One-Step Electrospun LTO Anode for Flexible Li-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
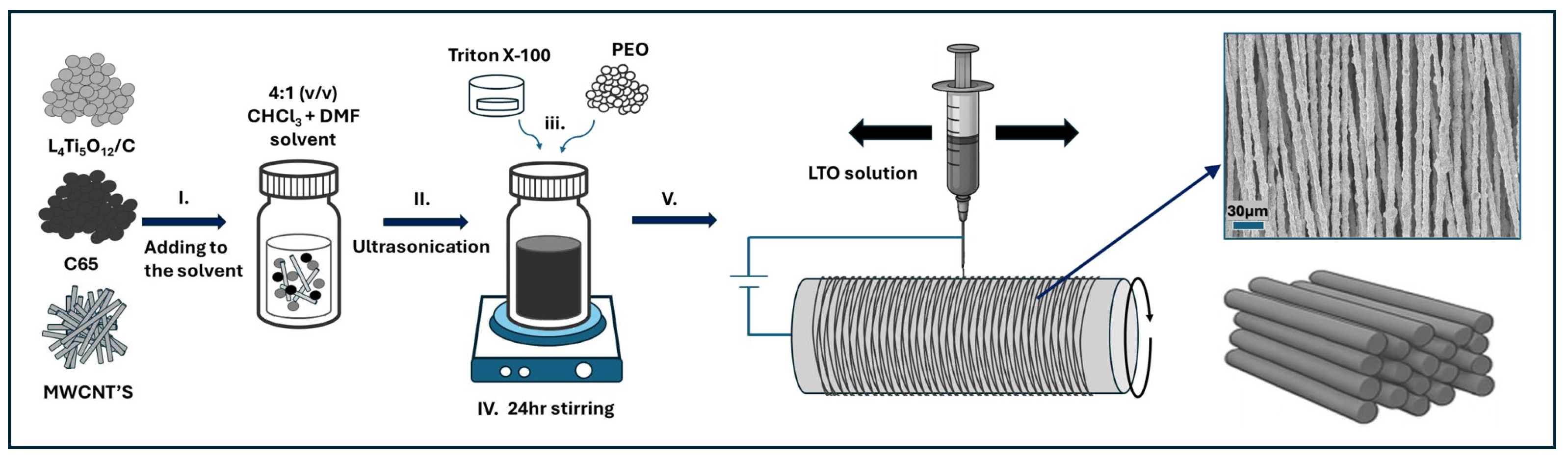
2.1. LTO Anode Preparation
2.2. Electrochemical Characterization of Electrospun LTO Anode
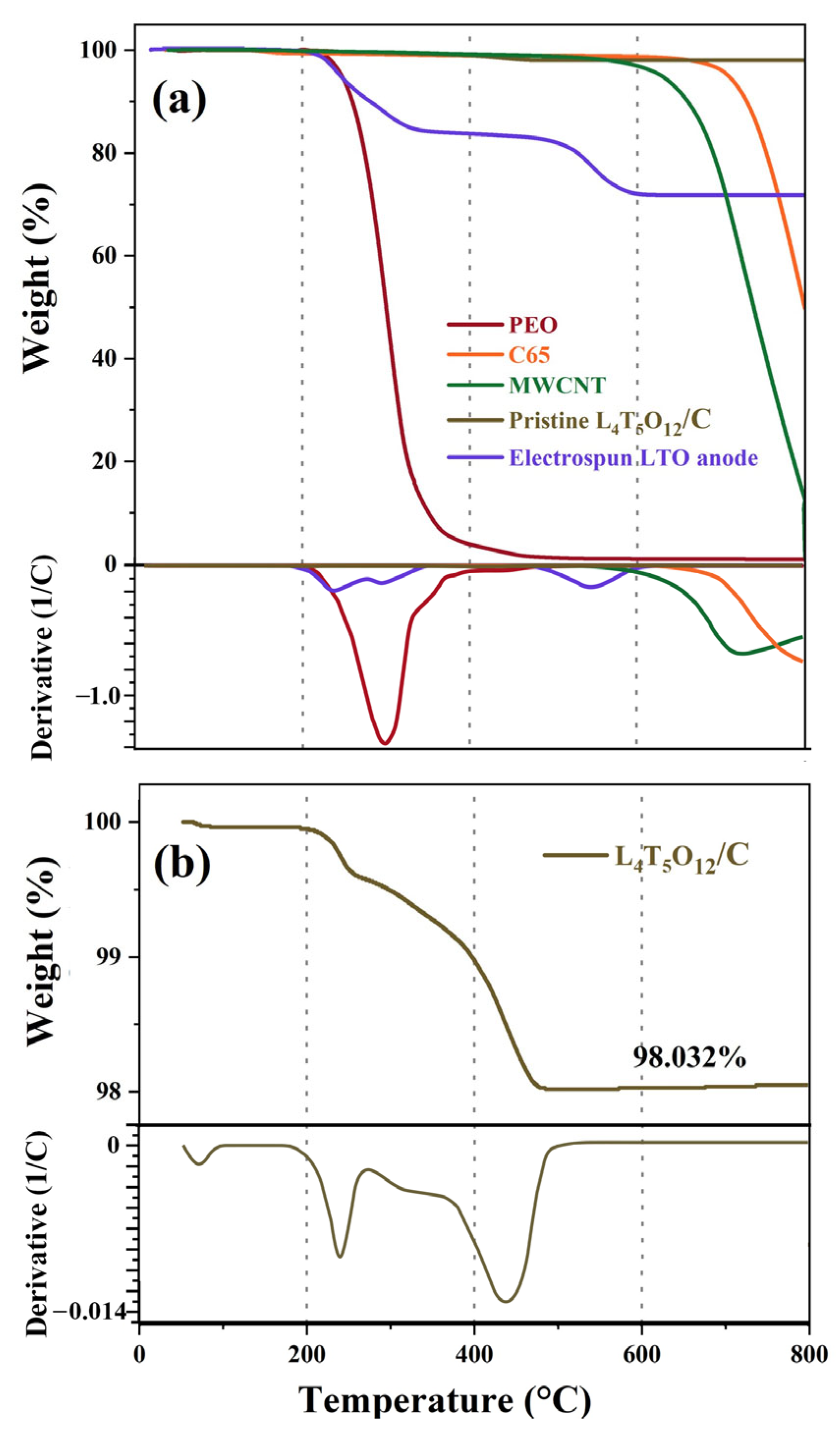
2.3. Structural Characterization
3. Results and Discussion
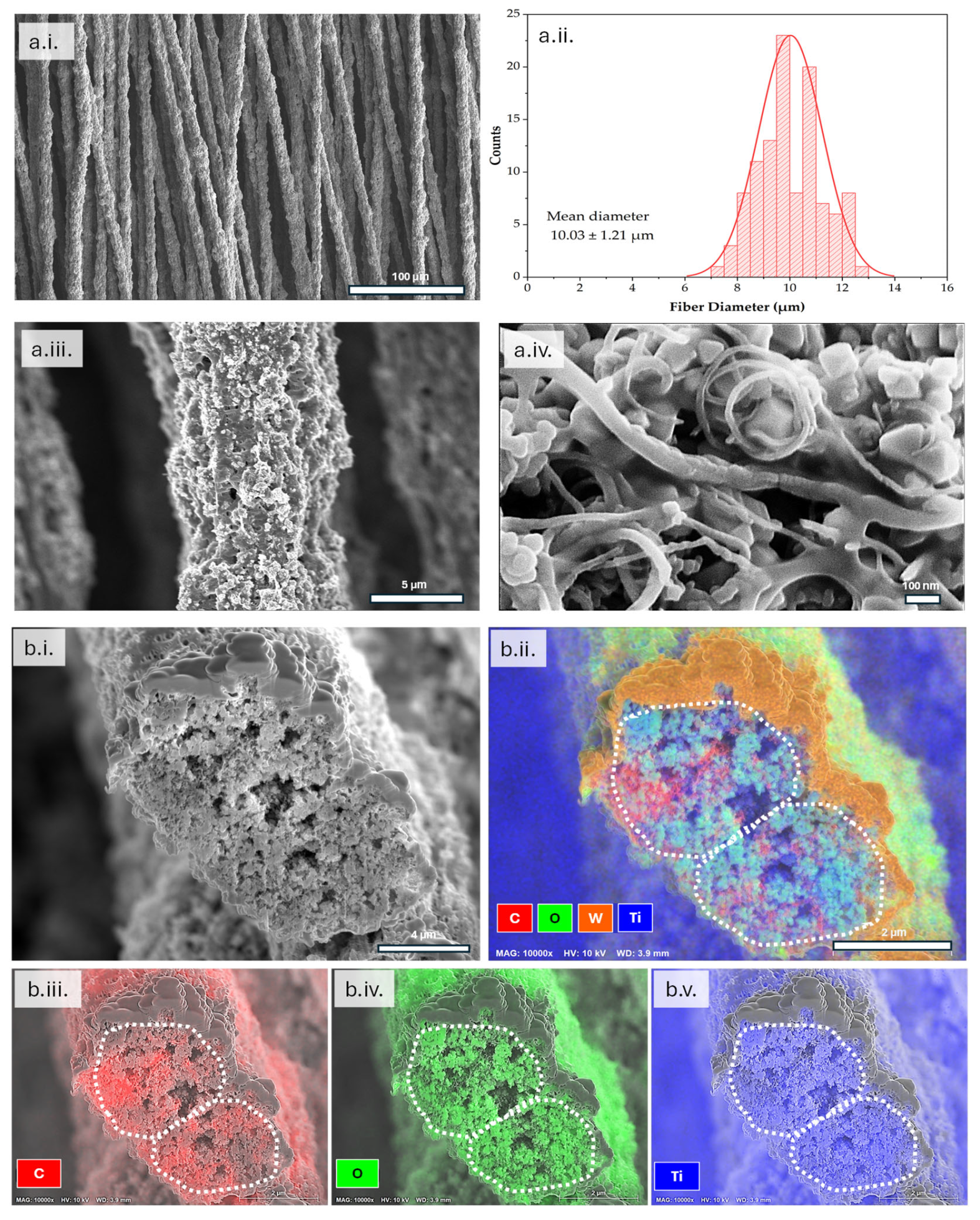
3.1. Morphology Characterization of Electrospun LTO Anode
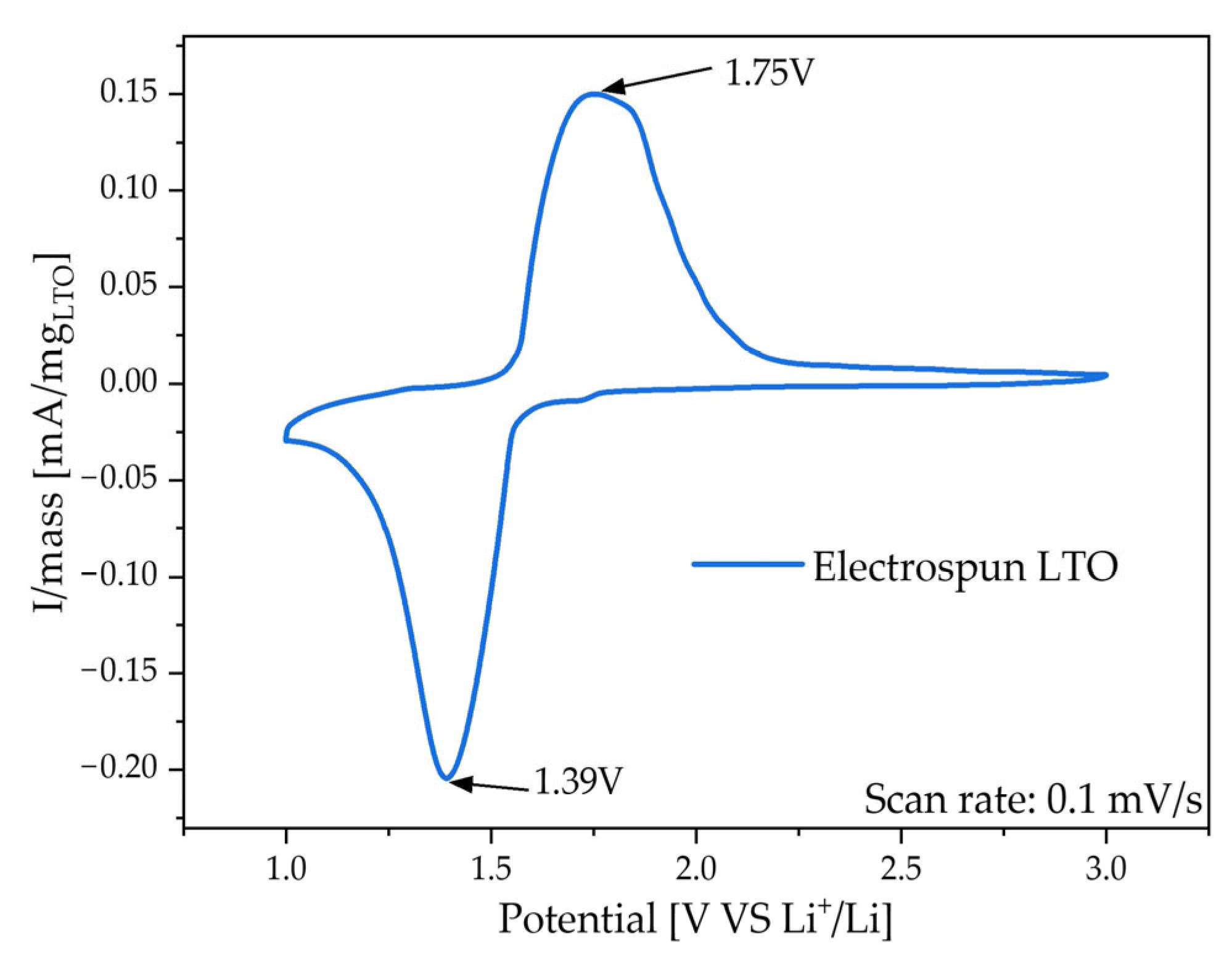
3.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LTO | Lithium Titanate Oxide |
| PEO | Polyethylene Oxide |
| MWCNT | Multi-Wall Carbon Nanotube |
| SEI | Solid Electrolyte Interphase |
| OCV | Open Circuit Voltage |
| EIS | Electrochemical Impedance Spectroscopy |
| (HR)SEM | (High Resolution) Scanning Electron Microscope |
| CV | Cyclic Voltammetry |
| EDS | Energy Dispersive X-Ray Spectroscopy |
| TGA | Thermogravimetric Analysis |
References
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, Z.; Sun, J.; Liu, Z.; Wang, J. High-Energy Batteries: Beyond Lithium-Ion and Their Long Road to Commercialisation; Springer: Singapore, 2022; Volume 14. [Google Scholar]
- Fan, X.; Liu, B.; Ding, J.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Flexible and Wearable Power Sources for Next—Generation Wearable Electronics. Batter. Supercaps 2020, 3, 1262–1274. [Google Scholar] [CrossRef]
- Zhang, Q.; Soham, D.; Lian, Z.; Wan, J. Advances in wearable energy storage and harvesting systems. Med-X 2025, 3, 3. [Google Scholar] [CrossRef]
- Wang, N.; Hou, W.; Chang, Y.; Song, H.; Li, H.; Li, Y.; Han, G. Binder-free hydrogen storage composite Co9S8/rGO: A prospective anode for flexible energy storage device with high energy density. Electrochim. Acta 2020, 354, 136734. [Google Scholar] [CrossRef]
- Wang, J.G.; Jin, D.; Zhou, R.; Li, X.; Liu, X.R.; Shen, C.; Xie, K.; Li, B.; Kang, F.; Wei, B. Highly Flexible Graphene/Mn3O4 Nanocomposite Membrane as Advanced Anodes for Li-Ion Batteries. ACS Nano 2016, 10, 6227–6234. [Google Scholar] [CrossRef]
