Study of the Carbochlorination Process with CaCl2 and Water Leaching for the Extraction of Lithium from Spent Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
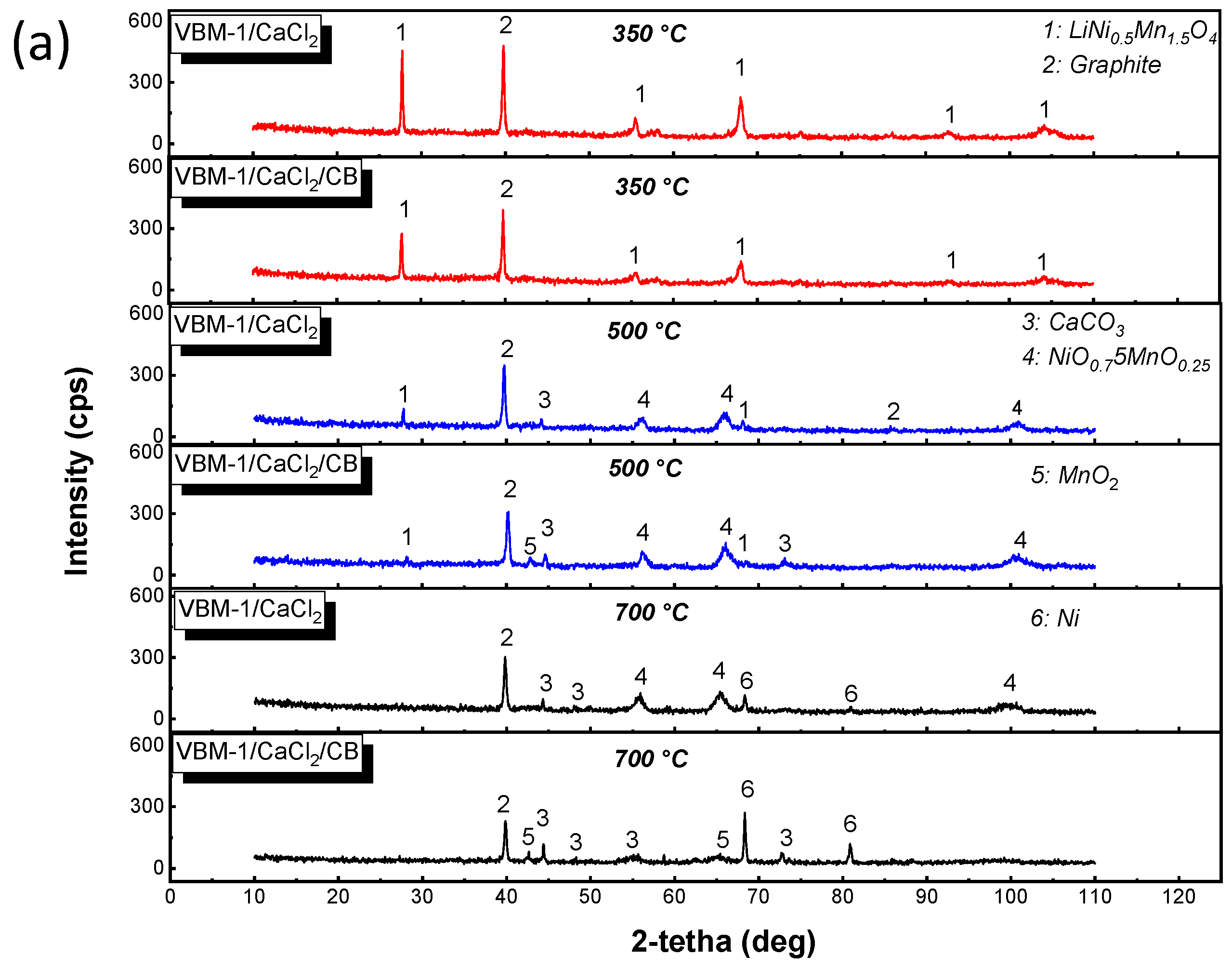
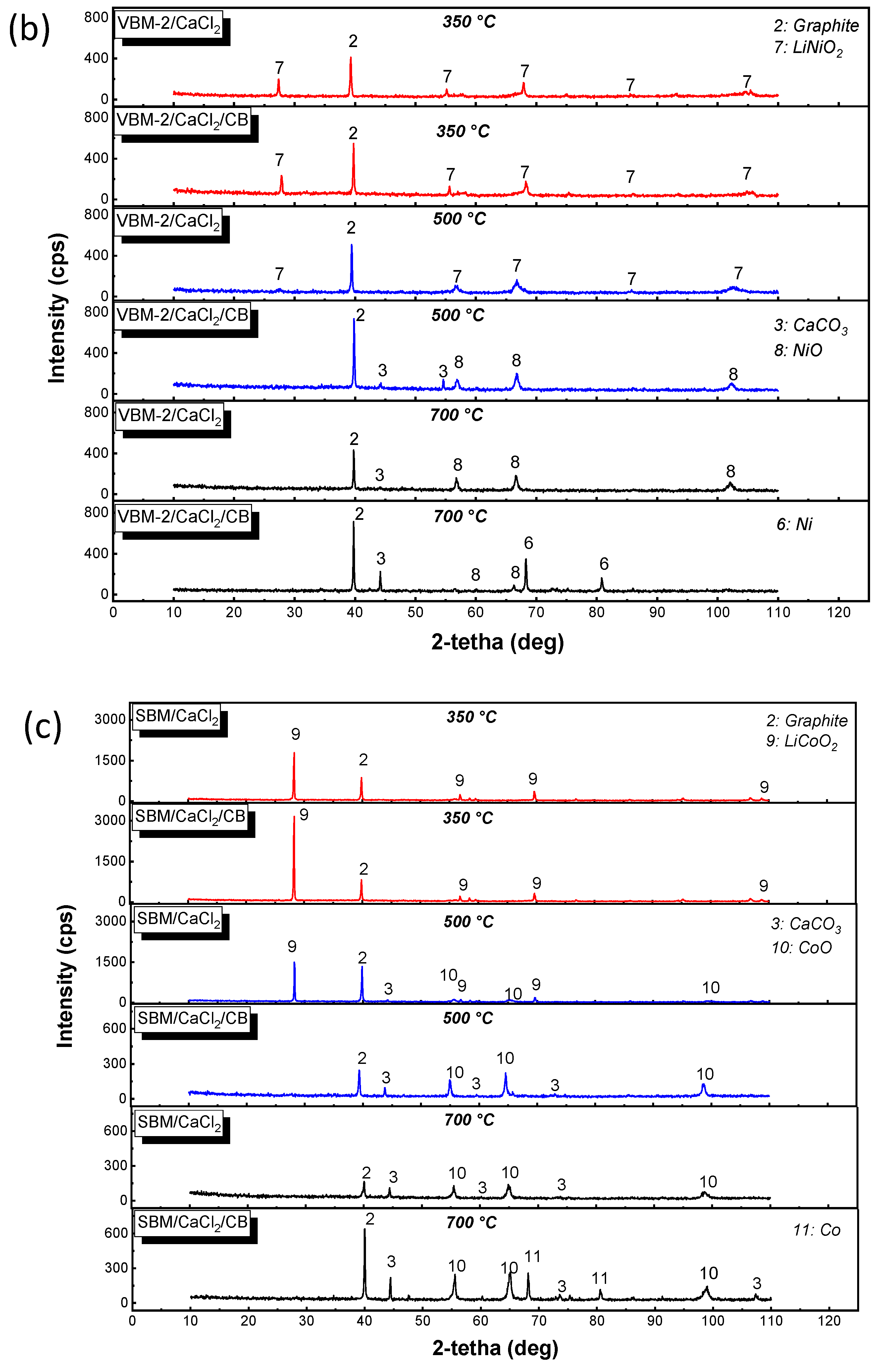
2.1. X-ray Diffraction (XRD)
2.2. Atomic Absorption Spectroscopy (AAS)
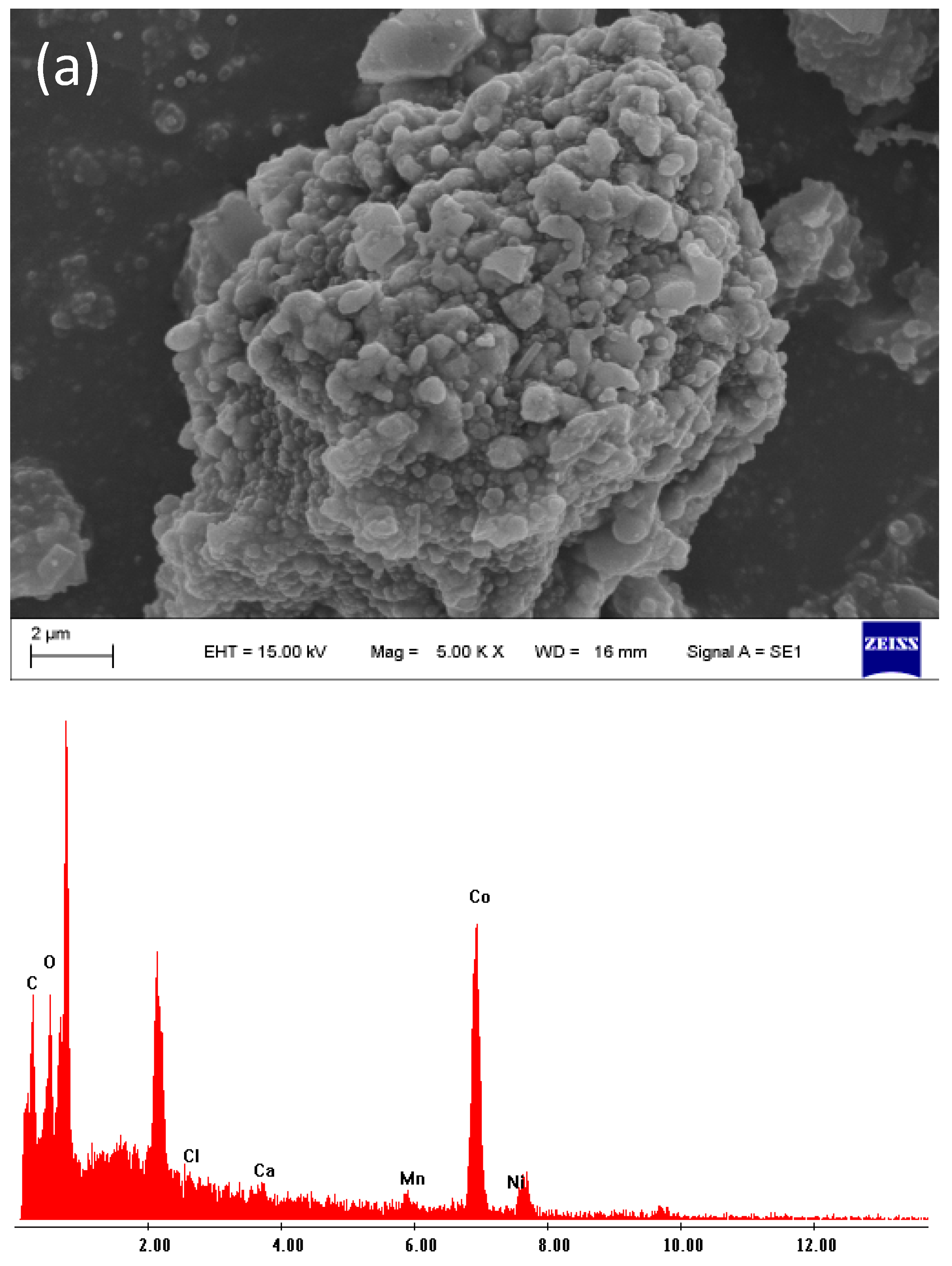
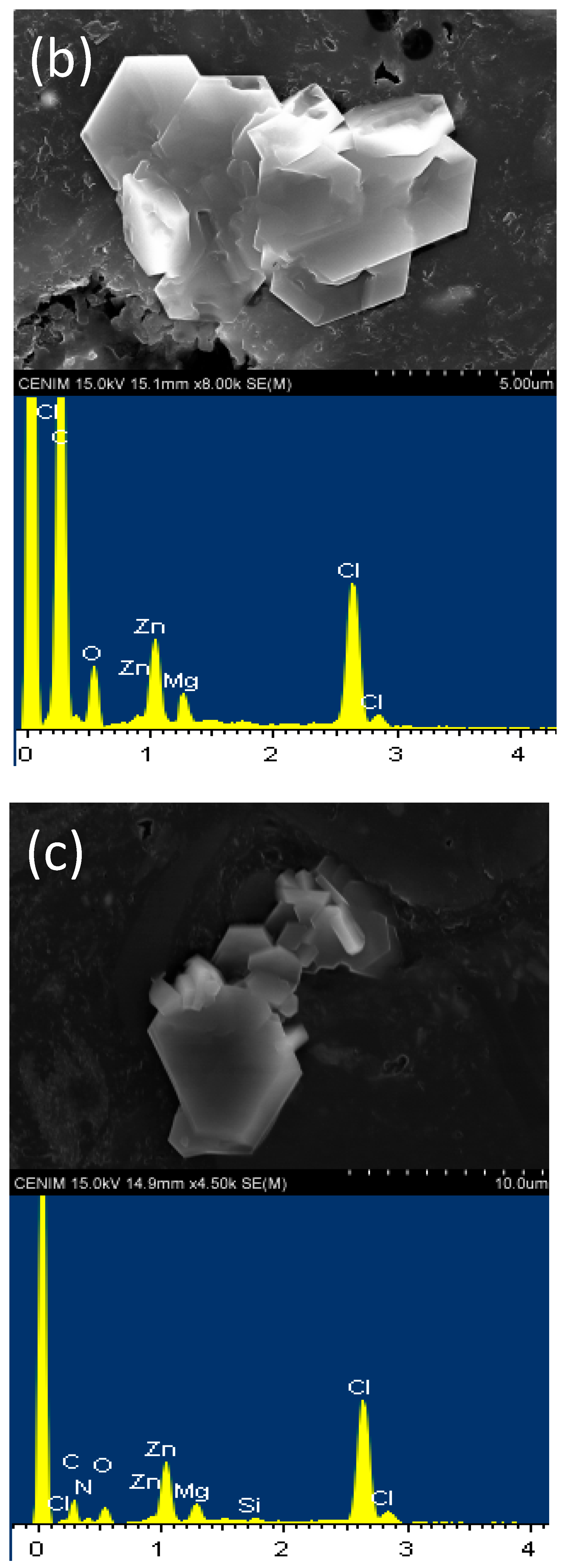
2.3. Scanning Electron Microscopy (SEM)
2.4. Thermogravimetric Analysis (TGA) and Differential Thermogravimetric Analysis (DTG)
3. Experimental Procedure
3.1. Non-Isothermal Experiments
3.2. Isothermal Experiments
4. Results and Discussion
4.1. Characterization of the Initial Black Mass Samples
4.2. Non-Isothermal Experiments
4.3. Isothermal Experiments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirza, M.; Abdulaziz, R.; Maskell, W.C.; Tan, C.; Shearing, P.R.; Brett, D.J.L. Recovery of Cobalt from Lithium-ion Batteries using Fluidised Cathode Molten Salt Electrolysis. Electrochim. Acta 2021, 391, 138846. [Google Scholar] [CrossRef]
- Moazzam, P.; Boroumand, Y.; Rabiei, P.; Baghbaderani, S.S.; Mokarian, P.; Mohagheghian, F.; Mohammed, L.J.; Razmjou, A. Lithium bioleaching: An emerging approach for the recovery of Li from spent lithium ion batteries. Chemosphere 2021, 277, 130196. [Google Scholar] [CrossRef] [PubMed]
