Enhancing Performance of LiFePO4 Battery by Using a Novel Gel Composite Polymer Electrolyte
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
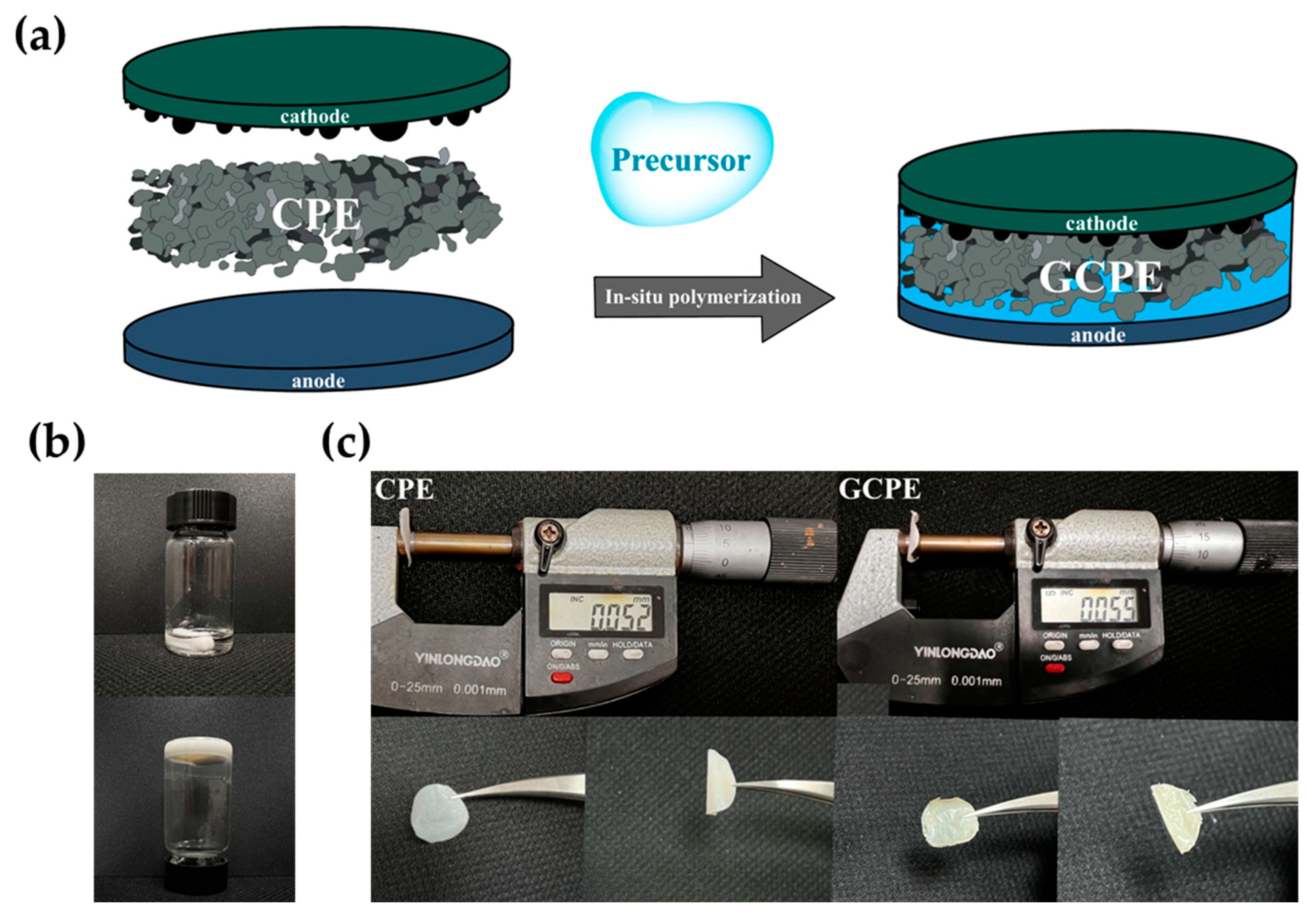
2.2. Preparation of Composite Polymer Electrolyte (CPE)
2.3. Preparation of Gel Composite Polymer Electrolyte (GCPE)
2.4. Preparation of Cathodes
2.5. Cell Assembly and Measurements
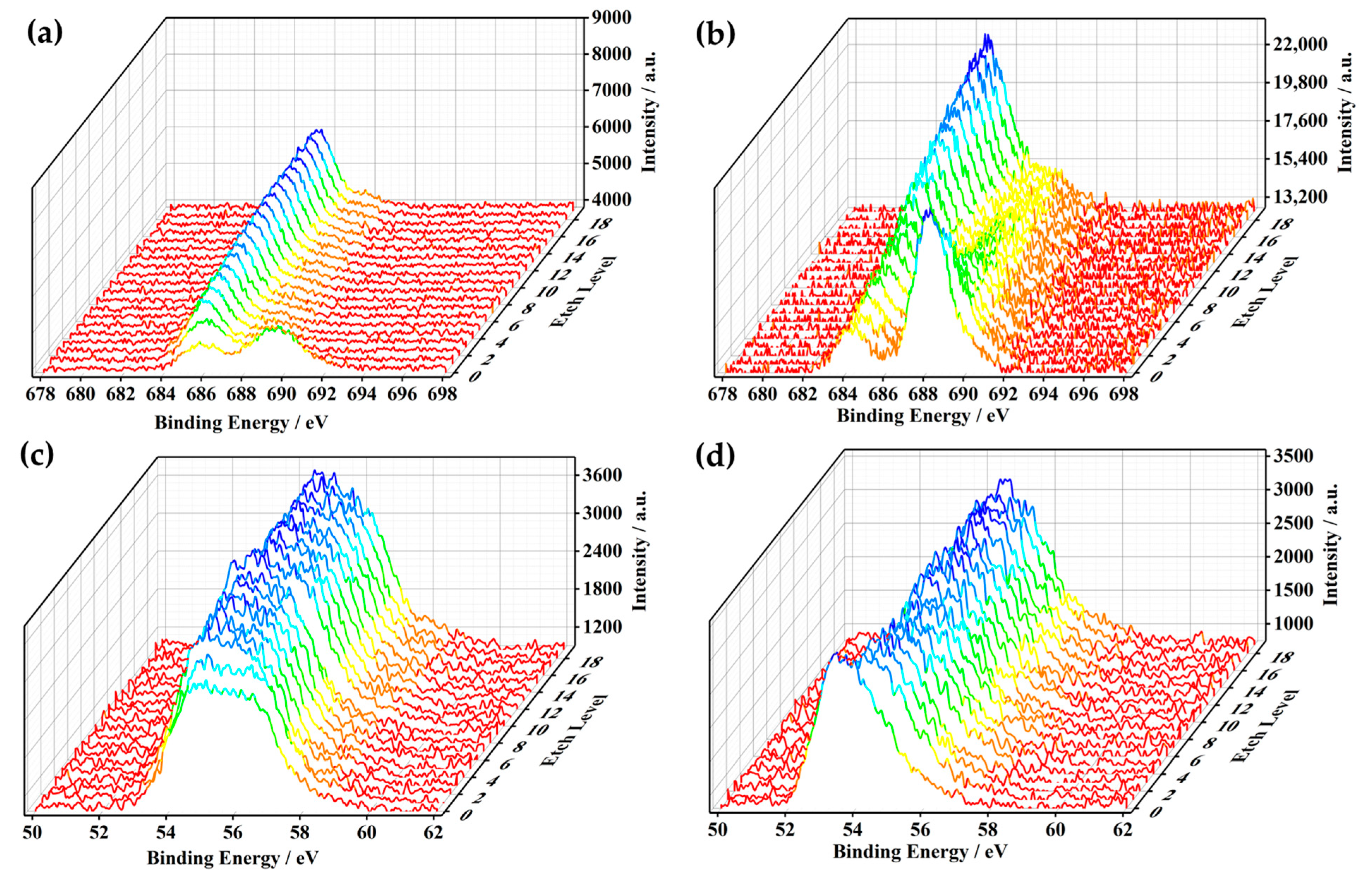
2.6. Characterization
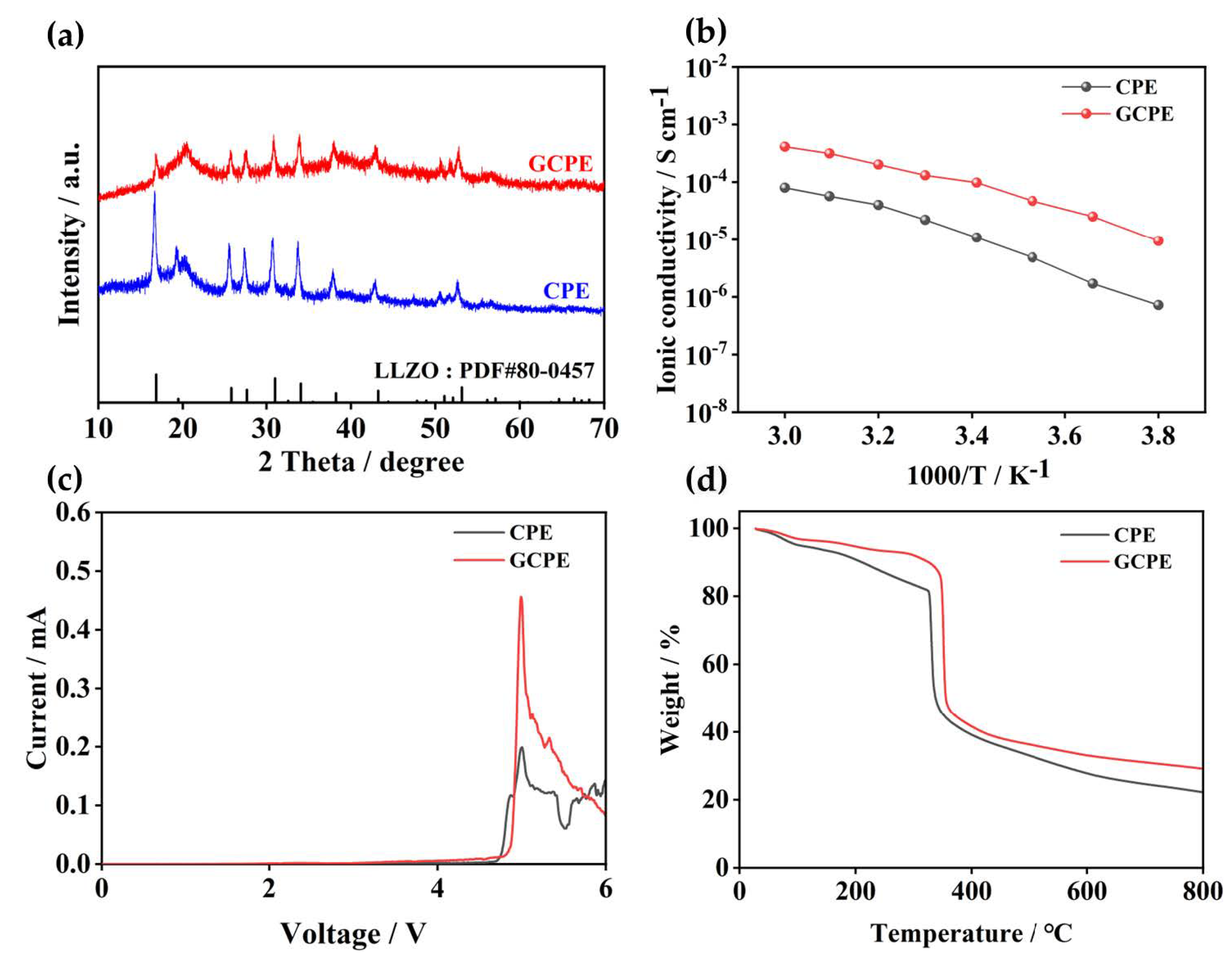
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Chen, R.; Li, Q.; Yu, X.; Chen, L.; Li, H. Approaching practically accessible solid-state batteries: Stability issues related to solid electrolytes and interfaces. Chem. Rev. 2019, 120, 6820–6877. [Google Scholar] [CrossRef]
- Liu, G.; Sun, Q.; Li, Q.; Zhang, J.; Ming, J. Electrolyte issues in lithium–sulfur batteries: Development, prospect, and challenges. Energy Fuels 2021, 35, 10405–10427. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.W.; Appleby, A.J. Solvent-free composite PEO-ceramic fiber/mat electrolytes for lithium secondary cells. J. Electrochem. Soc. 2004, 152, A205. [Google Scholar] [CrossRef]
- Yu, J.; Kwok, S.C.; Lu, Z.; Effat, M.B.; Lyu, Y.Q.; Yuen, M.M.; Ciucci, F. A ceramic-PVDF composite membrane with modified interfaces as an ion-conducting electrolyte for solid-state lithium-ion batteries operating at room temperature. ChemElectroChem 2018, 5, 2873–2881. [Google Scholar] [CrossRef]
- Kachmar, A.; Carignano, M.; Laino, T.; Iannuzzi, M.; Hutter, J. Mapping the free energy of lithium solvation in the Protic ionic liquid ethylammonuim nitrate: A metadynamics study. ChemSusChem 2017, 10, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, R.; Aravindan, V.; Srinivasan, M. Novel polymer electrolyte based on cob-web electrospun multi component polymer blend of polyacrylonitrile/poly (methyl methacrylate)/polystyrene for lithium ion batteries—Preparation and electrochemical characterization. J. Power Sources 2012, 202, 299–307. [Google Scholar] [CrossRef]
