Melamine-Sacrificed Pyrolytic Synthesis of Spiderweb-like Nanocages Encapsulated with Catalytic Co Atoms as Cathode for Advanced Li-S Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZIF-67
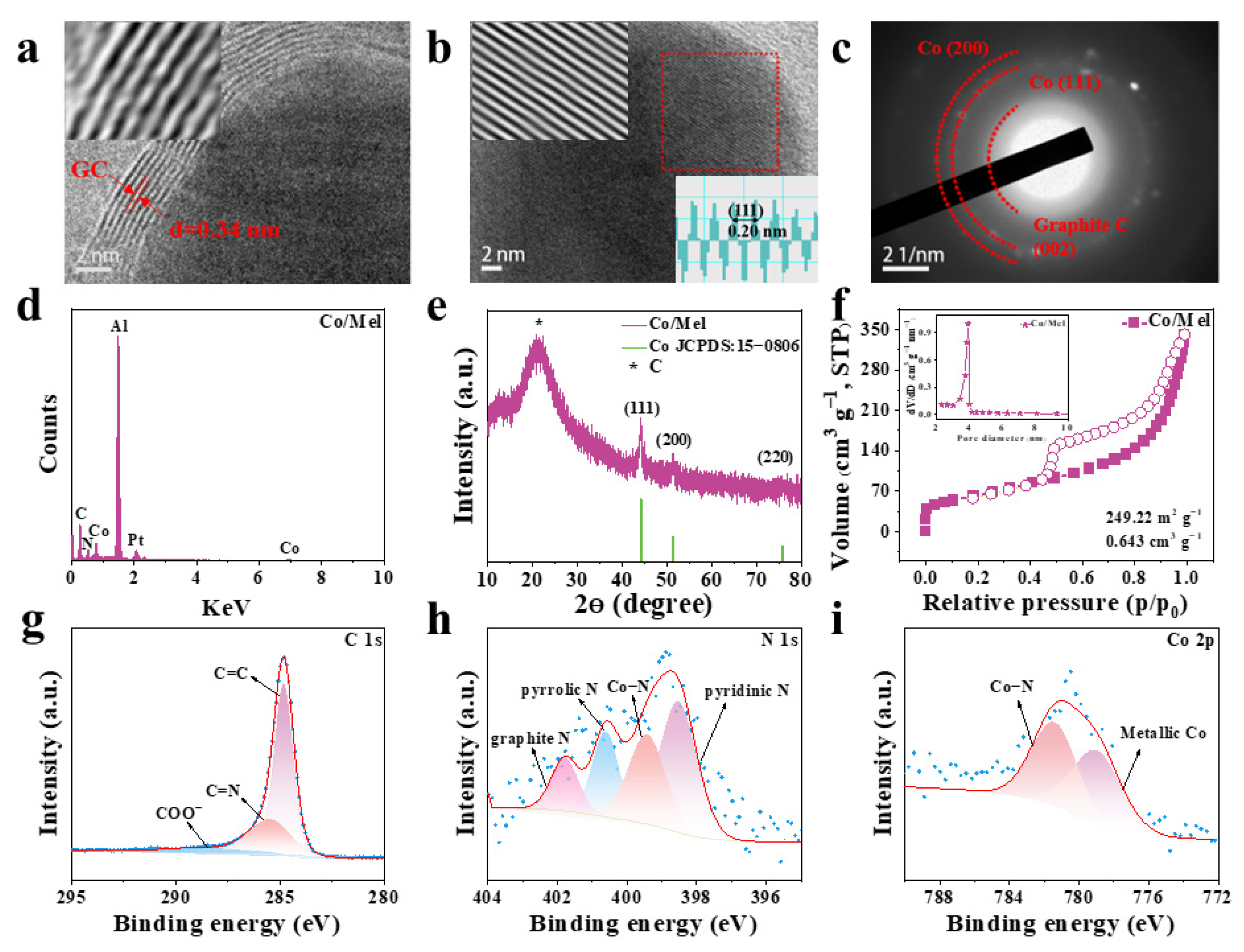
2.3. Synthesis of Co/Mel
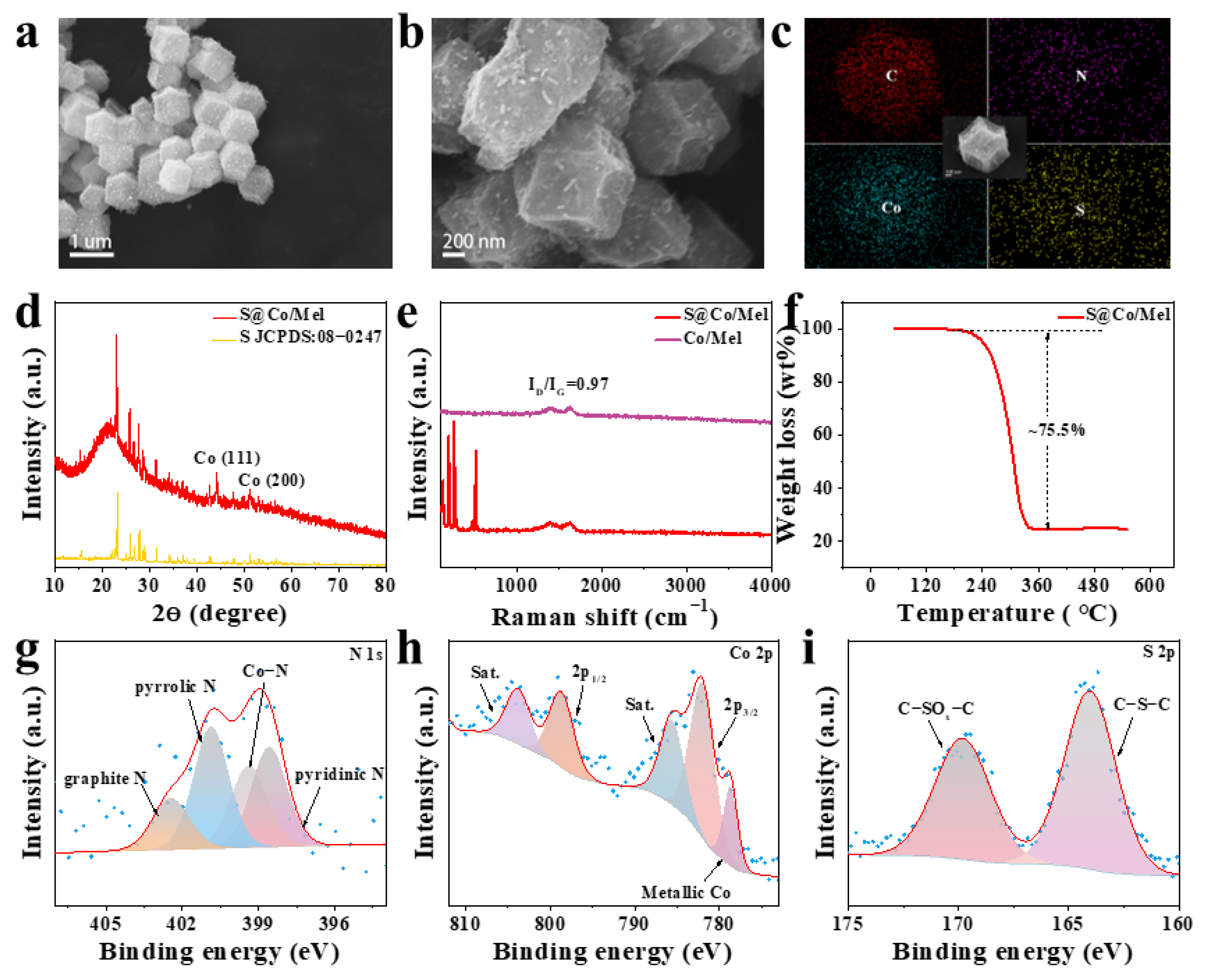
2.4. Synthesis of S@Co/Mel
2.5. Adsorption Experiment
2.6. Li2S Deposition
2.7. Electrochemical Measurement
2.8. Physical Characterization
2.9. In Situ Test
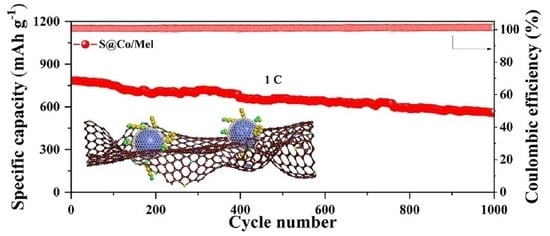
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mauger, A.; Julien, C.M.; Armand, M.; Zaghib, K. Tribute to john b. Goodenough: From magnetism to rechargeable batteries. Adv. Energy Mater. 2021, 11, 2000773. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Tian, H.; Su, D.; Zhang, Q.; Wang, G. Advances in lithium-sulfur batteries: From academic research to commercial viability. Adv. Mater. 2021, 33, 2003666. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Yan, W.; Zhu, Y.; Liu, L.; Fu, L.; Chen, Y.; Yu, N.; Wu, Y.; Wang, B.; Xiao, R. Li4Ti5O12 coating on copper foil as ion redistributor layer for stable lithium metal anode. Adv. Energy Mater. 2022, 12, 2103112. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, X.; Xiong, X.; Zhang, Q.; Peng, B.; Chen, Y.; Wang, Z.; Fu, L.; Wu, Y. A solid electrolyte based on electrochemical active Li4Ti5O12 with pvdf for solid state lithium metal battery. Adv. Energy Mater. 2022, 12, 2201991. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Qiao, L.; Santiago, A.; Judez, X.; de Buruaga, A.S.; Jimenez, G.; Armand, M.; Zhang, H.; Li, C. Perspective of polymer-based solid-state li-s batteries. Energy Mater. 2022, 2, 200003. [Google Scholar] [CrossRef]
- Ruan, J.; Sun, H.; Song, Y.; Pang, Y.; Yang, J.; Sun, D.; Zheng, S. Constructing 1D/2D interwoven carbonous matrix to enable high-efficiency sulfur immobilization in li-s battery. Energy Mater. 2022, 1, 100018. [Google Scholar] [CrossRef]
- Wu, W.; Pu, J.; Wang, J.; Shen, Z.; Tang, H.; Deng, Z.; Tao, X.; Pan, F.; Zhang, H. Biomimetic bipolar microcapsules derived fromstaphylococcus aureus for enhanced properties of lithium-sulfur battery cathodes. Adv. Energy Mater. 2018, 8, 1702373. [Google Scholar] [CrossRef]
- Yu, B.; Jung, J.; Park, K.; Goodenough, J.B. A new approach for recycling waste rubber products in Li-S batteries. Energy Environ. Sci. 2017, 10, 86–90. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.; Zhang, Q.; Mai, L. Nanostructured metal oxides and sulfides for lithium-sulfur batteries. Adv. Mater. 2017, 29, 1601759. [Google Scholar] [CrossRef] [PubMed]