- Mossali, E.; Picone, N.; Gentilini, L.; Rodrìguez, O.; Pérez, J.M.; Colledani, M. Lithium-ion batteries towards circular economy: A literature review of opportunities and issues of recycling treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A critical review of lithium-ion battery recycling processes from a circular economy perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Nasser, O.A.; Petranikova, M. Review of achieved purities after li-ion batteries hydrometallurgical treatment and impurities effects on the cathode performance. Batteries 2021, 7, 60. [Google Scholar] [CrossRef]
- Alcaraz, L.; Díaz-Guerra, C.; Calbet, J.; López, M.L.; López, F.A. Obtaining and Characterization of Highly Crystalline Recycled Graphites from Different Types of Spent Batteries. Materials 2022, 15, 3246. [Google Scholar] [CrossRef]
- Chandran, V.; Ghosh, A.; Patil, C.K.; Mohanavel, V.; Priya, A.K.; Rahim, R.; Madavan, R.; Muthuraman, U.; Karthick, A. Comprehensive review on recycling of spent lithium-ion batteries. Mater. Today Proc. 2021, 47, 167–180. [Google Scholar] [CrossRef]
- Bankole, O.E.; Lei, L. Battery Recycling Technologies: Recycling Waste Lithium Ion Batteries with the Impact on the Environment In-View. J. Environ. Ecol. 2017, 4, 14. [Google Scholar] [CrossRef]
- Magalini, F.; Kuehr, R.; Baldé, C.P. eWaste en América Latina. GSMA Lat. Am. 2015, 1–38. [Google Scholar]
