small-Angle and Wide-Angle X-ray Scattering. ACS Nano 2019, 13, 11538–11551. [Google Scholar] [CrossRef]
- Zilberman, I.; Sturm, J.; Jossen, A. Reversible self-discharge and calendar aging of 18650 nickel-rich, silicon-graphite lithium-ion cells. J. Power Sources 2019, 425, 217–226. [Google Scholar] [CrossRef]
- Sturm, J.; Rheinfeld, A.; Zilberman, I.; Spingler, F.B.; Kosch, S.; Frie, F.; Jossen, A. Modeling and simulation of inhomogeneities in a 18650 nickel-rich, silicon-graphite lithium-ion cell during fast charging. J. Power Sources 2019, 412, 204–223. [Google Scholar] [CrossRef]
- Kreissl, J.J.A.; Petit, J.; Oppermann, R.; Cop, P.; Gerber, T.; Joos, M.; Abert, M.; Tübke, J.; Miyazaki, K.; Abe, T.; et al. Electrochemical Lithiation/Delithiation of ZnO in 3D-Structured Electrodes: Elucidating the Mechanism and the Solid Electrolyte Interphase Formation. ACS Appl. Mater. Interfaces 2021, 13, 35625–35638. [Google Scholar] [CrossRef]
- The NIST Mass Spectral Search Program, version 2.3; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017.
- Ai, W.; Kirkaldy, N.; Jiang, Y.; Offer, G.; Wang, H.; Wu, B. A composite electrode model for lithium-ion batteries with silicon/graphite negative electrodes. J. Power Sources 2022, 527, 231142. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Chevrier, V.L. Alloy negative electrodes for Li-ion batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef]
- Chevrier, V.L.; Dahn, J.R. First Principles Model of Amorphous Silicon Lithiation. J. Electrochem. Soc. 2009, 156, A454. [Google Scholar] [CrossRef]
- Zhang, W.J. Lithium insertion/extraction mechanism in alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 877–885. [Google Scholar] [CrossRef]
- Safari, M.; Morcrette, A.; Teyssot, A.; Delacort, C. Multimodal Physics-Based Aging Model for Life Prediction of Li-Ion Batteries. J. Electrochem. Soc. 2009, 156, A145. [Google Scholar] [CrossRef]
- Bazlen, S.; Heugel, P.; von Kessel, O.; Commerell, W.; Tübke, J. Influence of charging protocols on the charging capability and aging of lithium-ion cells with silicon-containing anodes. J. Energy Storage 2022, 49, 104044. [Google Scholar] [CrossRef]
- Ohzuku, T.; Iwakoshi, Y.; Sawai, K. Formation of Lithium–Graphite Intercalation Compounds in Nonaqueous Electrolytes and Their Application as a Negative Electrode for a Lithium Ion (Shuttlecock) Cell. J. Electrochem. Soc. 1993, 140, 2490. [Google Scholar]
- Schweidler, S.; de Biasi, L.; Schiele, A.; Hartmann, P.; Brezesinski, T.; Janek, J. Volume Changes of Graphite Anodes Revisited: A Combined Operando X-ray Diffraction and In Situ Pressure Analysis Study. J. Phys. Chem. C 2018, 122, 8829–8835. [Google Scholar] [CrossRef]
- Spingler, F.B.; Kücher, S.; Phillips, R.; Moyassari, E.; Jossen, A. Electrochemically Stable In Situ Dilatometry of NMC, NCA and Graphite Electrodes for Lithium-Ion Cells Compared to XRD Measurements. J. Electrochem. Soc. 2021, 168, 040515. [Google Scholar] [CrossRef]
- von Kessel, O.; Hoehl, T.; Heugel, P.; Brauchle, F.; Vrankovic, D.; Birke, K.P. Electrochemical-Mechanical Parameterization and Modeling of Expansion, Pressure, and Porosity Evolution in NMC811|SiOx-Graphite Lithium-Ion Cells. J. Electrochem. Soc. 2023. [Google Scholar] [CrossRef]