- Bonnick, P.; Muldoon, J. The dr jekyll and mr hyde of lithium sulfur batteries. Energy Environ. Sci. 2020, 13, 4808–4833. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H. Ionic liquids for electrochemical energy storage devices applications. J. Mater. Sci. Technol. 2019, 35, 674–686. [Google Scholar] [CrossRef]
- Geng, P.; Du, M.; Wu, C.; Luo, T.; Zhang, Y.; Pang, H. Ppy-constructed core-shell structures from mofs for confining lithium polysulfides. Inorg. Chem. Front. 2022, 9, 2389–2394. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Sun, Z.; Fan, X.; Wang, Z.; Shi, Y.; Hu, G.; Li, F. Tunable interaction between metal-organic frameworks and electroactive components in lithium-sulfur batteries: Status and perspectives. Adv. Energy Mater. 2021, 11, 2100387. [Google Scholar] [CrossRef]
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; El Gabaly, F.; Yoon, H.P.; et al. Tunable electrical conductivity in metal-organic framework thin-film devices. Science 2014, 343, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Hendon, C.H.; Rieth, A.J.; Korzyński, M.D.; Dincă, M. Grand challenges and future opportunities for metal-organic frameworks. ACS Cent. Sci. 2017, 3, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Stavila, V.; Talin, A.A.; Allendorf, M.D. Mof-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014, 43, 5994–6010. [Google Scholar] [CrossRef]
- Yang, M.; Jiao, L.; Dong, H.; Zhou, L.; Teng, C.; Yan, D.; Ye, T.; Chen, X.; Liu, Y.; Jiang, H. Conversion of bimetallic mof to ru-doped cu electrocatalysts for efficient hydrogen evolution in alkaline media. Sci. Bull. 2021, 66, 257–264. [Google Scholar] [CrossRef]
- Downes, C.A.; Marinescu, S.C. Electrocatalytic metal-organic frameworks for energy applications. Chemsuschem 2017, 10, 4374–4392. [Google Scholar] [CrossRef]
- Zhao, M.; Lu, Q.; Ma, Q.; Zhang, H. Two-dimensional metal-organic framework nanosheets. Small Methods 2017, 1, 1600030. [Google Scholar] [CrossRef]
- Wang, F.; Feng, T.; Jin, X.; Zhou, Y.; Xu, Y.; Gao, Y.; Li, H.; Lei, J. Atomic co/ni active sites assisted mof-derived rich nitrogen-doped carbon hollow nanocages for enhanced lithium storage. Chem. Eng. J. 2021, 420, 127583. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Lv, W.; Wen, K.; Xu, C.; Zhang, W.; Li, Y.; Qin, W.; He, W. From metal–organic framework to Li2S@C-Co-N nanoporous architecture: A high-capacity cathode for lithium-sulfur batteries. ACS Nano 2016, 10, 10981–10987. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Liu, X.; Zhu, K.; Wu, S.; Zhou, H. Metal-organic framework-based separator for lithium-sulfur batteries. Nat. Energy 2016, 1, 1–6. [Google Scholar] [CrossRef]