- Hu, L.; Liu, N.; Eskilsson, M.; Zheng, G.; McDonough, J.; Wågberg, L.; Cui, Y. Silicon-conductive nanopaper for Li-ion batteries. Nano Energy 2013, 2, 138–145. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Liu, B.; Chen, D.; Tong, Y.; Shen, G. Flexible energy-storage devices: Design consideration and recent progress. Adv. Mater. 2014, 26, 4763–4782. [Google Scholar] [CrossRef]
- Mados, E.; Atar, I.; Gratz, Y.; Israeli, M.; Kondrova, O.; Fourman, V.; Sherman, D.; Golodnitsky, D.; Sitt, A. Polymer-based LFP cathode/current collector microfiber-meshes with bi- and interlayered architectures for Li-ion battery. J. Power Sources 2024, 603, 234397. [Google Scholar] [CrossRef]
- Opra, D.P.; Gnedenkov, S.V.; Sokolov, A.A.; Minaev, A.N.; Kuryavyi, V.G.; Sinebryukhov, S.L. Facile synthesis of nanostructured transition metal oxides as electrodes for Li-ion batteries. AIP Conf. Proc. 2017, 1874, 397–402. [Google Scholar]
- Kavan, L.; Procházka, J.; Spitler, T.M.; Kalbáč, M.; Zukalová, M.; Drezen, T.; Grätzel, M. Li Insertion into Li4Ti5O12 (Spinel). J. Electrochem. Soc. 2003, 150, A1000. [Google Scholar] [CrossRef]
- Ronci, F.; Reale, P.; Scrosati, B.; Panero, S.; Rossi Albertini, V.; Perfetti, P.; Di Michiel, M.; Merino, J.M. High-resolution in-situ structural measurements of the Li4/3Ti5/3O4 “zero-strain” insertion material. J. Phys. Chem. B 2002, 106, 3082–3086. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Yamamoto, N. Zero—Strain Insertion Material of Li[Li1/3Ti5/3]O4 for Rechargeable Lithium Cells. J. Electrochem. Soc. 1995, 142, 1431–1435. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, D.; Qilu; Duo, X.; Sheng, X. A review of spinel lithium titanate (Li4Ti5O12) as electrode material for advanced energy storage devices. Ceram. Int. 2021, 47, 5870–5895. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material-fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Zaghib, K.; Dontigny, M.; Guerfi, A.; Charest, P.; Rodrigues, I.; Mauger, A.; Julien, C.M. Safe and fast-charging Li-ion battery with long shelf life for power applications. J. Power Sources 2011, 196, 3949–3954. [Google Scholar] [CrossRef]