- Romo, L.A.; López-Fernández, A.; García-Díaz, I.; Fernández, P.; Urbieta, A.; López, F.A. From spent alkaline batteries to ZnxMn3−xO4 by a hydrometallurgical route: Synthesis and characterization. RSC Adv. 2018, 8, 33496–33505. [Google Scholar] [CrossRef]
- Ji, Y.; Jafvert, C.T.; Zhao, F. Recovery of cathode materials from spent lithium-ion batteries using eutectic system of lithium compounds. Resour. Conserv. Recycl. 2021, 170, 105551. [Google Scholar] [CrossRef]
- Barbosa, L.; Luna-Lama, F.; Peña, Y.G.; Caballero, A. Simple and Eco-Friendly Fabrication of Electrode Materials and Their Performance in High-Voltage Lithium-Ion Batteries. ChemSusChem 2020, 13, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.M.; Barbosa, J.C.; Gonçalves, R.; Castro, H.; Del Campo, F.J.; Lanceros-Méndez, S. Recycling and environmental issues of lithium-ion batteries: Advances, challenges and opportunities. Energy Storage Mater. 2021, 37, 433–465. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-lópez, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Choubey, P.K.; Kim, M.S.; Srivastava, R.R.; Lee, J.C.; Lee, J.Y. Advance review on the exploitation of the prominent energy-storage element: Lithium. Part I: From mineral and brine resources. Miner. Eng. 2016, 89, 119–137. [Google Scholar] [CrossRef]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef]