- Zhang, H.; Xin, S.; Li, J.; Cui, H.; Liu, Y.; Yang, Y.; Wang, M. Synergistic regulation of polysulfides immobilization and conversion by mof-derived cop-hnc nanocages for high-performance lithium-sulfur batteries. Nano Energy 2021, 85, 106011. [Google Scholar] [CrossRef]
- Jeon, Y.; Lee, J.; Jo, H.; Hong, H.; Lee, L.Y.S.; Piao, Y. Co/Co3O4-embedded n-doped hollow carbon composite derived from a bimetallic mof/zno core-shell template as a sulfur host for li-s batteries. Chem. Eng. J. 2021, 407, 126967. [Google Scholar] [CrossRef]
- Xie, Y.; Cao, J.; Wang, X.; Li, W.; Deng, L.; Ma, S.; Zhang, H.; Guan, C.; Huang, W. MOF-derived bifunctional Co0.85Se nanoparticles embedded in n-doped carbon nanosheet arrays as efficient sulfur hosts for lithium-sulfur batteries. Nano Lett. 2021, 21, 8579–8586. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, G.; Shao, L.; Yuan, Z.; Jing, Q.; Huang, K.; Huang, Z.; Zhao, X.; Zou, G. Controlled synthesis of nickel encapsulated into nitrogen-doped carbon nanotubes with covalent bonded interfaces: The structural and electronic modulation strategy for an efficient electrocatalyst in dye-sensitized solar cells. Chem. Mater. 2017, 29, 9680–9694. [Google Scholar] [CrossRef]
- Wu, A.; Liu, D.; Tong, L.; Yu, L.; Yang, H. Magnetic properties of nanocrystalline Fe/Fe3C composites. Crystengcomm 2011, 13, 876–882. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, L.; Liu, X.; Huang, Q.; Wang, Y.; Hou, C.; Hou, Y.; Wang, J.; Dang, F.; Zhang, J. Metal-organic-framework derived core-shell n-doped carbon nanocages embedded with cobalt nanoparticles as high-performance anode materials for lithium-ion batteries. Adv. Funct. Mater. 2020, 30, 2006188. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Liu, P.; Wang, H.; Xu, M.; Bao, S. Anthozoan-like porous nanocages with nano-cobalt-armed cnt multifunctional layers as a cathode material for highly stable na-s batteries. Inorg. Chem. Front. 2022, 9, 645–651. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, R.; Feng, J.; Zhang, Q.; Huang, J.; Fu, G.; Zheng, M.; Ren, B.; Dong, Q. Conductive lewis base matrix to recover the missing link of li2S8 during the sulfur redox cycle in Li-S battery. Chem. Mater. 2015, 27, 2048–2055. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, F.; Lu, H.; Hong, X.; Jiang, H.; Wu, Y.; Li, Y. Hollow zn/co zif particles derived from core-shell zif-67@zif-8 as selective catalyst for the semi-hydrogenation of acetylene. Angew. Chem. Int. Ed. 2015, 54, 10889–10893. [Google Scholar] [CrossRef]