- Wu, K.; Yang, J.; Qiu, X.Y.; Xu, J.M.; Zhang, Q.Q.; Jin, J.; Zhuang, Q.C. Study of spinel Li4Ti5O12electrode reaction mechanism byelectrochemical impedance spectroscopy. Electrochim. Acta 2013, 108, 841–851. [Google Scholar] [CrossRef]
- Vasileiadis, A.; de Klerk, N.J.J.; Smith, R.B.; Ganapathy, S.; Harks, P.P.R.M.L.; Bazant, M.Z.; Wagemaker, M. Toward Optimal Performance and In-Depth Understanding of Spinel Li4Ti5O12 Electrodes through Phase Field Modeling. Adv. Funct. Mater. 2018, 28, 1705992. [Google Scholar] [CrossRef]
- Park, S.K.; Copic, D.; Zhao, T.Z.; Rutkowska, A.; Wen, B.; Sanders, K.; He, R.; Kim, H.K.; De Volder, M. 3D Porous Cu-Composites for Stable Li-Metal Battery Anodes. ACS Nano 2023, 17, 14658–14666. [Google Scholar] [CrossRef]
- Patnaik, S.G.; Jadon, A.; Tran, C.C.H.; Estève, A.; Guay, D.; Pech, D. High Areal Capacity Porous Sn-Au Alloys with Long Cycle Life for Li-ion Microbatteries. Sci. Rep. 2020, 10, 10405. [Google Scholar] [CrossRef]
- Hu, L.; La Mantia, F.; Wu, H.; Xie, X.; McDonough, J.; Pasta, M.; Cui, Y. Lithium-ion textile batteries with large areal mass loading. Adv. Energy Mater. 2011, 1, 1012–1017. [Google Scholar] [CrossRef]
- Wang, A. Three-Dimensional Hierarchical Porous Electrode Structure for Improved Performance in Battery Applications. Int. J. High Sch. Res. 2022, 4, 1–5. [Google Scholar] [CrossRef]
- Lu, H.W.; Zeng, W.; Li, Y.S.; Fu, Z.W. Fabrication and electrochemical properties of three-dimensional net architectures of anatase TiO2 and spinel Li4Ti5O12 nanofibers. J. Power Sources 2007, 164, 874–879. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X.; Luo, W.; Sun, Y.; Yang, Z.; Hu, C.; Huang, Y. Electrospun Conformal Li4Ti5O12/C Fibers for High-Rate Lithium-Ion Batteries. ChemElectroChem 2014, 1, 611–616. [Google Scholar] [CrossRef]
- Zhu, N.; Liu, W.; Xue, M.; Xie, Z.; Zhao, D.; Zhang, M.; Chen, J.; Cao, T. Graphene as a conductive additive to enhance the high-rate capabilities of electrospun Li4Ti5O12 for lithium-ion batteries. Electrochim. Acta 2010, 55, 5813–5818. [Google Scholar] [CrossRef]
- Sandhya, C.P.; John, B.; Gouri, C. Synthesis and electrochemical characterisation of electrospun lithium titanate ultrafine fibres. J. Mater. Sci. 2013, 48, 5827–5832. [Google Scholar] [CrossRef]
