Research Progress on the Influence of Cathode Materials on Thermal Runaway Behavior of Lithium-Ion Batteries
Abstract
1. Introduction
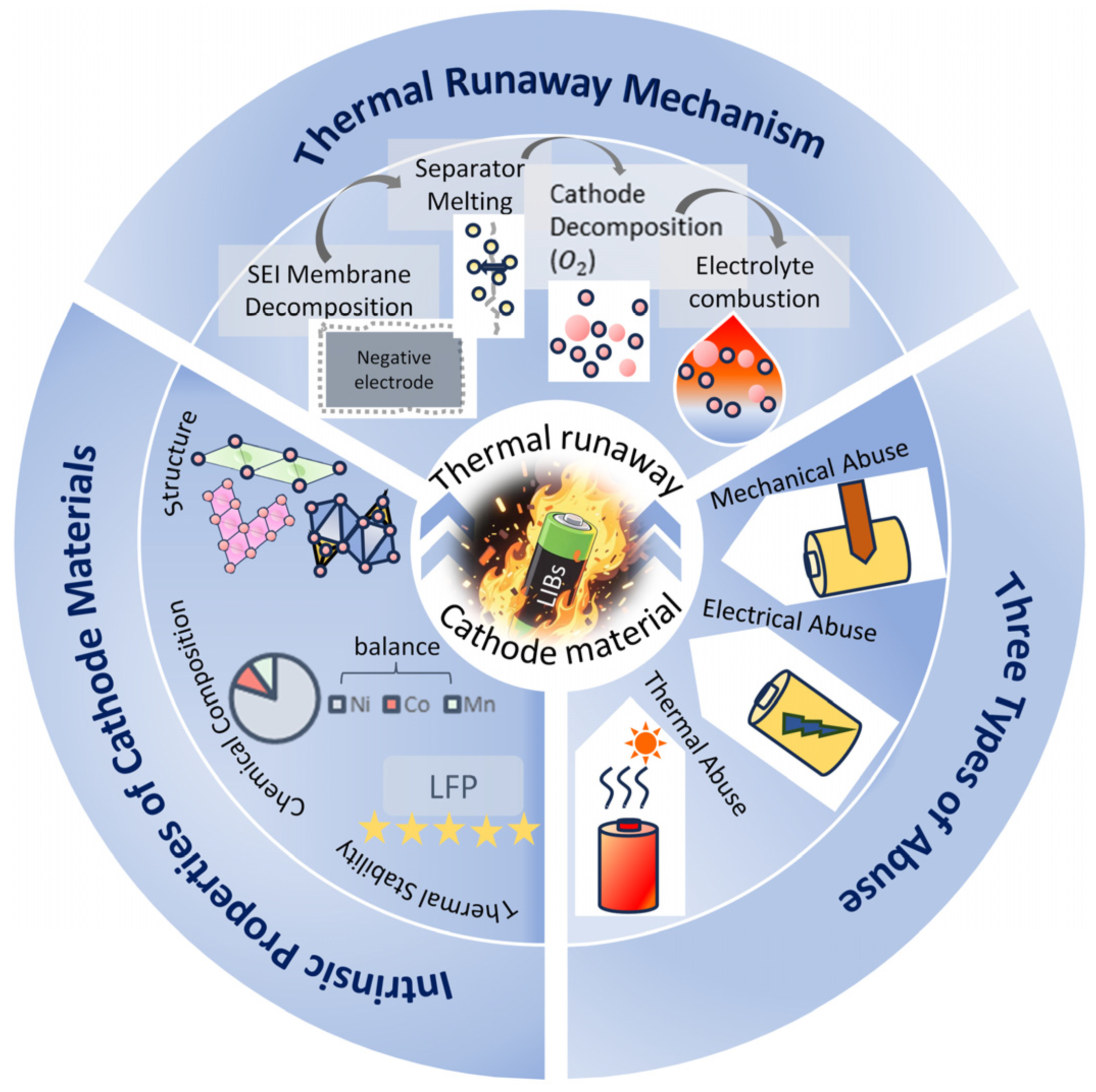
2. Thermal Runaway Mechanism Characteristics of Cathode Materials
2.1. Triggering and Evolution Mechanism of Thermal Runaway
2.2. Role of Cathode Materials in Thermal Runaway