- Pagliaro, M.; Meneguzzo, F. Lithium battery reusing and recycling: A circular economy insight. Heliyon 2019, 5, e01866. [Google Scholar] [CrossRef]
- González, Y.C.; Barrios, O.C.; González, J.A.; Barbosa, L.I. Study on the carboreduction of the cathode material present in spent LIBs to produce Li2CO3 and CoO. Miner. Eng. 2022, 184, 107665. [Google Scholar] [CrossRef]
- Zhou, L.F.; Yang, D.; Du, T.; Gong, H.; Luo, W.B. The Current Process for the Recycling of Spent Lithium Ion Batteries. Front. Chem. 2020, 8, 1–7. [Google Scholar] [CrossRef]
- Yang, H.; Deng, B.; Jing, X.; Li, W.; Wang, D. Direct recovery of degraded LiCoO2 cathode material from spent lithium-ion batteries: Efficient impurity removal toward practical applications. Waste Manag. 2021, 129, 85–94. [Google Scholar] [CrossRef]
- Guo, Y.; Li, F.; Zhu, H.; Li, G.; Huang, J.; He, W. Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl). Waste Manag. 2016, 51, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Pinna, E.G.; Ruiz, M.C.; Ojeda, M.W.; Rodriguez, M.H. Cathodes of spent Li-ion batteries: Dissolution with phosphoric acid and recovery of lithium and cobalt from leach liquors. Hydrometallurgy 2017, 167, 66–71. [Google Scholar] [CrossRef]
