Band-Gap Engineering: Lithium Effect on the Electronic Properties of Hydrogenated 3C-SiC (1 1 0) Surfaces
Abstract
1. Introduction
2. Methods
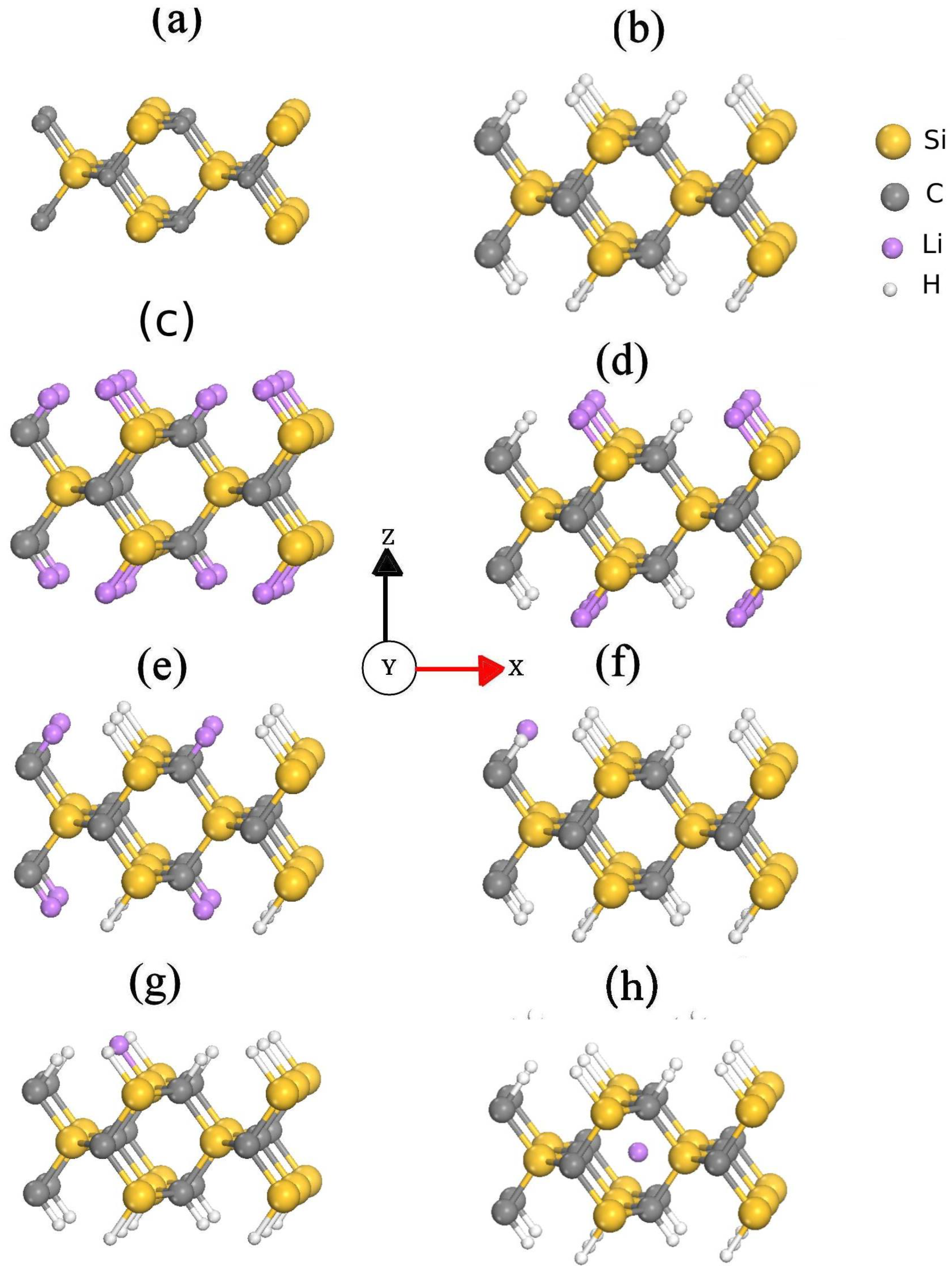
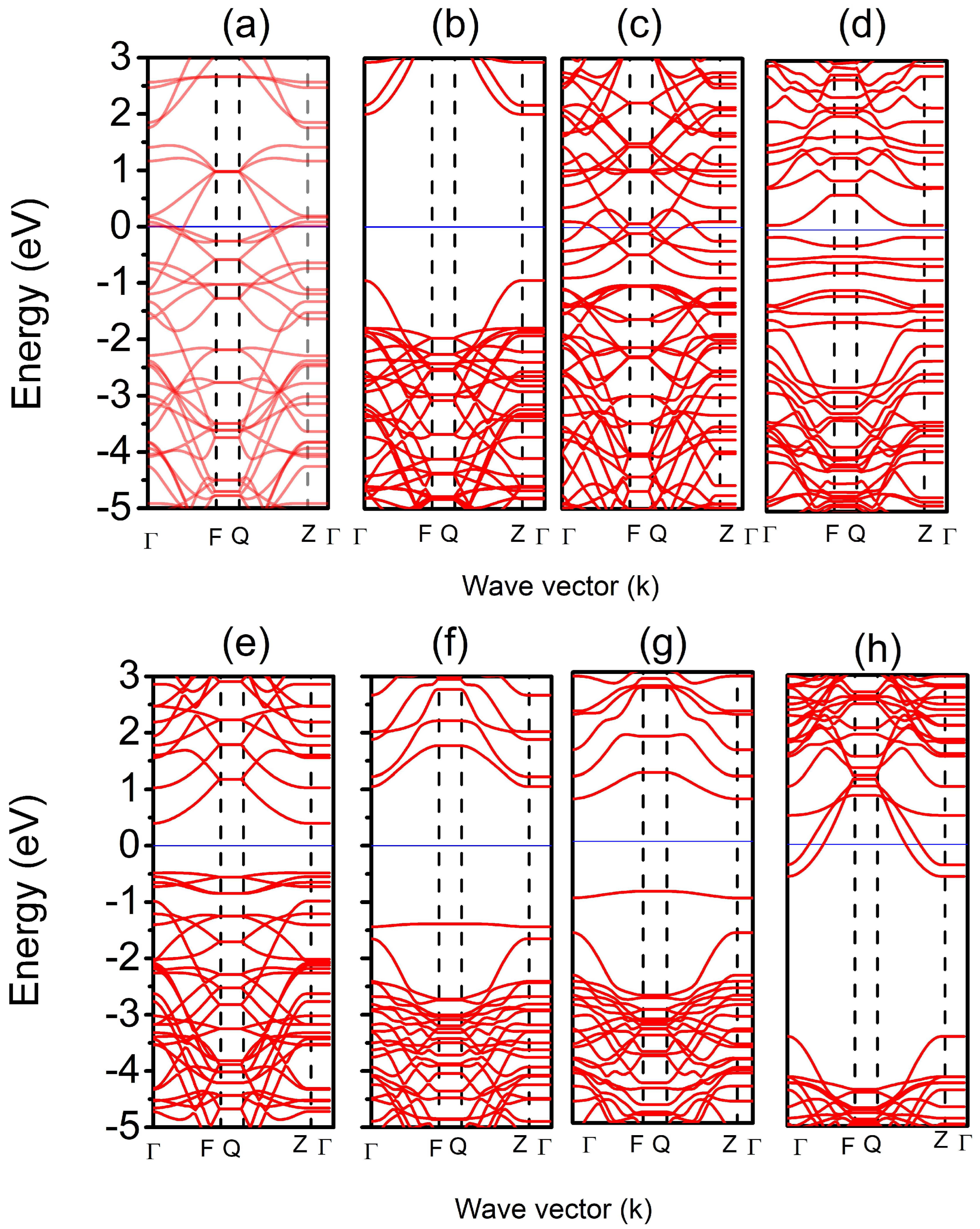
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, Z.; Wang, C.; Wan, C.; Wang, R.; Dai, X.; Wei, J.; Xia, H.; Li, W.; Zhang, W.; Cao, S.; et al. Strain-Driven Auto-Detachable Patterning of Flexible Electrodes. Adv. Mater. 2022, 34, 2202877. [Google Scholar] [CrossRef]
- Miao, L.; Wang, R.; Di, S.; Qian, Z.; Zhang, L.; Xin, W.; Liu, M.; Zhu, Z.; Chu, S.; Du, Y.; et al. Aqueous Electrolytes with Hydrophobic Organic Cosolvents for Stabilizing Zinc Metal Anodes. ACS Nano 2022, 16, 9667–9678. [Google Scholar] [CrossRef]
- Yang, K.; Liu, D.; Qian, Z.; Jiang, D.; Wang, R. Computational auxiliary for the progress of sodium-ion solid-state electrolytes. ACS Nano 2021, 15, 17232–17246. [Google Scholar] [CrossRef] [PubMed]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, Y.; Chen, Q. High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal-organic framework. Nat. Commun. 2014, 5, 5261. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Niaei, A.H.F.; Searles, D.J.; Hankel, M. Three-Dimensional Silicon Carbide from Siligraphene as a High Capacity Lithium Ion Battery Anode Material. J. Phys. Chem. C 2019, 123, 45. [Google Scholar] [CrossRef]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A5019. [Google Scholar] [CrossRef]
- Edge, J.S.; O’Kane, S.; Prosser, R.; Kirkaldy, N.D.; Patel, A.N.; Hales, A.; Ghosh, A.; Ai, W.; Chen, J.; Yang, J.; et al. Lithium ion battery degradation: What you need to know. Phys. Chem. Chem. Phys. 2021, 23, 8200. [Google Scholar] [CrossRef]
- Pender, J.P.; Jha, G.; Youn, D.H.; Ziegler, J.M.; Andoni, I.; Choi, E.J.; Heller, A.; Dunn, B.S.; Weiss, P.S.; Penner, R.M.; et al. Electrode Degradation in Lithium-Ion Batteries. ACS Nano 2020, 14, 1243. [Google Scholar] [CrossRef]