2.2.1. Hazards of Cathode Materials
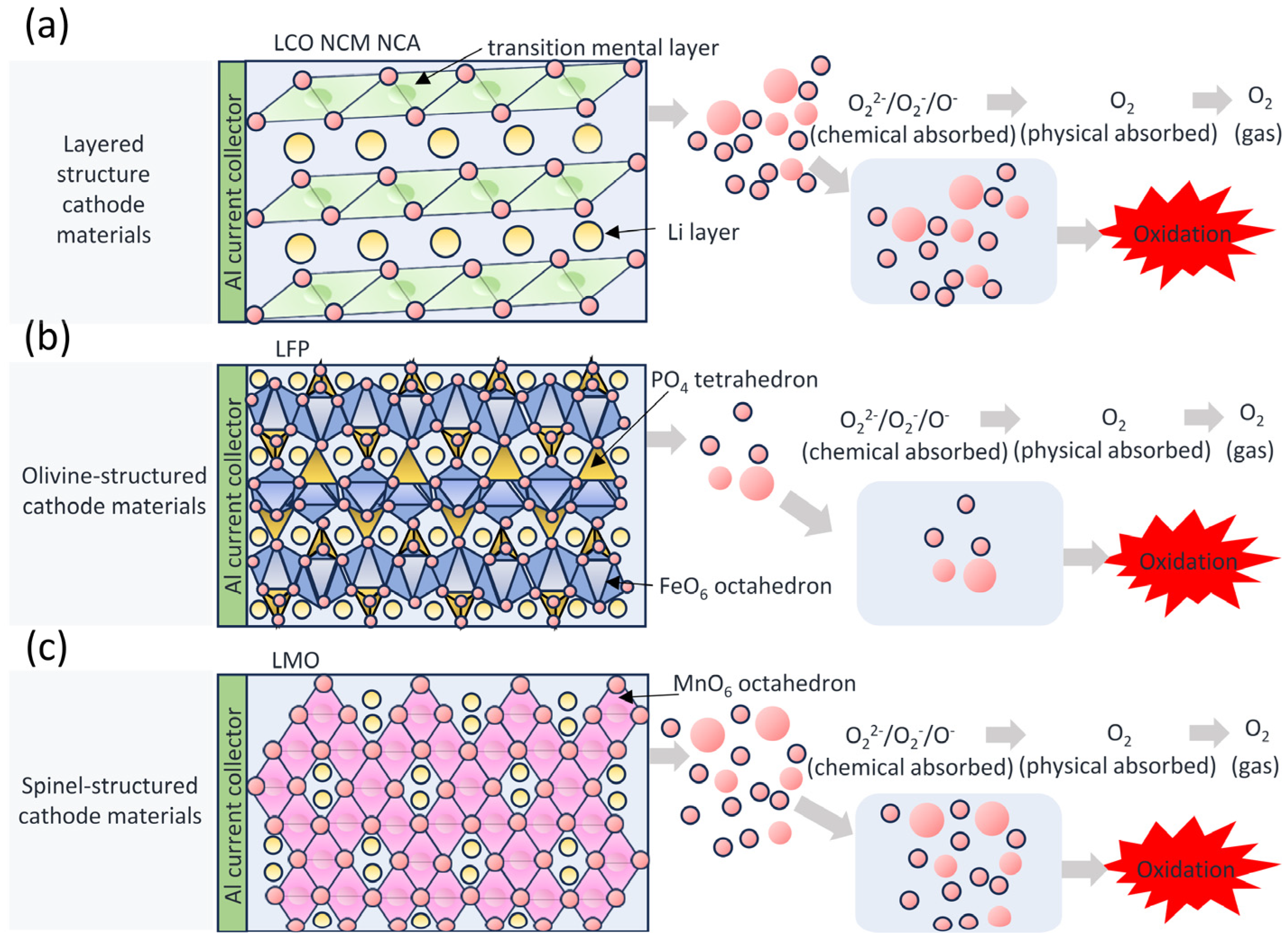
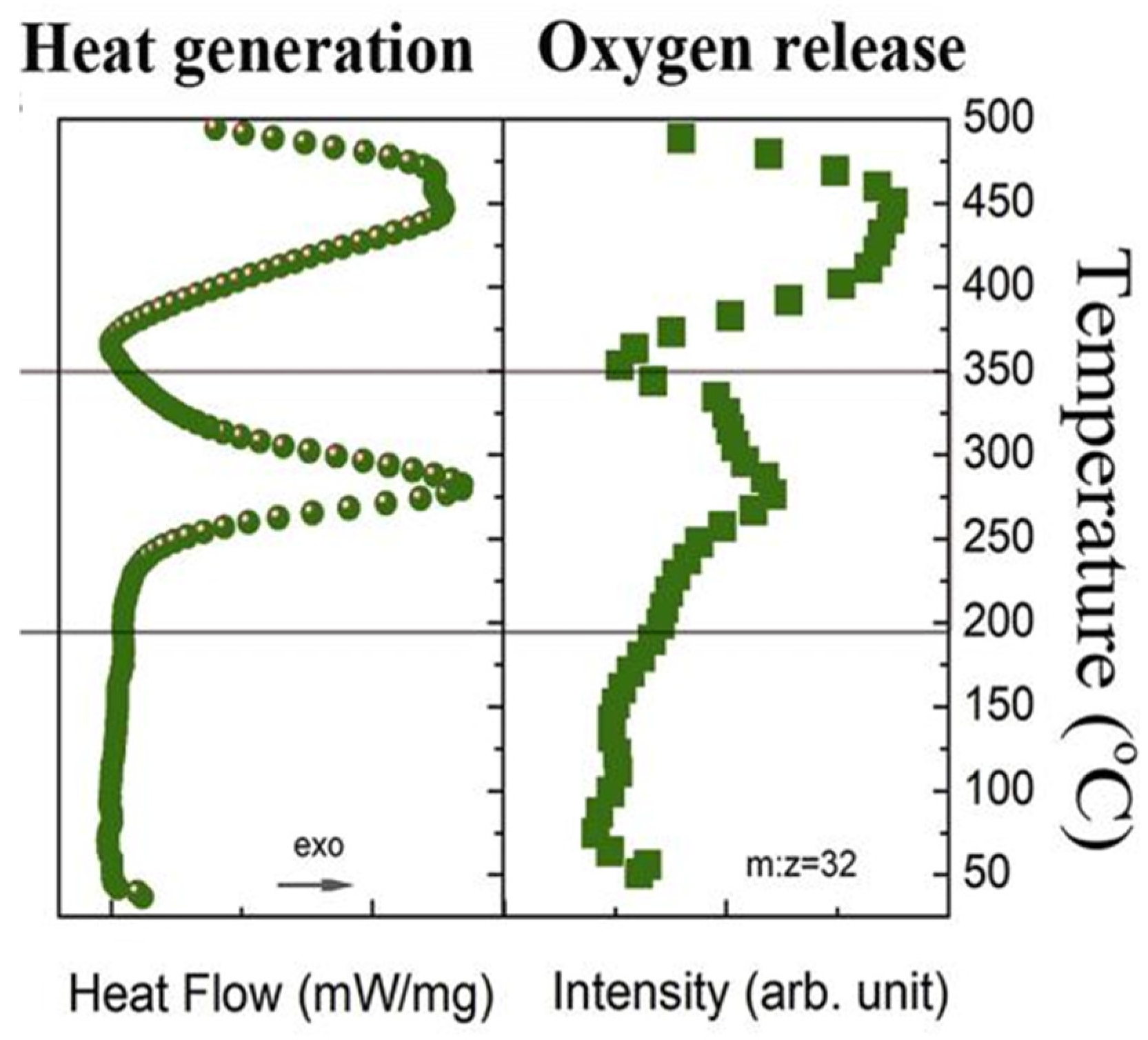
2.2.2. Sources and Hazards of Oxygen
2.2.3. Differences in Oxygen Release of Cathode Materials
3. Influence of Intrinsic Properties on Thermal Runaway Behavior of Lithium-Ion Batteries