- Jung, Y.; Yoo, B.; Park, S.; Kim, Y.; Son, S. Study on Roasting for Selective Lithium Leaching of Cathode Active Materials from Spent Lithium-Ion Batteries. Metals 2021, 11, 1336. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, Y.; Song, S.; Xu, R.; Sun, W.; Xu, S.; Yang, Y. Strengthening Valuable Metal Recovery from Spent Lithium-Ion Batteries by Environmentally Friendly Reductive Thermal Treatment and Electrochemical Leaching. ACS Sustain. Chem. Eng. 2021, 9, 7053–7062. [Google Scholar] [CrossRef]
- Yue, Y.; Wei, S.; Yongjie, B.; Chenyang, Z.; Shaole, S.; Yuehua, H. Recovering Valuable Metals from Spent Lithium Ion Battery via a Combination of Reduction Thermal Treatment and Facile Acid Leaching. ACS Sustain. Chem. Eng. 2018, 6, 10445–10453. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Li, H.; Chen, Y.; Wang, C. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J. Power Sources 2017, 351, 192–199. [Google Scholar] [CrossRef]
- Barrios, O.C.; González, Y.C.; Barbosa, L.I.; Orosco, P. Chlorination roasting of the cathode material contained in spent lithium-ion batteries to recover lithium, manganese, nickel and cobalt. Miner. Eng. 2022, 176, 107321. [Google Scholar] [CrossRef]
- Movahedian, A.; Raygan, S.; Pourabdoli, M. The chlorination kinetics of zirconium dioxide mixed with carbon black. Thermochim. Acta 2011, 512, 93–97. [Google Scholar] [CrossRef]