- Zhu, J.; Chroneos, A.; Schwingenschlögl, U. Silicene/germanene on MgX2(X = Cl, Br, and I) for Li-ion battery applications. Nanoscale 2017, 8, 7272. [Google Scholar] [CrossRef]
- Akinwande, D.; Brennan, C.J.; Bunch, J.S.; Egberts, P.; Felts, J.R.; Gao, H.; Huang, R.; Kim, J.-S.; Li, T.; Li, Y.; et al. A review on mechanics and mechanical properties of 2D materials—Graphene and beyond. Extrem. Mech. Lett. 2017, 13, 42. [Google Scholar] [CrossRef]
- Vishnoi, P.; Pramoda, K.; Rao, C.N.R. 2D Elemental Nanomaterials Beyond Graphene. ChemNanoMat 2019, 5, 1062–1091. [Google Scholar] [CrossRef]
- Calabretta, C.; Scuderi, V.; Anzalone, R.; Mauceri, M.; Crippa, D.; Cannizzaro, A.; Boninelli, S.; Via, F.L. Effect of Nitrogen and Aluminum Doping on 3C-SiC Heteroepitaxial Layers Grown on 4° Off-Axis Si (100). Materials 2021, 14, 440. [Google Scholar] [CrossRef]
- Huczko, A.; Dąbrowska, A.; Savchyn, V.; Popov, A.I.; Karbovnyk, I. Silicon carbide nanowires: Synthesis and cathodoluminescence. Phys. Status Solidi B 2009, 246, 2806–2808. [Google Scholar] [CrossRef]
- Khan, A.A.; Ahmad, R.; Ahmad, I. Silicon carbide and III-Nitrides nanosheets: Promising anodes for Mg-ion batteries. Mater. Chem. Phys. 2021, 257, 123785. [Google Scholar] [CrossRef]
- Xiang, K.; Wang, X.; Chen, M.; Shen, Y.; Shu, H.; Yang, X. Industrial waste silica preparation of silicon carbide composites and their applications in lithium-ion battery anode. J. Alloys Compd. 2017, 695, 100–105. [Google Scholar] [CrossRef]
- Amy, F.; Chabal, Y.J. Interaction of H, O2, and H2O with 3C-SiC surfaces. J. Chem. Phys. 2003, 119, 6201. [Google Scholar] [CrossRef]
- Cicero, G.; Catellani, A. Towards SiC surface functionalization: An ab initio study. J. Chem. Phys. 2005, 122, 214716. [Google Scholar] [CrossRef]
- Coletti, C.; Frewin, C.L.; Saddow, S.E.; Hetzel, M.; Virojanadara, C.; Starke, U. Surface studies of hydrogen etched 3C-SiC (001) on Si(001). Appl. Phys. Lett. 2007, 91, 061914. [Google Scholar] [CrossRef]