- Liang, B.; Tang, S.; Jiang, Q.; Chen, C.; Chen, X.; Li, S.; Yan, X. Preparation and characterization of PEO-PMMA polymer composite electrolytes doped with nano-Al2O3. Electrochim. Acta 2015, 169, 334–341. [Google Scholar] [CrossRef]
- Lu, Y.; Tu, Z.; Archer, L.A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 2014, 13, 961–969. [Google Scholar] [CrossRef]
- Song, S.; Qin, X.; Ruan, Y.; Li, W.; Xu, Y.; Zhang, D.; Thokchom, J. Enhanced performance of solid-state lithium-air batteries with continuous 3D garnet network added composite polymer electrolyte. J. Power Sources 2020, 461, 228146. [Google Scholar] [CrossRef]
- Han, X.; Gong, Y.; Fu, K.K.; He, X.; Hitz, G.T.; Dai, J.; Hu, L. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 2017, 16, 572–579. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Qiu, M.; Li, X.; Li, C.; Li, R.; Chen, Y. Research progress in electrospinning engineering for all-solid-state electrolytes of lithium metal batteries. J. Energy Chem. 2021, 61, 253–268. [Google Scholar] [CrossRef]
- Heubner, C.; Liebmann, T.; Voigt, K.; Weiser, M.; Matthey, B.; Junker, N.; Michaelis, A. Scalable fabrication of nanostructured tin oxide anodes for high-energy lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 27019–27029. [Google Scholar] [CrossRef]
- Li, W.; Song, B.; Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 2017, 46, 3006–3059. [Google Scholar] [CrossRef]
- Tan, S.J.; Yue, J.; Tian, Y.F.; Ma, Q.; Wan, J.; Xiao, Y.; Guo, Y.G. In-situ encapsulating flame-retardant phosphate into robust polymer matrix for safe and stable quasi-solid-state lithium metal batteries. Energy Storage Mater. 2021, 39, 186–193. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Dou, Q.; Wong, K.W.; Ng, K.M. Li7La3Zr2O12 ceramic nanofiber-incorporated composite polymer electrolytes for lithium metal batteries. J. Mater. Chem. A 2019, 7, 3391–3398. [Google Scholar] [CrossRef]