- Yang, Z.; Trask, S.E.; Wu, X.; Ingram, B.J. Effect of Si Content on Extreme Fast Charging Behavior in Silicon–Graphite Composite Anodes. Batteries 2023, 9, 138. [Google Scholar] [CrossRef]
- Peschel, C.; Horsthemke, F.; Leißing, M.; Wiemers-Meyer, S.; Henschel, J.; Winter, M.; Nowak, S. Analysis of Carbonate Decomposition During Solid Electrolyte Interphase Formation in Isotope–Labeled Lithium Ion Battery Electrolytes: Extending the Knowledge about Electrolyte Soluble Species. Batter. Supercaps 2020, 3, 1183–1192. [Google Scholar] [CrossRef]
- Rinkel, B.L.D.; Hall, D.S.; Temprano, I.; Grey, C.P. Electrolyte Oxidation Pathways in Lithium-Ion Batteries. J. Am. Chem. Soc. 2020, 142, 15058–15074. [Google Scholar] [CrossRef]
- Han, J.G.; Kim, K.; Lee, Y.; Choi, N.S. Scavenging Materials to Stabilize LiPF6-Containing Carbonate-Based Electrolytes for Li-Ion Batteries. Adv. Mater. 2019, 31, e1804822. [Google Scholar] [CrossRef]
- Hasa, I.; Haregewoin, A.M.; Zhang, L.; Tsai, W.Y.; Guo, J.; Veith, G.M.; Ross, P.N.; Kostecki, R. Electrochemical Reactivity and Passivation of Silicon Thin-Film Electrodes in Organic Carbonate Electrolytes. ACS Appl. Mater. Interfaces 2020, 12, 40879–40890. [Google Scholar] [CrossRef]
- Heiskanen, S.K.; Kim, J.; Lucht, B.L. Generation and Evolution of the Solid Electrolyte Interphase of Lithium-Ion Batteries. Joule 2019, 3, 2322–2333. [Google Scholar] [CrossRef]
- Pender, J.P.; Jha, G.; Youn, D.H.; Ziegler, J.M.; Andoni, I.; Choi, E.J.; Heller, A.; Dunn, B.S.; Weiss, P.S.; Penner, R.M.; et al. Electrode Degradation in Lithium-Ion Batteries. ACS Nano 2020, 14, 1243–1295. [Google Scholar] [CrossRef]
- Kim, S.H.; Dong, K.; Zhao, H.; El-Zoka, A.A.; Zhou, X.; Woods, E.V.; Giuliani, F.; Manke, I.; Raabe, D.; Gault, B. Understanding the Degradation of a Model Si Anode in a Li-Ion Battery at the Atomic Scale. J. Phys. Chem. Lett. 2022, 13, 8416–8421. [Google Scholar] [CrossRef]
- Yamada, Y.; Iriyama, Y.; Abe, T.; Ogumi, Z. Kinetics of Electrochemical Insertion and Extraction of Lithium Ion at SiO. J. Electrochem. Soc. 2010, 157, A26. [Google Scholar] [CrossRef]
- Nagao, Y.; Sakaguchi, H.; Honda, H.; Funkunaga, T. Structural Analysis of Pure and Electrochemically Lithiated SiO Using Neutron Elastic Scattering. J. Electrochem. Soc. 2004, 151, A1572. [Google Scholar] [CrossRef]
- Miyachi, M.; Yamamoto, H.; Kawai, H.; Ohta, T. Analysis of SiO Anodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A2089–A2091. [Google Scholar] [CrossRef]
- Daubinger, P.; Schelter, M.; Petersohn, R.; Nagler, F.; Hartmann, S.; Herrmann, M.; Giffin, G.A. Impact of Bracing on Large Format Prismatic Lithium–Ion Battery Cells during Aging. Adv. Energy Mater. 2022, 12, 2102448. [Google Scholar] [CrossRef]




| Anode | |||||||
|---|---|---|---|---|---|---|---|
| Gr | SiO | CMC | SBR | CB | Thickness | Porosity | |
| wt.-% | µm | % | |||||
| Graphite | 94.0 | 0.0 | 2.0 | 2.0 | 2.0 | 76 | 39.42 |
| 2.5 SiO | 91.5 | 2.5 | 2.0 | 2.0 | 2.0 | 68 | 40.13 |