- Catellani, A.; Galli, G. Theoretical studies of silicon carbide surfaces. Prog. Surf. Sci. 2002, 69, 101. [Google Scholar] [CrossRef]
- Catellani, A.; Calzolari, A. Functionalization of SiC(110) Surfaces via Porphyrin Adsorption: Ab Initio Results. J. Phys. Chem. C 2012, 116, 886. [Google Scholar] [CrossRef]
- Wenzien, B.; Käckell, P.K.; Bechstedt, F. Ab initio calculation of the atomic and electronic structure for the clean 3C-SiC(110) 1 × 1 surface. Surf. Sci. 1994, 307, 989. [Google Scholar] [CrossRef]
- Gavrilenko, V.I. Calculated differential reflectance of the (110) surface of cubic silicon carbide. Appl. Phys. Lett. 1995, 67, 16. [Google Scholar] [CrossRef]
- Park, T.; Park, C.; Jung, J.; Yun, G.J. Investigation of Silicon Carbide Oxidation Mechanism Using ReaxFF Molecular Dynamics Simulation. J. Spacecr. Rocket. 2020, 57, 1328–1334. [Google Scholar] [CrossRef]
- Trejo, A.; Cuevas, J.L.; Salazar, F.; Carvajal, E.; Cruz-Irisson, M. Ab-initio study of anisotropic and chemical surface modifications of β-SiC nanowires. J. Mol. Model. 2013, 19, 2043–2048. [Google Scholar] [CrossRef]
- Soler, J.M.; Artacho, E.; Gale, J.D.; García, A.; Junquera, J.; Ordejón, P.; Sánchez-Portal, D. The SIESTA method for ab initio order-N materials simulation. J. Phys. Condens. Matter 2002, 14, 2745. [Google Scholar] [CrossRef]
- Calvino, M.; Trejo, A.; Cuevas, J.; Carvajal, E.; Duchén, G.; Cruz-Irisson, M. A Density Functional Theory study of the chemical surface modification of β-SiC nanopores. Mater. Sci. Eng. B 2012, 177, 1482. [Google Scholar] [CrossRef]
- Cuevas, J.L.; de Santiago, F.; Ramírez, J.; Alejandro Trejo, A.M.; Pérez, L.A.; Cruz-Irisson, M. First principles band-gap engineering of [1 1 0] oriented 3C-SiC nanowires. Comput. Mater. Sci. 2018, 142, 268. [Google Scholar] [CrossRef]