- Li, Z.; Xie, H.X.; Zhang, X.Y.; Guo, X. In situ thermally polymerized solid composite electrolytes with a broad electrochemical window for all-solid-state lithium metal batteries. J. Mater. Chem. A 2020, 8, 3892–3900. [Google Scholar] [CrossRef]
- Xu, S.; Sun, Z.; Sun, C.; Li, F.; Chen, K.; Zhang, Z.; Li, F. Homogeneous and fast ion conduction of PEO-based solid-state electrolyte at low temperature. Adv. Funct. Mater. 2020, 30, 2007172. [Google Scholar] [CrossRef]
- Yang, T.; Zheng, J.; Cheng, Q.; Hu, Y.Y.; Chan, C.K. Composite polymer electrolytes with Li7La3Zr2O12 garnet-type nanowires as ceramic fillers: Mechanism of conductivity enhancement and role of doping and morphology. ACS Appl. Mater. Interfaces 2017, 9, 21773–21780. [Google Scholar] [CrossRef]
- Hanai, K.; Ueno, M.; Imanishi, N.; Hirano, A.; Yamamoto, O.; Takeda, Y. Interfacial resistance of the LiFePO4-C/PEO-LiTFSI composite electrode for dry-polymer lithium-ion batteries. J. Power Sources 2011, 196, 6756–6761. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- He, K.; Chen, C.; Fan, R.; Liu, C.; Liao, C.; Xu, Y.; Li, R.K. Polyethylene oxide/garnet-type Li6. 4La3Zr1. 4Nb0. 6O12 composite electrolytes with improved electrochemical performance for solid state lithium rechargeable batteries. Compos. Sci. Technol. 2019, 175, 28–34. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, C.; Wang, L.; Zhang, W.; Zhang, L. Modification of Cycle Life Model for Normal Aging Trajectory Prediction of Lithium-Ion Batteries at Different Temperatures and Discharge Current Rates. World Electr. Veh. J. 2022, 13, 59. [Google Scholar] [CrossRef]
- Doux, J.M.; Yang, Y.; Tan, D.H.; Nguyen, H.; Wu, E.A.; Wang, X.; Meng, Y.S. Pressure effects on sulfide electrolytes for all solid-state batteries. J. Mater. Chem. A 2020, 8, 5049–5055. [Google Scholar] [CrossRef]