3.1. Influence of Structure on Thermal Runaway Behavior
3.1.1. Structural Type Determines Thermal Stability
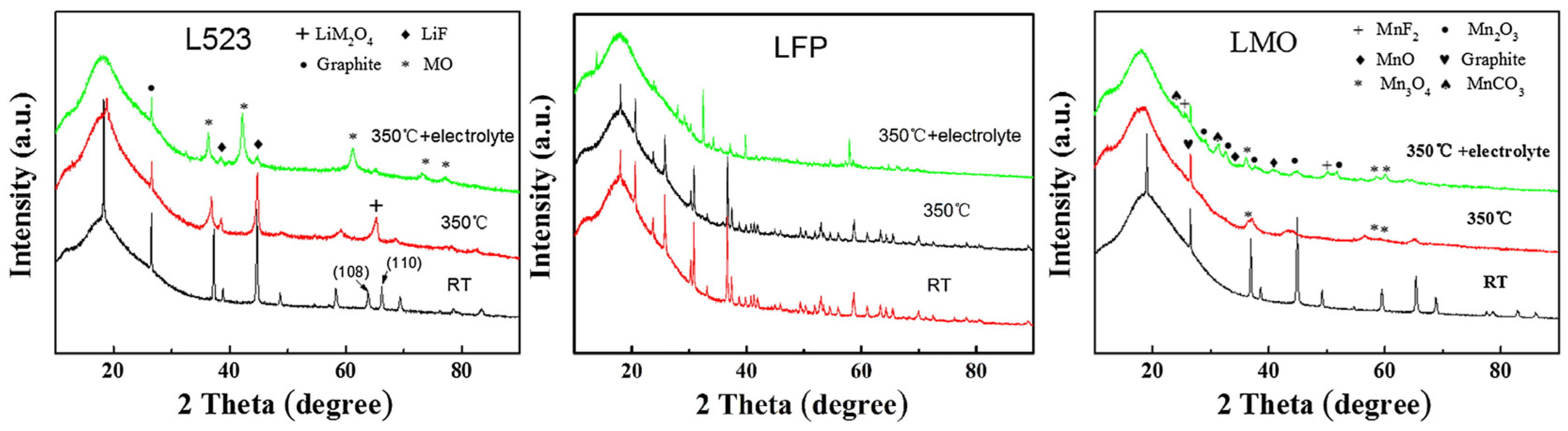
3.1.2. Mechanisms of Destabilization of Layered Structures
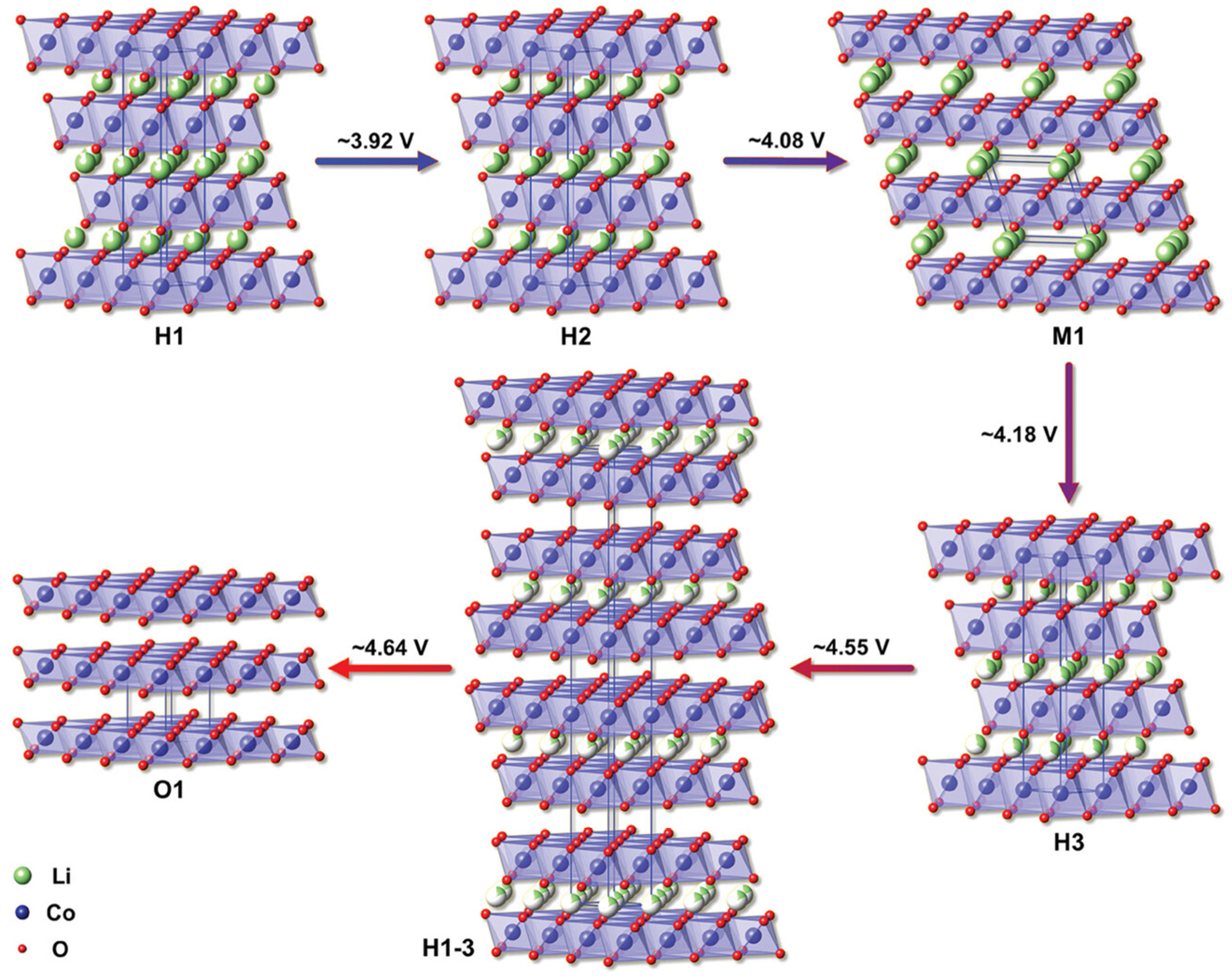
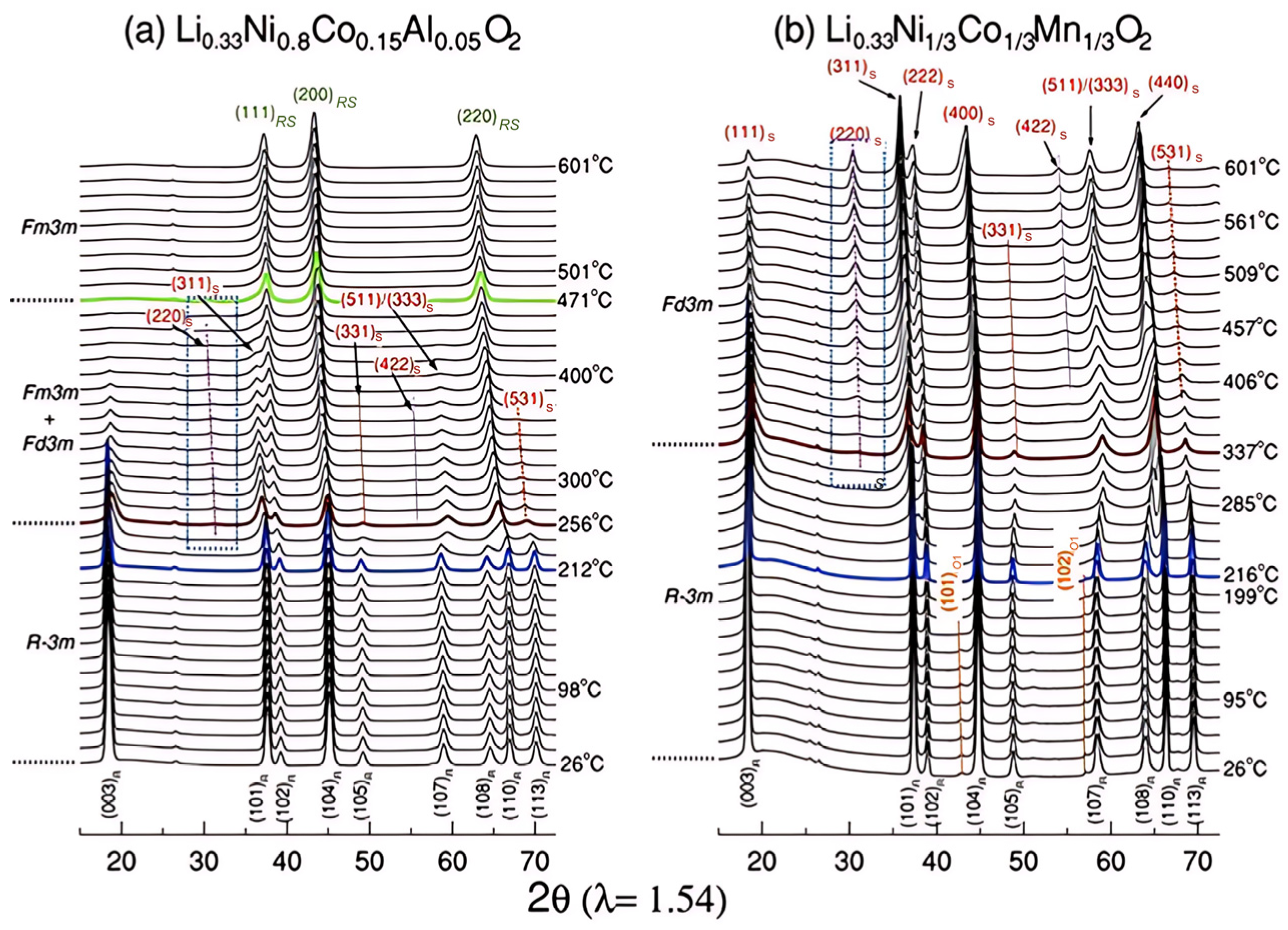
3.1.3. Influence of Phase Transition Path Differences on Thermal Runaway Behavior