- González, J.; Bohé, A.; Pasquevich, D.; Del, M. β-Ta2O5 carbochlorination with different types of carbon. Can. Metall. Q. 2002, 41, 29–40. [Google Scholar] [CrossRef]
- Banik, R.; Suresh, N.; Mandre, N.R. Selective Flocculation as a Pre-Concentration Process—An Overview. Miner. Process. Extr. Metall. Rev. Int. J. 1995, 14, 169–177. [Google Scholar] [CrossRef]
- Barbosa, L.I.; González, J.A.; Ruiz, M.D.C. Extraction of lithium from β-spodumene using chlorination roasting with calcium chloride. Thermochim. Acta 2015, 605, 63–67. [Google Scholar] [CrossRef]
- Qu, X.; Xie, H.; Chen, X.; Tang, Y.; Zhang, B.; Xing, P.; Yin, H. Recovery of LiCoO2 from Spent Lithium-Ion Batteries through a Low-Temperature Ammonium Chloride Roasting Approach: Thermodynamics and Reaction Mechanisms. ACS Sustain. Chem. Eng. 2020, 8, 6524–6532. [Google Scholar] [CrossRef]
- Jian, Y.; Zongliang, Z.; Gang, Z.; Liangxing, J.; Fangyang, L.; Ming, J.; Yanqing, L. Process study of chloride roasting and water leaching for the extraction of valuable metals from spent lithium-ion batteries. Hydrometallurgy 2021, 203, 105638. [Google Scholar] [CrossRef]
- Li, W.; Song, B.; Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 2017, 46, 3006–3059. [Google Scholar] [CrossRef]
- Túnez, F.M.; González, J.; Ruiz, M.D.C. Aparato de Laboratorio para Realizar Termogravimetrías en Atmósferas Corrosivas y no Corrosivas. AR Patent 053676 A1, P060100450, 2007. [Google Scholar]