- Woods, J.; Bhattarai, N.; Chapagain, P.; Yang, Y.; Neupane, S. In situ transmission electron microscopy observations of rechargeable lithium ion batteries. Nano Energy 2019, 56, 619–640. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, W.; Chen, X.; Das, D.; Ruess, R.; Gautam, A.; Janek, J. Influence of crystallinity of lithium thiophosphate solid electrolytes on the performance of solid-state batteries. Adv. Energy Mater. 2021, 11, 2100654. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, X.; Xie, J.; Shen, C.; Jin, Y.; Huang, C.; Cheng, J. Scale-up processing of a safe quasi-solid-state lithium battery by cathode-supported solid electrolyte coating. Mater. Today Energy 2021, 21, 100841. [Google Scholar] [CrossRef]
- Huang, L.; Liu, L.; Lu, L.; Feng, X.; Han, X.; Li, W.; Ouyang, M. A review of the internal short circuit mechanism in lithium-ion batteries: Inducement, detection and prevention. Int. J. Energy Res. 2021, 45, 15797–15831. [Google Scholar] [CrossRef]
- Mu, W.; Liu, X.; Wen, Z.; Liu, L. Numerical simulation of the factors affecting the growth of lithium dendrites. J. Energy Storage 2019, 26, 100921. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Liang, Z.; Lee, H.W.; Sun, J.; Wang, H.; Cui, Y. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 2016, 11, 626–632. [Google Scholar] [CrossRef]
- Love, C.T.; Baturina, O.A.; Swider-Lyons, K.E. Observation of lithium dendrites at ambient temperature and below. ECS Electrochem. Lett. 2015, 4, A24. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, J.; Han, W.; Zhang, J.; Ding, G.; Dong, S.; Cui, G. In situ polymerization for integration and interfacial protection towards solid state lithium batteries. J. Electrochem. Soc. 2020, 167, 070527. [Google Scholar] [CrossRef]