- Hu, L.; Lu, Y.; Zhang, T.; Huang, T.; Zhu, Y.; Qian, Y. Ultramicroporous carbon through an activation-free approach for li-s and na-s batteries in carbonate-based electrolyte. ACS Appl. Mater. Inter. 2017, 9, 13813–13818. [Google Scholar] [CrossRef] [PubMed]
- Vizintin, A.; Chabanne, L.; Tchernychova, E.; Arčon, I.; Stievano, L.; Aquilanti, G.; Antonietti, M.; Fellinger, T.; Dominko, R. The mechanism of li2s activation in lithium-sulfur batteries: Can we avoid the polysulfide formation? J. Power Sources 2017, 344, 208–217. [Google Scholar] [CrossRef]
- Xia, B.Y.; Yan, Y.; Li, N.; Wu, H.B.; Lou, X.W.; Wang, X. A metal-organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006. [Google Scholar] [CrossRef]
- Tian, H.; Tian, H.; Wang, S.; Chen, S.; Zhang, F.; Song, L.; Liu, H.; Liu, J.; Wang, G. High-power lithium-selenium batteries enabled by atomic cobalt electrocatalyst in hollow carbon cathode. Nat. Commun. 2020, 11, 5025. [Google Scholar] [CrossRef]
- Du, Z.; Chen, X.; Hu, W.; Chuang, C.; Xie, S.; Hu, A.; Yan, W.; Kong, X.; Wu, X.; Ji, H.; et al. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium-sulfur batteries. J. Am. Chem. Soc. 2019, 141, 3977–3985. [Google Scholar] [CrossRef]
- Garapati, M.S.; Sundara, R. Enhancing polysulfide confinement and redox kinetics by electrocatalytic interlayer for highly stable lithium-sulfur batteries. Electrochim. Acta 2020, 362, 137035. [Google Scholar] [CrossRef]
- Zhou, T.; Du, Y.; Yin, S.; Tian, X.; Yang, H.; Wang, X.; Liu, B.; Zheng, H.; Qiao, S.; Xu, R. Nitrogen-doped cobalt phosphate@nanocarbon hybrids for efficient electrocatalytic oxygen reduction. Energy Environ. Sci. 2016, 9, 2563–2570. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, B.; Chang, S.; Yang, C.; Hu, Y.; Cao, S.; Lu, J.; Zhang, L.; Ye, H. Effects of annealing temperature on electrochemical performance of snsx embedded in hierarchical porous carbon with n-carbon coating by in-situ structural phase transformation as anodes for lithium ion batteries. J. Mater. Sci. Technol. 2021, 84, 191–199. [Google Scholar] [CrossRef]