- Abureden, S.; Hassan, F.M.; Lui, G.; Ahn, W.; Sy, S.; Yu, A.; Chen, Z. Multigrain electrospun nickel doped lithium titanate nanofibers with high power lithium ion storage. J. Mater. Chem. A 2016, 4, 12638–12647. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, X.; Sunarso, J.; Cai, R.; Chu, S.; Miao, J.; Zhou, W.; Shao, Z. Two-Step Fabrication of Li4Ti5O12-Coated Carbon Nanofibers as a Flexible Film Electrode for High-Power Lithium-Ion Batteries. ChemElectroChem 2017, 4, 2286–2292. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, X. Electrospun carbon nanofibers containing silicon particles as an energy-storage medium. Carbon 2009, 47, 3219–3226. [Google Scholar] [CrossRef]
- Self, E.C.; McRen, E.C.; Pintauro, P.N. High Performance Particle/Polymer Nanofiber Anodes for Li-ion Batteries using Electrospinning. ChemSusChem 2016, 9, 208–215. [Google Scholar] [CrossRef]
- Self, E.C.; Wycisk, R.; Pintauro, P.N. Electrospun titania-based fibers for high areal capacity Li-ion battery anodes. J. Power Sources. 2015, 282, 187–193. [Google Scholar] [CrossRef]
- Aravindan, V.; Suresh, P.; Sundaramurthy, J.; Ling, W.C.; Ramakrishna, S.; Madhavi, S. Electrospun NiO nanofibers as high performance anode material for Li-ion batteries. J. Power Sources 2013, 227, 284–290. [Google Scholar] [CrossRef]
- Nan, X.; Zhang, Y.; Shen, J.; Liang, R.; Wang, J.; Jia, L.; Yang, X.; Yu, W.; Zhang, Z. A Review of the Establishment of Effective Conductive Pathways of Conductive Polymer Composites and Advances in Electromagnetic Shielding. Polymers 2024, 16, 2539. [Google Scholar] [CrossRef]
- Li, J.; Ma, P.C.; Chow, W.S.; To, C.K.; Tang, B.Z.; Kim, J.K. Correlations between percolation threshold, dispersion state, and aspect ratio of carbon nanotubes. Adv. Funct. Mater. 2007, 17, 3207–3215. [Google Scholar] [CrossRef]
- Burmistrov, I.; Gorshkov, N.; Ilinykh, I.; Muratov, D.; Kolesnikov, E.; Anshin, S.; Mazov, I.; Issi, J.P.; Kusnezov, D. Improvement of carbon black based polymer composite electrical conductivity with additions of MWCNT. Compos. Sci. Technol. 2016, 129, 79–85. [Google Scholar] [CrossRef]