- Yu, X.; Wang, L.; Ma, J.; Sun, X.; Zhou, X.; Cui, G. Selectively wetted rigid–flexible coupling polymer electrolyte enabling superior stability and compatibility of high-voltage lithium metal batteries. Adv. Energy Mater. 2020, 10, 1903939. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, Y.; Zhang, J.; Wu, X.; Yi, C.; Zhang, L.; Qi, S. Synergistic Effect of TMSPi and FEC in Regulating the Electrode/Electrolyte Interfaces in Nickel-Rich Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 11517–11527. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Z.; Amine, K. Fluorinated electrolytes for Li-ion battery: An FEC-based electrolyte for high voltage LiNi0.5Mn1.5O4/graphite couple. Electrochem. Commun. 2013, 35, 76–79. [Google Scholar] [CrossRef]
- Li, Q.; Liu, G.; Cheng, H.; Sun, Q.; Zhang, J.; Ming, J. Low-Temperature Electrolyte Design for Lithium-Ion Batteries: Prospect and Challenges. Chem. Eur. J. 2021, 27, 15842–15865. [Google Scholar] [CrossRef] [PubMed]
- Veith, G.M.; Doucet, M.; Sacci, R.L.; Vacaliuc, B.; Baldwin, J.K.; Browning, J.F. Determination of the solid electrolyte interphase structure grown on a silicon electrode using a fluoroethylene carbonate additive. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.; Hu, N.; Wang, S.; Geng, Z.; Deng, W. Enhancing Performance of LiFePO4 Battery by Using a Novel Gel Composite Polymer Electrolyte. Batteries 2023, 9, 51. https://doi.org/10.3390/batteries9010051
Wu K, Hu N, Wang S, Geng Z, Deng W. Enhancing Performance of LiFePO4 Battery by Using a Novel Gel Composite Polymer Electrolyte. Batteries. 2023; 9(1):51. https://doi.org/10.3390/batteries9010051
Chicago/Turabian StyleWu, Ke, Naiqi Hu, Shuchan Wang, Zhiyuan Geng, and Wenwen Deng. 2023. "Enhancing Performance of LiFePO4 Battery by Using a Novel Gel Composite Polymer Electrolyte" Batteries 9, no. 1: 51. https://doi.org/10.3390/batteries9010051
APA StyleWu, K., Hu, N., Wang, S., Geng, Z., & Deng, W. (2023). Enhancing Performance of LiFePO4 Battery by Using a Novel Gel Composite Polymer Electrolyte. Batteries, 9(1), 51. https://doi.org/10.3390/batteries9010051