- Hu, L.; Dai, C.; Lim, J.M.; Chen, Y.; Lian, X.; Wang, M.; Li, Y.; Xiao, P.; Henkelman, G.; Xu, M. A highly efficient double-hierarchical sulfur host for advanced lithium-sulfur batteries. Chem. Sci. 2018, 9, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Lim, J.; Wang, M.; Hu, L.; Chen, Y.; Chen, Z.; Chen, H.; Bao, S.; Shen, B.; Li, Y.; et al. Honeycomb-like spherical cathode host constructed from hollow metallic and polar Co9S8 tubules for advanced lithium-sulfur batteries. Adv. Funct. Mater. 2018, 28, 1704443. [Google Scholar] [CrossRef]
- Ma, H.; Liu, X.; Liu, N.; Zhao, Y.; Zhang, Y.; Bakenov, Z.; Wang, X. Defect-rich porous tubular graphitic carbon nitride with strong adsorption towards lithium polysulfides for high-performance lithium-sulfur batteries. J. Mater. Sci. Technol. 2022, 115, 140–147. [Google Scholar] [CrossRef]
- Guo, B.; Bandaru, S.; Dai, C.; Chen, H.; Zhang, Y.; Xu, Q.; Bao, S.; Chen, M.; Xu, M. Self-supported FeCoS4 nanotube arrays as binder-free cathodes for lithium-sulfur batteries. ACS Appl. Mater. Inter. 2018, 10, 43707–43715. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Wu, P.; Xu, N.; Wang, L.; Li, M.; Dai, A.; Amine, K.; Mai, L.; Lu, J. Silica restricting the sulfur volatilization of nickel sulfide for high-performance lithium-ion batteries. Adv. Energy Mater. 2019, 9, 1901153. [Google Scholar] [CrossRef]
- Cheng, R.; Guan, Y.; Luo, Y.; Zhang, C.; Xia, Y.; Wei, S.; Zhao, M.; Lin, Q.; Li, H.; Zheng, S.; et al. Guanine-assisted n-doped ordered mesoporous carbons as efficient capacity decaying suppression materials for lithium-sulfur batteries. J. Mater. Sci. Technol. 2022, 101, 155–164. [Google Scholar] [CrossRef]
- Zhao, J.; Qi, Y.; Yang, Q.; Huang, T.; Wang, H.; Wang, Y.; Niu, Y.; Liu, Y.; Bao, S.; Xu, M. Chessboard structured electrode design for li-s batteries based on mxene nanosheets. Chem. Eng. J. 2022, 429, 131997. [Google Scholar] [CrossRef]
- Zech, C.; Hönicke, P.; Kayser, Y.; Risse, S.; Grätz, O.; Stamm, M.; Beckhoff, B. Polysulfide driven degradation in lithium-sulfur batteries during cycling—Quantitative and high time-resolution operando x-ray absorption study for dissolved polysulfides probed at both electrode sides. J. Mater. Chem. A 2021, 9, 10231–10239. [Google Scholar] [CrossRef]
- Gong, Z.; Wu, Q.; Wang, F.; Li, X.; Fan, X.; Yang, H.; Luo, Z. A hierarchical micro/mesoporous carbon fiber/sulfur composite for high-performance lithium-sulfur batteries. Rsc Adv. 2016, 6, 37443–37451. [Google Scholar] [CrossRef]
- Peng, H.; Hou, T.; Zhang, Q.; Huang, J.; Cheng, X.; Guo, M.; Yuan, Z.; He, L.; Wei, F. Strongly coupled interfaces between a heterogeneous carbon host and a sulfur-containing guest for highly stable lithium-sulfur batteries: Mechanistic insight into capacity degradation. Adv. Mater. Interfaces 2014, 1, 1400227. [Google Scholar] [CrossRef]