| 5 SiO | 89.0 | 5.0 | 2.0 | 2.0 | 2.0 | 63 | 37.80 |
| 7.5 SiO | 86.5 | 7.5 | 2.0 | 2.0 | 2.0 | 60 | 38.29 |
| 10 SiO | 84.0 | 10.0 | 2.0 | 2.0 | 2.0 | 56 | 37.37 |
| 25 SiO | 69.0 | 25.0 | 2.0 | 2.0 | 2.0 | 45 | 39.35 |
| Cathode | |||||||
| NMC | PVDF | CB | Thickness | Porosity | |||
| wt.-% | µm | % | |||||
| NMC622 | 94.0 | 3.0 | 3.0 | 73 | 29.00 | ||
| Half Cell | Full Cell | |
|---|---|---|
| FCE | ||
| % | ||
| Graphite | 92.97 | 89.97 |
| 2.5 SiO | 91.87 | 87.84 |
| 5 SiO | 90.69 | 86.66 |
| 7.5 SiO | 89.89 | 85.86 |
| 10 SiO | 89.41 | 84.39 |
| NMC622 | 90.87 | - |
| New | After Formation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anode | ||||||||||||
| Gr | 2.5% | 5% | 7.5% | 10% | Gr | 2.5% | 5% | 7.5% | 10% | |||
| ICP | Lithium | wt.-% | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 1.00 | 0.83 | 0.87 | 1.04 | 1.16 |
| Nickel | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ||
| Manganese | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ||
| Cobalt | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | ||
| Phosphorus | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.04 | ||
| Photometry | Silicon | 0 | 1.263 | 2.446 | 3.711 | 4.955 | 0 | 1.165 | 2.369 | 3.493 | 4.620 | |
| TGA | in N | 2.8 | 2.6 | 2.6 | 2.6 | 2.6 | 3.2 | 2.9 | 2.6 | 2.4 | 2.0 | |
| in O | 96.7 | 93.7 | 90.9 | 89.1 | 85.6 | 95.5 | 92.0 | 88.4 | 85.4 | 82.9 | ||
| Residue | 0.6 | 3.7 | 6.4 | 8.1 | 11.8 | 1.4 | 5.0 | 8.0 | 12.2 | 15.1 | ||
| Cathode | ||||||||||||
| TGA | in N | wt.-% | 5.1 | 5.1 | 5.1 | 5.1 | 5.1 | 8.0 | 7.5 | 7.6 | 7.9 | 7.7 |
| in O | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | ||
| Residue | 94.3 | 94.3 | 94.3 | 94.3 | 94.3 | 91.7 | 92.4 | 92.2 | 92.1 | 92.1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heugel, P.; Petit, J.; Klein, F.; Tübke, J. Investigation of the Influence of Silicon Oxide Content on Electrolyte Degradation, Gas Evolution, and Thickness Change in Silicon Oxide/Graphite Composite Anodes for Li-Ion Cells Using Operando Techniques. Batteries 2023, 9, 449. https://doi.org/10.3390/batteries9090449
Heugel P, Petit J, Klein F, Tübke J. Investigation of the Influence of Silicon Oxide Content on Electrolyte Degradation, Gas Evolution, and Thickness Change in Silicon Oxide/Graphite Composite Anodes for Li-Ion Cells Using Operando Techniques. Batteries. 2023; 9(9):449. https://doi.org/10.3390/batteries9090449
Chicago/Turabian StyleHeugel, Philipp, Jan Petit, Franziska Klein, and Jens Tübke. 2023. "Investigation of the Influence of Silicon Oxide Content on Electrolyte Degradation, Gas Evolution, and Thickness Change in Silicon Oxide/Graphite Composite Anodes for Li-Ion Cells Using Operando Techniques" Batteries 9, no. 9: 449. https://doi.org/10.3390/batteries9090449
APA StyleHeugel, P., Petit, J., Klein, F., & Tübke, J. (2023). Investigation of the Influence of Silicon Oxide Content on Electrolyte Degradation, Gas Evolution, and Thickness Change in Silicon Oxide/Graphite Composite Anodes for Li-Ion Cells Using Operando Techniques. Batteries, 9(9), 449. https://doi.org/10.3390/batteries9090449