- Zhao, B.L.; Li, N.; Langner, A.; Steinhart, M.; Tan, T.Y.; Pippel, E.; Hofmeister, H.; Tu, K.; Gösele, U. Crystallization of Amorphous SiO2 Microtubes Catalyzed by Lithium. Adv. Funct. Mater. 2007, 17, 1952–1957. [Google Scholar] [CrossRef]
- González, J.; Del Ruiz, M.C.; Bohé, A.; Pasquevich, D. Oxidation of carbons in the presence of chlorine. Carbon N. Y. 1999, 37, 1979–1988. [Google Scholar] [CrossRef]
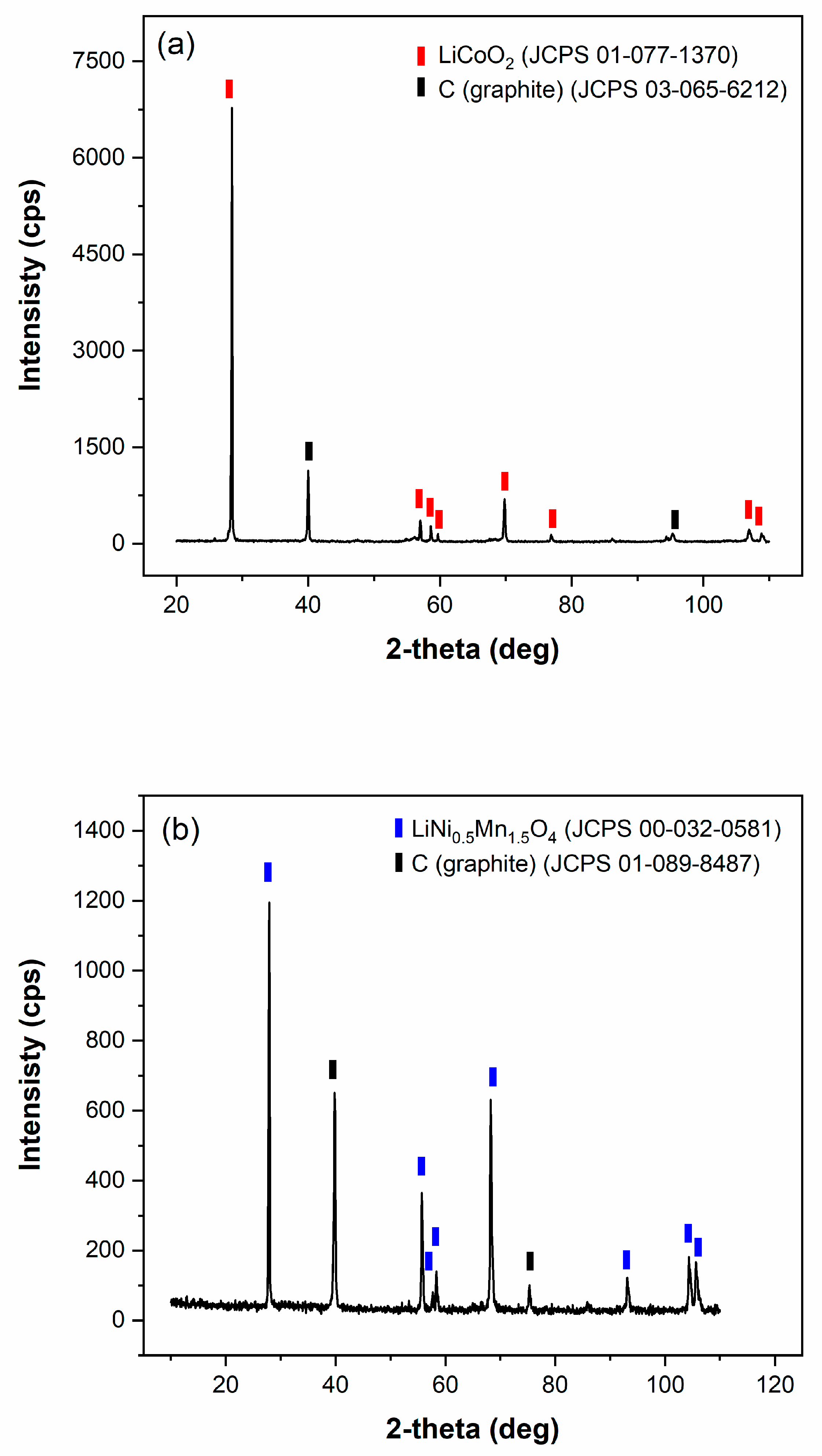
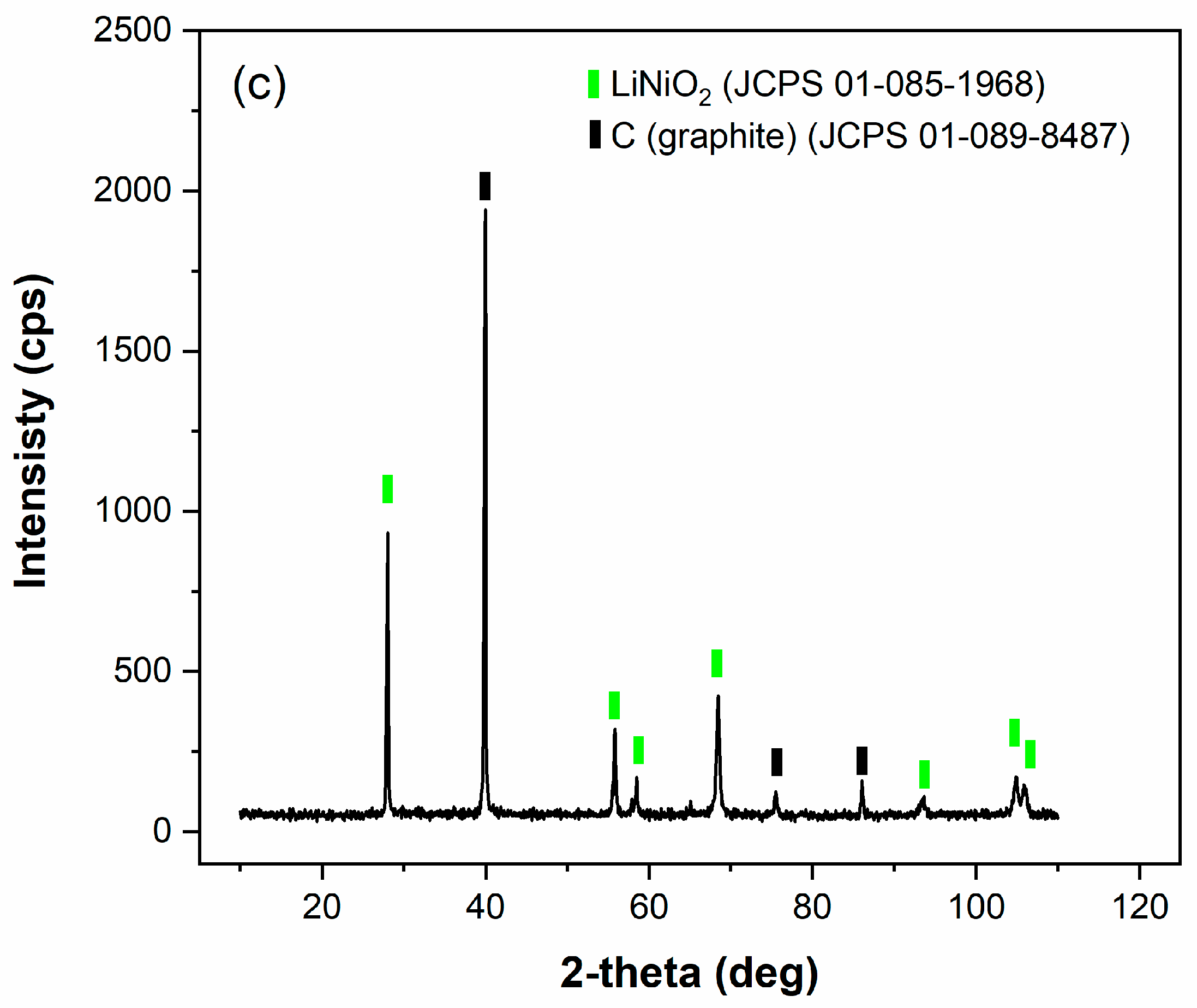

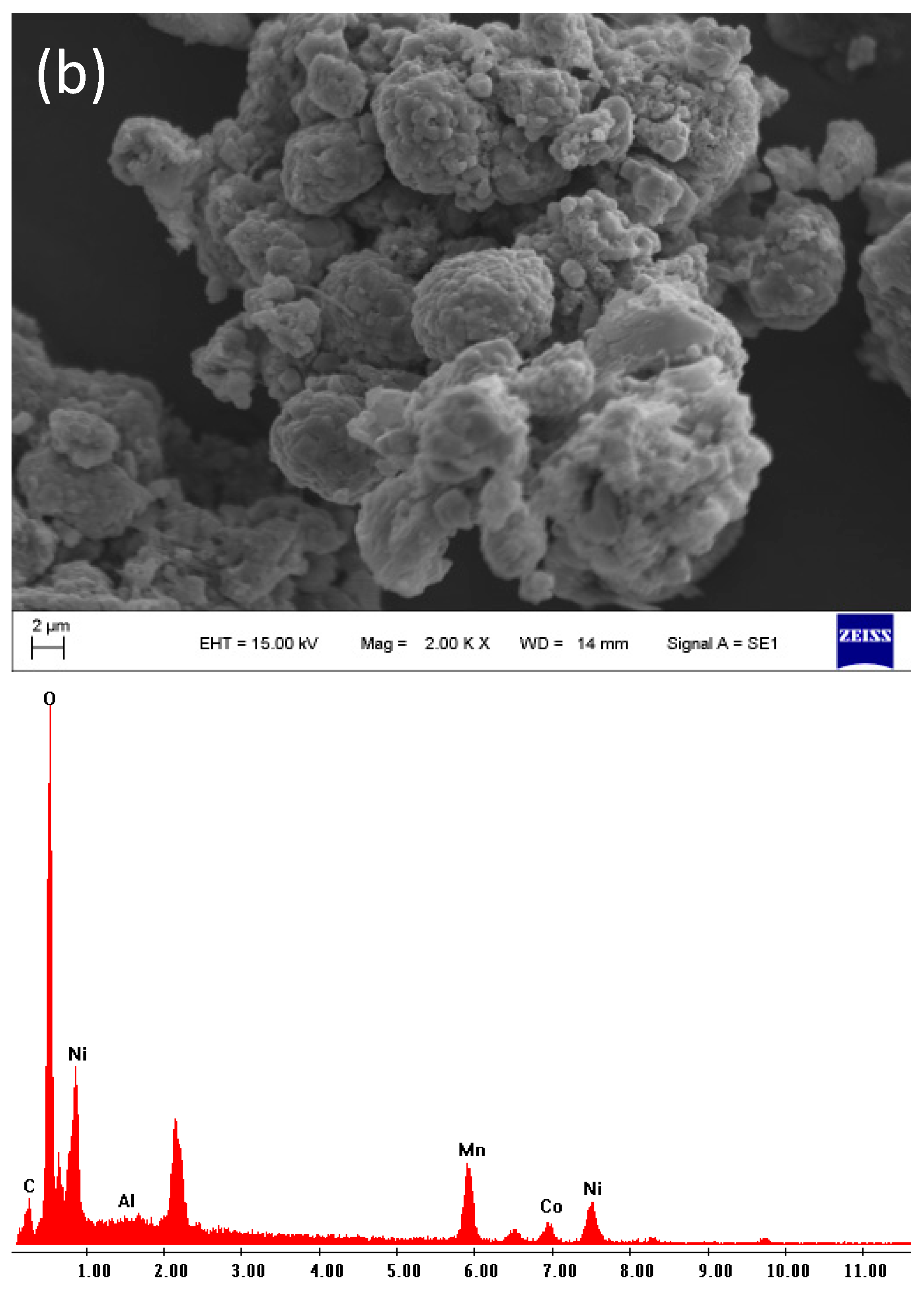
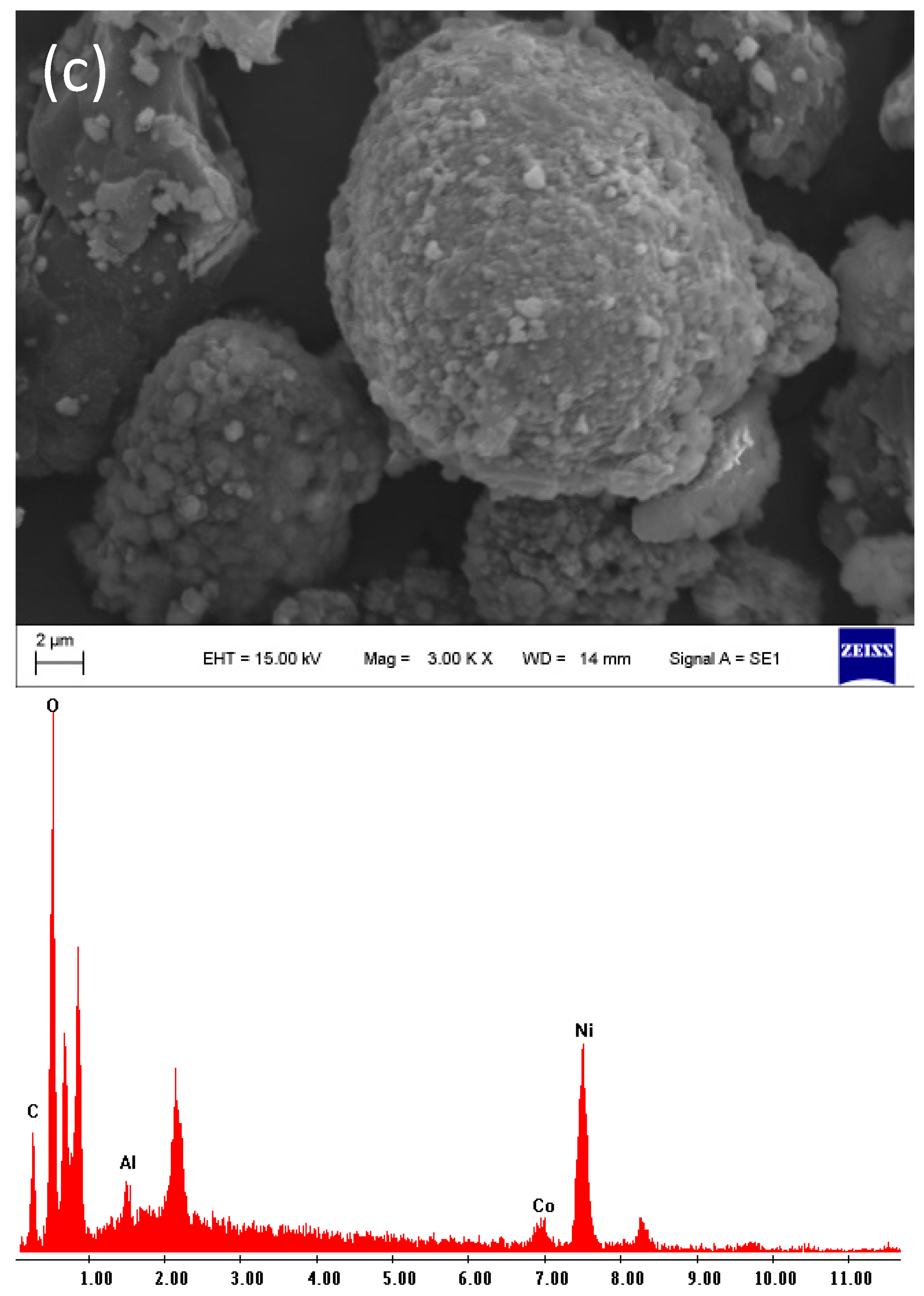






| Sample | Li | Co | Mn | Ni | Cu | C |
|---|---|---|---|---|---|---|
| SBM 1 | 4.5 | 30 | 4.30 | 0.90 | 0.30 | 33.3 |
| VBM-1 | 3.11 | 5.62 | 10.1 | 11.2 | 2.2 | 32 |
| VBM-2 | 5.2 | 3.2 | n.d. | 41.2 | 1.2 | 30.9 |
| Temperature (°C) | BM/CaCl2 | Δm (%) | BM/CaCl2/Carbon Black | Δm (%) |
|---|---|---|---|---|
| 350 | VBM-1 | 7 | VBM-1 | 24 |
| VBM-2 | 12 | VBM-2 | 20 | |
| SBM | 4 | SBM | 27 | |
| 500 | VBM-1 | 10 | VBM-1 | 27 |
| VBM-2 | 14 | VBM-2 | 25 | |
| SBM | 6 | SBM | 27 | |
| 700 | VBM-1 | 12 | VBM-1 | 30 |
| VBM-2 | 15 | VBM-2 | 30 | |
| SBM | 7 | SBM | 31 |
| Temperature (°C) | BM/CaCl2 | X (%) | BM/CaCl2/Carbon Black | X (%) |
|---|---|---|---|---|
| 500 | VBM-1 | 35 | VBM-1 | 76 |
| VBM-2 | 35 | VBM-2 | 80 | |