3.2. Effect of Chemical Composition on Thermal Runaway Behavior
3.2.1. Bifacial Characterization of High-Nickel Cathode Materials
3.2.2. Stabilizing Effects and Limitations of Cobalt and Manganese
3.2.3. Synergies and Optimal Ratios of Chemical Elements
3.3. Influence of Thermal Stability on Thermal Runaway Behavior
3.3.1. Centrality and Characterization Parameters of Thermal Stability
3.3.2. Measurement of Thermal Stability
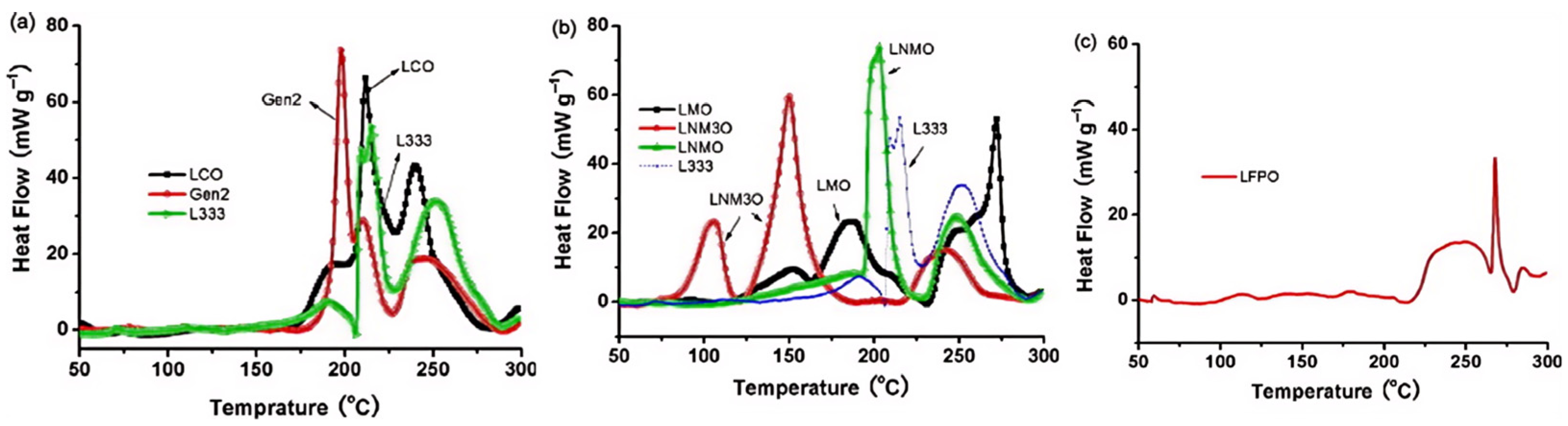
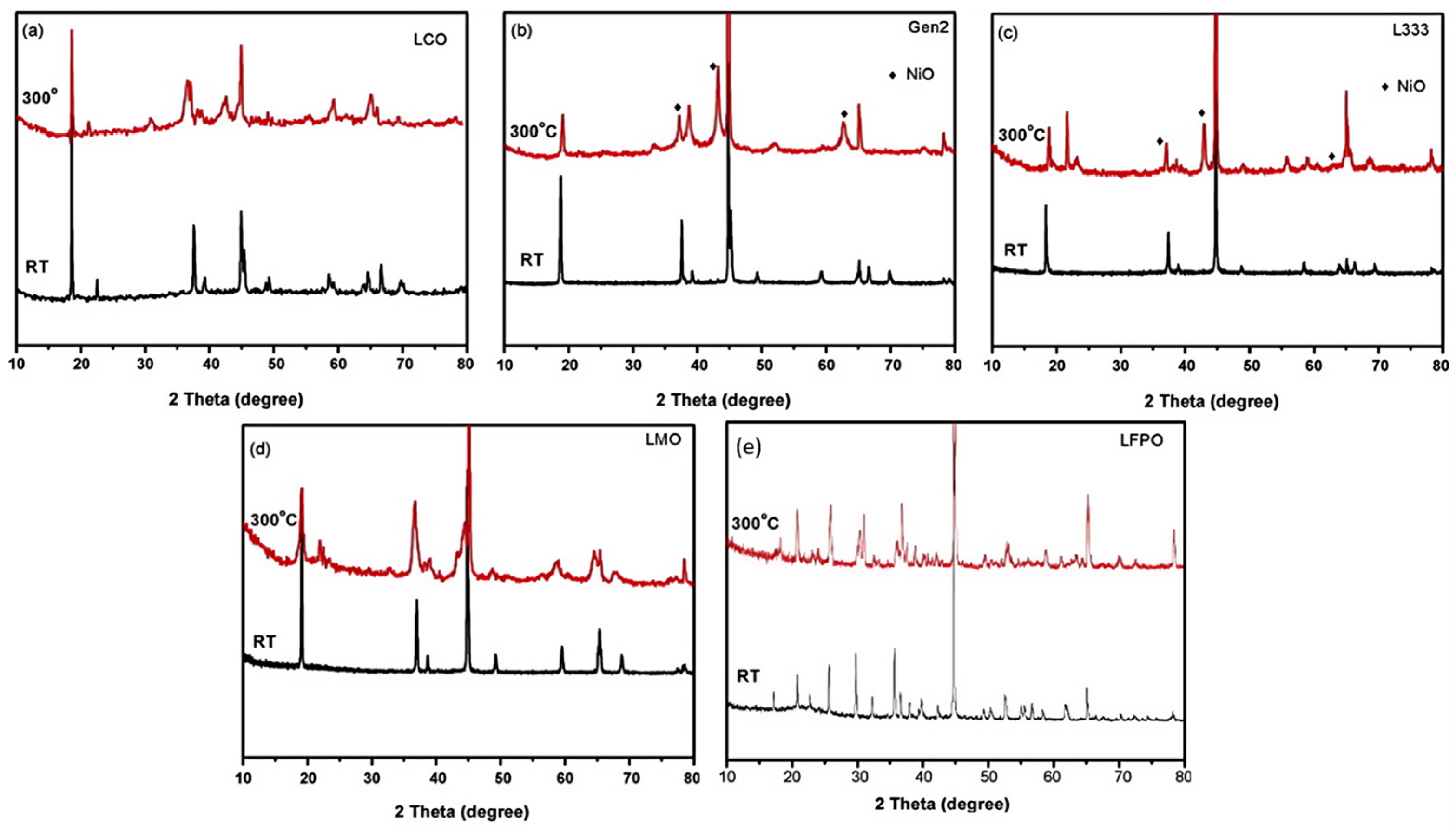
3.3.3. Thermal Stability of Cathode Materials and Its Impact on Thermal Runaway Behavior
4. Thermal Runaway Behavior of Cathode Materials Under Three Abuses
5. Impact of Cathode Materials on System-Level Safety
5.1. Role of Cathode Materials in Module-Level Thermal Runaway Propagation
5.2. Modulation of System-Level Hazard Characteristics by Cathode Materials
6. Summary and Outlook
6.1. Summary
- During thermal runaway events, cathode materials act as primary contributors. Oxygen liberated through their thermal decomposition serves as a key accelerator of the runaway process; greater oxygen evolution is directly proportional to intensified exothermic reactions. With LFP cathodes exhibiting negligible oxygen evolution, they demonstrate significantly reduced hazard potential compared to alternative cathode chemistries.