- Dyall, K.G.; Taylor, P.R.; Faegri, K., Jr.; Partridge, H. All electron molecular Dirac Hartree Fock calculations: The group IV tetrahydrides CH4, SiH4, GeH4, SnH4, and PbH4. J. Chem. Phys. 1991, 95, 2583. [Google Scholar] [CrossRef]
- Wan, W.; Zhang, Q.; Cui, Y.; Wang, E. First principles study of lithium insertion in bulk silicon. J. Phys. Condens. Matter 2010, 22, 415501. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L. Structural Changes in Silicon Anodes during Lithium Insertion/Extraction. Electrochem. Solid-State Lett. 2004, 7, A93. [Google Scholar] [CrossRef]
- Sheehan, W.F., Jr.; Schomaker, V. The Si–C Bond Distance in Si(CH3)4. J. Am. Chem. Soc. 1952, 74, 3956. [Google Scholar] [CrossRef]
- Boyd, D.R.J. Infrared Spectrum of Trideuterosilane and the Structure of the Silane Molecule. J. Chem. Phys. 1955, 23, 922. [Google Scholar] [CrossRef]
- Kim, H.; Chou, C.Y.; Ekerdt, J.G.; Hwang, G.S. Structure and Properties of Li-Si Alloys: A First-Principles Study. J. Phys. Chem. C 2011, 115, 2514–2521. [Google Scholar] [CrossRef]
- Bravo-Zhivotovskii, D.; Yuzefovich, M.; Sigal, N.; Korogodsky, G.; Klinkhammer, K.; Tumanskii, B.; Shames, A.; Apeloig, Y. The Synthesis of the First Compound with Li-Si-Hg Bonding: [{Li(iPr3Si)2Si}2Hg]—A Source for the [Li(iPr3Si)2Si] Radical. Angew. Chem. Int. 2002, 41, 649–651. [Google Scholar] [CrossRef]
- McKean, D.C.; Boggs, J.E.; Schäfer, L. CH bond length variations due to the intramolecular environment: A comparison of the results obtained by the method of isolated CH stretching frequencies and by ab initio gradient calculations. J. Mol. Struct. 1984, 116, 313. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, M.; Liu, H. Predicted two-dimensional electrides: Lithium-carbon monolayer sheet. Phys. Lett. A 2015, 379, 2511. [Google Scholar] [CrossRef]



| Parameter | Value in Calculation |
|---|---|
| Exchange and correlation energy | GGA-RPBE |
| Cutoff energy | 350 eV |
| k-point mesh | 8 × 8 × 1 |
| Force tolerance | 0.001 eV/Å |
| Basis set | DZP |