- Mao, Y.; Li, G.; Guo, Y.; Li, Z.; Liang, C.; Peng, X.; Lin, Z. Foldable interpenetrated metal-organic frameworks/carbon nanotubes thin film for lithium–sulfur batteries. Nat. Commun. 2017, 8, 14628. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, H.; Cheng, C.; Yan, T.; Gao, W.; Mao, J.; Dai, K.; Zhang, L. Boosting polysulfide redox conversion of li-s batteries by one-step-synthesized co-mo bimetallic nitride. J. Energy Chem. 2021, 61, 336–346. [Google Scholar] [CrossRef]
- Liu, B.; Fan, K.; Li, J.; Liu, G.; Liu, Y.; Yang, C.; Tong, H.; Han, K.; Qian, D. Carbon nanotubes-intercalated co-n-c as a robust sulfur host for lithium-sulfur batteries. Mater. Res. Bull. 2020, 121, 110625. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Sun, F.; Jiang, G.; Zheng, N.; Xu, C.; Li, Y. Core-shell polyhedrons of carbon nanotubes-grafted graphitic carbon@nitrogen doped carbon as efficient sulfur immobilizers for lithium-sulfur batteries. Appl. Surf. Sci. 2018, 450, 364–371. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, H.; Shen, Q. Modification of cobalt-containing mof-derived mesoporous carbon as an effective sulfur-loading host for rechargeable lithium-sulfur batteries. J. Alloy. Compd. 2019, 772, 843–851. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Jamil, S.; Tang, W.; Zhao, J.; Liu, H.; Bao, S.; Liu, Y.; Xu, M. Melamine-Sacrificed Pyrolytic Synthesis of Spiderweb-like Nanocages Encapsulated with Catalytic Co Atoms as Cathode for Advanced Li-S Batteries. Batteries 2022, 8, 161. https://doi.org/10.3390/batteries8100161
Wang H, Jamil S, Tang W, Zhao J, Liu H, Bao S, Liu Y, Xu M. Melamine-Sacrificed Pyrolytic Synthesis of Spiderweb-like Nanocages Encapsulated with Catalytic Co Atoms as Cathode for Advanced Li-S Batteries. Batteries. 2022; 8(10):161. https://doi.org/10.3390/batteries8100161
Chicago/Turabian StyleWang, Han, Sidra Jamil, Wenwen Tang, Jing Zhao, Hui Liu, Shujuan Bao, Yijun Liu, and Maowen Xu. 2022. "Melamine-Sacrificed Pyrolytic Synthesis of Spiderweb-like Nanocages Encapsulated with Catalytic Co Atoms as Cathode for Advanced Li-S Batteries" Batteries 8, no. 10: 161. https://doi.org/10.3390/batteries8100161
APA StyleWang, H., Jamil, S., Tang, W., Zhao, J., Liu, H., Bao, S., Liu, Y., & Xu, M. (2022). Melamine-Sacrificed Pyrolytic Synthesis of Spiderweb-like Nanocages Encapsulated with Catalytic Co Atoms as Cathode for Advanced Li-S Batteries. Batteries, 8(10), 161. https://doi.org/10.3390/batteries8100161