- Ma, P.C.; Liu, M.Y.; Zhang, H.; Wang, S.Q.; Wang, R.; Wang, K.; Wong, Y.K.; Tang, B.Z.; Hong, S.H.; Paik, K.W.; et al. Enhanced electrical conductivity of nanocomposites containing hybrid fillers of carbon nanotubes and carbon black. ACS Appl. Mater. Interfaces 2009, 1, 1090–1096. [Google Scholar] [CrossRef]
- Wen, B.R. Nanostructured Li4Ti5O12 as Anode Material for Lithium Ion Batteries. Master’s Thesis, Faculty of Science, The University of New South Wales, Kensington, NSW, Australia, 2012. [Google Scholar]
- Ragones, H.; Vinegrad, A.; Ardel, G.; Goor, M.; Kamir, Y.; Dorfman, M.M.; Gladkikh, A.; Golodnitsky, D. On the Road to a Multi-Coaxial-Cable Battery: Development of a Novel 3D-Printed Composite Solid Electrolyte. J. Electrochem. Soc. 2020, 167, 070503. [Google Scholar] [CrossRef]
- Li, Q.; Church, J.S.; Kafi, A.; Naebe, M.; Fox, B.L. An improved understanding of the dispersion of multi-walled carbon nanotubes in non-aqueous solvents. J. Nanopart. Res. 2014, 16, 2513. [Google Scholar] [CrossRef]
- Singh, N.K.; Verma, M.L.; Minakshi, M. PEO nanocomposite polymer electrolyte for solid state symmetric capacitors. Bull. Mater. Sci. 2015, 38, 1577–1588. [Google Scholar] [CrossRef]
- Freiberg, A.T.S.; Sicklinger, J.; Solchenbach, S.; Gasteiger, H.A. Li2CO3 decomposition in Li-ion batteries induced by the electrochemical oxidation of the electrolyte and of electrolyte impurities. Electrochim. Acta 2020, 346, 136271. [Google Scholar] [CrossRef]
- Mahajan, A.; Kingon, A.; Kukovecz, Á.; Konya, Z.; Vilarinho, P.M. Studies on the thermal decomposition of multiwall carbon nanotubes under different atmospheres. Mater. Lett. 2013, 90, 165–168. [Google Scholar] [CrossRef]
- Zhu, G.-N.; Wang, C.-X.; Xia, Y.-Y. A Comprehensive Study of Effects of Carbon Coating on Li4Ti5O12 Anode Material for Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, A102. [Google Scholar] [CrossRef]
- Yuan, T.; Yu, X.; Cai, R.; Zhou, Y.; Shao, Z. Synthesis of pristine and carbon-coated Li4Ti5O12 and their low-temperature electrochemical performance. J. Power Sources 2010, 195, 4997–5004. [Google Scholar] [CrossRef]