| DM tolerance | 0.0001 |
| SCF iterations | 1000 |
| Geometry optimization | cg |
| Passivation Scheme | Angles () | Bond Length (Å) | Experimental Value (Å) |
|---|---|---|---|
| Pristine | = 113.74 = 113.7 = 89.72 | Si–C = 1.91 | |
| Full-H | = 109.4 = 109.4 = 109.7 | Si–C = 1.89 Si–H = 1.52 C–H = 1.11 | |
| Full-Li | = 109.6 = 109.6 = 108.9 | Si–C = 1.89 Si–Li = 1.58 C–Li = 1.19 | |
| CH+SiLi | = 106.11 = 109.34 = 107.46 | Si–C = 1.95 Si–Li = 2.56 C–H = 1.12 | |
| CLi+SiH | = 109.6 = 109.6 = 109.1 | Si–C = 1.8, 1.9 Si–H = 1.65 C–Li = 2.07 | Si–C = 1.93 [32] Si–H = 1.89 [33] Si–Li = 2.57–3.09 [34,35] C–H = 1.08–1.10 [32,36] C–Li = 2.02 [37] |
| H+1LiC | = 109.9 = 110.9 = 104.11 | Si–C = 1.92 Si–H = 1.56 C–H = 1.11 C–Li = 2.1 | |
| H+1LiSi | = 109.1 = 109.1 = 108.3 | Si–C = 1.89 Si–H = 1.54 Si–Li = 2.54 C–H = 1.11 | |
| H+Li | = 108.1 = 111.9 = 108.3 | Si–C = 1.96 Si–H = 1.51 C–H = 1.11 C–Li = 1.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas, J.L.; Martinez, M.O.; Thirumuruganandham, S.P. Band-Gap Engineering: Lithium Effect on the Electronic Properties of Hydrogenated 3C-SiC (1 1 0) Surfaces. Batteries 2022, 8, 247. https://doi.org/10.3390/batteries8110247
Cuevas JL, Martinez MO, Thirumuruganandham SP. Band-Gap Engineering: Lithium Effect on the Electronic Properties of Hydrogenated 3C-SiC (1 1 0) Surfaces. Batteries. 2022; 8(11):247. https://doi.org/10.3390/batteries8110247
Chicago/Turabian StyleCuevas, Jose Luis, Miguel Ojeda Martinez, and Saravana Prakash Thirumuruganandham. 2022. "Band-Gap Engineering: Lithium Effect on the Electronic Properties of Hydrogenated 3C-SiC (1 1 0) Surfaces" Batteries 8, no. 11: 247. https://doi.org/10.3390/batteries8110247
APA StyleCuevas, J. L., Martinez, M. O., & Thirumuruganandham, S. P. (2022). Band-Gap Engineering: Lithium Effect on the Electronic Properties of Hydrogenated 3C-SiC (1 1 0) Surfaces. Batteries, 8(11), 247. https://doi.org/10.3390/batteries8110247