| SBM | 60 | SBM | 95 | |
| 700 | VBM-1 | 90 | VBM-1 | 99 |
| VBM-2 | 90 | VBM-2 | 99 | |
| SBM | 95 | SBM | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, Y.C.; Alcaraz, L.; Alguacil, F.J.; González, J.; Barbosa, L.; López, F.A. Study of the Carbochlorination Process with CaCl2 and Water Leaching for the Extraction of Lithium from Spent Lithium-Ion Batteries. Batteries 2023, 9, 12. https://doi.org/10.3390/batteries9010012
González YC, Alcaraz L, Alguacil FJ, González J, Barbosa L, López FA. Study of the Carbochlorination Process with CaCl2 and Water Leaching for the Extraction of Lithium from Spent Lithium-Ion Batteries. Batteries. 2023; 9(1):12. https://doi.org/10.3390/batteries9010012
Chicago/Turabian StyleGonzález, Yarivith C., Lorena Alcaraz, Francisco J. Alguacil, Jorge González, Lucía Barbosa, and Félix A. López. 2023. "Study of the Carbochlorination Process with CaCl2 and Water Leaching for the Extraction of Lithium from Spent Lithium-Ion Batteries" Batteries 9, no. 1: 12. https://doi.org/10.3390/batteries9010012
APA StyleGonzález, Y. C., Alcaraz, L., Alguacil, F. J., González, J., Barbosa, L., & López, F. A. (2023). Study of the Carbochlorination Process with CaCl2 and Water Leaching for the Extraction of Lithium from Spent Lithium-Ion Batteries. Batteries, 9(1), 12. https://doi.org/10.3390/batteries9010012