- Agrawal, R.; Hao, Y.; Song, Y.; Chen, C.; Wang, C. Hybridization of lithium-ion batteries and electrochemical capacitors: Fabrication and challenges. Energy Harvest. Storage Mater. Devices Appl. VI 2015, 9493, 94930B. [Google Scholar]
- Appetecchi, G.B.; Aihara, Y.; Scrosati, B. Investigation of swelling phenomena in PEO-based polymer electrolytes: II. Chemical and electrochemical characterization. Solid State Ionics 2004, 170, 63–72. [Google Scholar] [CrossRef]
- Montanino, M.; Moreno, M.; Carewska, M.; Maresca, G.; Simonetti, E.; Lo Presti, R.; Alessandrini, F.; Appetecchi, G.B. Mixed organic compound-ionic liquid electrolytes for lithium battery electrolyte systems. J. Power Sources 2014, 269, 608–615. [Google Scholar] [CrossRef]
- Castiglione, F.; Ragg, E.; Mele, A.; Appetecchi, G.B.; Montanino, M.; Passerini, S. Molecular environment and enhanced diffusivity of Li+ ions in lithium-salt-doped ionic liquid electrolytes. J. Phys. Chem. Lett. 2011, 2, 153–157. [Google Scholar] [CrossRef]
- Diddens, D.; Heuer, A. Simulation study of the lithium ion transport mechanism in ternary polymer electrolytes: The critical role of the segmental mobility. J. Phys. Chem. B 2014, 118, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Han, X.; Liu, Y.; Azhar, A.; Na, J.; Nanjundan, A.K.; Wang, S.; Yu, J.; Yamauchi, Y. Progress in Solid Polymer Electrolytes for Lithium-Ion Batteries and Beyond. Small 2022, 18, 1–36. [Google Scholar] [CrossRef]
- Guchok, O.; Ardel, G.; Keidar, T.D.; Nakar, H.; Ragones, H.; Kaplan, D.; Zheng, A.; Greenbaum, S.; Lounev, I.; Greenbaum, A.; et al. Understanding of the ion transport in blended TPU-PEO polymer electrolytes. J. Solid State Electrochem. 2025. [Google Scholar] [CrossRef]
- Zaghib, K.; Simoneau, M.; Armand, M.; Gauthier, M. Electrochemical study of Li4Ti5O12 as negative electrode for Li-ion polymer rechargeable batteries. J. Power Sources 1999, 81–82, 300–305. [Google Scholar] [CrossRef]
- Sun, X.; Radovanovic, P.V.; Cui, B. Advances in spinel Li4Ti5O12 anode materials for lithium-ion batteries. New J. Chem. 2015, 39, 38–63. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, S.W.; Van Hoang, H.; Doh, C.H.; Kim, D.W. Study on the cycling performance of Li4Ti5O 12 electrode in the ionic liquid electrolytes containing an additive. Bull. Korean Chem. Soc. 2011, 32, 105–108. [Google Scholar] [CrossRef]
- Huynh, L.T.N.; Ha, C.T.D.; Nguyen, V.D.; Nguyen, D.Q.; Le, M.L.P.; Tran, V.M. Structure and Electrochemical Properties of Li4Ti5O12 Prepared via Low-Temperature Precipitation. J. Chem. 2019, 2019, 1727859. [Google Scholar] [CrossRef]
- Zaghib, K.; Armand, M.; Gauthier, M. Electrochemistry of Anodes in Solid—State Li—Ion Polymer Batteries. J. Electrochem. Soc. 1998, 145, 3135–3140. [Google Scholar] [CrossRef]
- Elia, G.A.; Ulissi, U.; Jeong, S.; Passerini, S.; Hassoun, J. Exceptional long-life performance of lithium-ion batteries using ionic liquid-based electrolytes. Energy Environ. Sci. 2016, 9, 3210–3220. [Google Scholar] [CrossRef]
- Ara, M.; Meng, T.; Nazri, G.-A.; Salley, S.O.; Simon Ng, K.Y. Ternary Imidazolium-Pyrrolidinium-Based Ionic Liquid Electrolytes for Rechargeable Li-O2 Batteries. J. Electrochem. Soc. 2014, 161, A1969–A1975. [Google Scholar] [CrossRef]
- Diard, J.-P.; Le Gorrec, B.; Montella, C. Diffusion Impedances. In Handbook of Electrochemical Impedance Spectroscopy; Electrical Circuits Containing CPEs, Bio-Logic: Seyssinet-Pariset, France, 2013. [Google Scholar]
- Cabanel, R.; Barral, G.; Diard, J.P.; Le Gorrec, B.; Montella, C. Determination of the diffusion coefficient of an inserted species by impedance spectroscopy: Application to the H/HxNb2O5 system. J. Appl. Electrochem. 1993, 23, 93–97. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy. Ann. Biomed. Eng. 1992, 20, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Waluś, S.; Barchasz, C.; Bouchet, R.; Alloin, F. Electrochemical impedance spectroscopy study of lithium–sulfur batteries: Useful technique to reveal the Li/S electrochemical mechanism. Electrochim. Acta 2020, 359, 136944. [Google Scholar] [CrossRef]
- Gateman, S.M.; Gharbi, O.; Gomes de Melo, H.; Ngo, K.; Turmine, M.; Vivier, V. On the use of a constant phase element (CPE) in electrochemistry. Curr. Opin. Electrochem. 2022, 36, 101133. [Google Scholar] [CrossRef]
- Jagger, B.; Pasta, M. Solid electrolyte interphases in lithium metal batteries. Joule 2023, 7, 2228–2244. [Google Scholar] [CrossRef]
- Peled, E. The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems—The Solid Electrolyte Interphase Model. J. Electrochem. Soc. 1979, 126, 2047–2051. [Google Scholar] [CrossRef]
- Perez-Beltran, S.; Kuai, D.; Balbuena, P.B. SEI Formation and Lithium-Ion Electrodeposition Dynamics in Lithium Metal Batteries via First-Principles Kinetic Monte Carlo Modeling. ACS Energy Lett. 2024, 9, 5268–5278. [Google Scholar] [CrossRef] [PubMed]
- Hosen, M.S.; Gopalakrishnan, R.; Kalogiannis, T.; Jaguemont, J.; Van Mierlo, J.; Berecibar, M. Impact of Relaxation Time on Electrochemical Impedance Spectroscopy Characterization of the Most Common Lithium Battery Technologies—Experimental Study and Chemistry-Neutral Modeling. World Electr. Veh. J. 2021, 12, 77. [Google Scholar] [CrossRef]


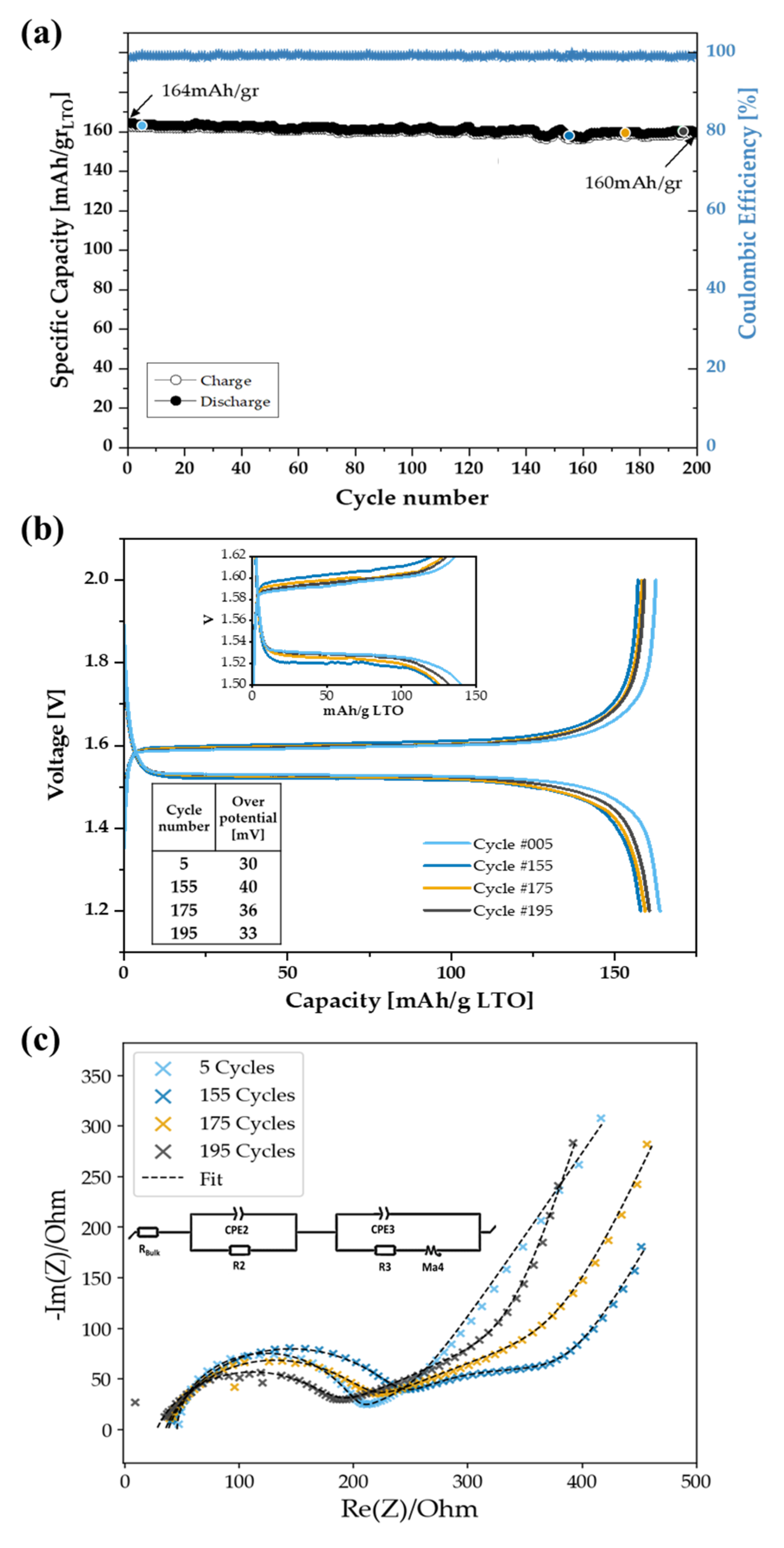
| Cycle | Rbulk [Ω] | R2 [Ω] | Q2 | R3 [Ω] | Q3 | αd |
|---|---|---|---|---|---|---|
| 5 | 46 | 133 | 5.76 | 49 | 5.0 | 0.65 |
| 155 | 38 | 206 | 1.3 | 83.6 | 9.79 | 0.72 |
| 175 | 35 | 180 | 1.56 | 83.8 | 1.00 | 0.77 |
| 195 | 28 | 158 | 1.85 | 71.5 | 1.35 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mados, E.E.; Amit, R.; Kluska, N.; Golodnitsky, D.; Sitt, A. One-Step Electrospun LTO Anode for Flexible Li-Ion Batteries. Batteries 2025, 11, 405. https://doi.org/10.3390/batteries11110405
Mados EE, Amit R, Kluska N, Golodnitsky D, Sitt A. One-Step Electrospun LTO Anode for Flexible Li-Ion Batteries. Batteries. 2025; 11(11):405. https://doi.org/10.3390/batteries11110405
Chicago/Turabian StyleMados, Edi Edna, Roni Amit, Noy Kluska, Diana Golodnitsky, and Amit Sitt. 2025. "One-Step Electrospun LTO Anode for Flexible Li-Ion Batteries" Batteries 11, no. 11: 405. https://doi.org/10.3390/batteries11110405
APA StyleMados, E. E., Amit, R., Kluska, N., Golodnitsky, D., & Sitt, A. (2025). One-Step Electrospun LTO Anode for Flexible Li-Ion Batteries. Batteries, 11(11), 405. https://doi.org/10.3390/batteries11110405