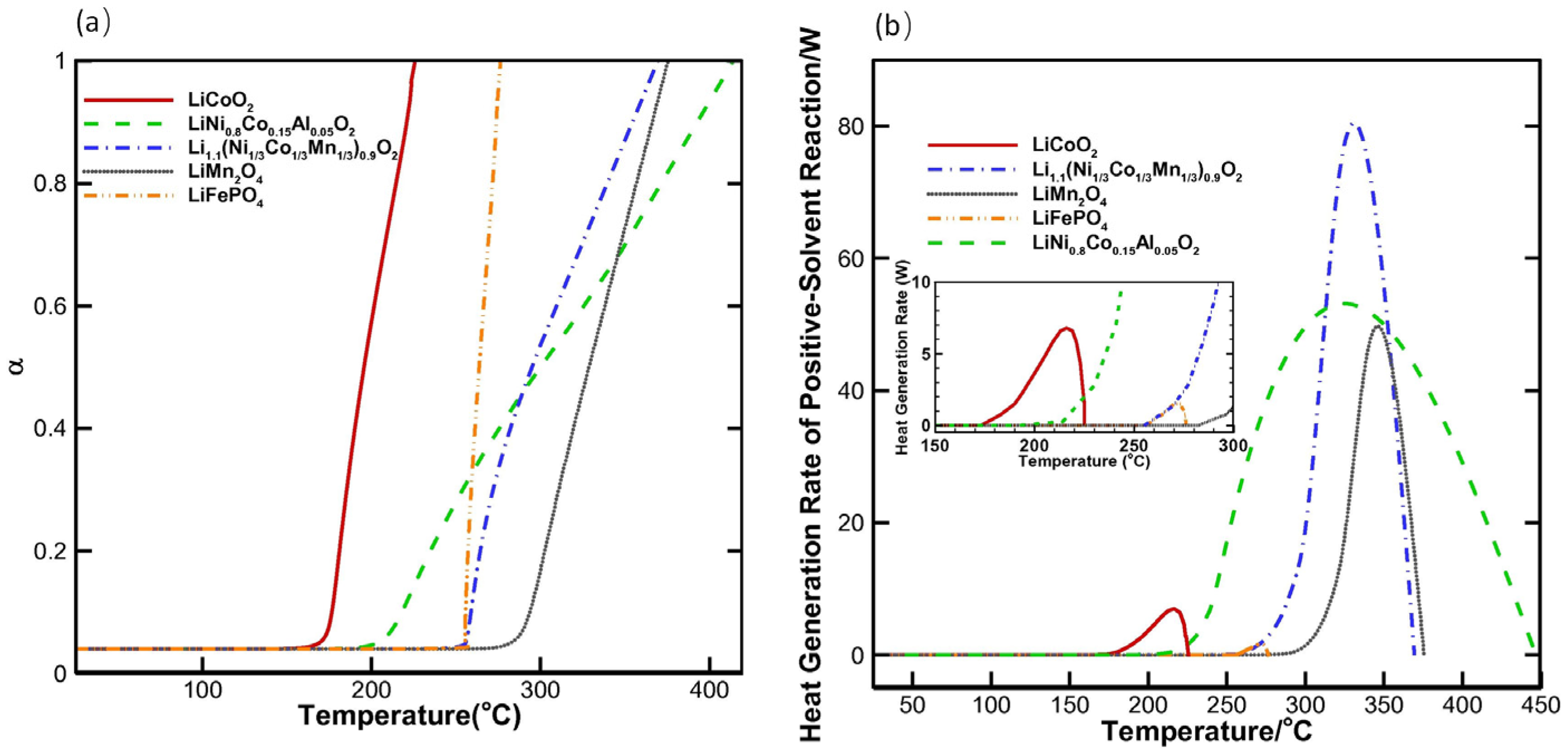
- Crystal configuration fundamentally governs the inherent thermal resilience of cathode materials, with structural stability typically ranking: olivine > spinel > layered. The strong covalent bonding of PO4 tetrahedra in the structure of olivine locks oxygen with excellent thermal stability. The (PO4)3− polyanion can convert the heat released from oxygen into phase transition energy and realize flame retardancy. Delithiation-induced structural collapse in layered cathode materials is the root cause of high risk, and the layered structure requires phase transition modulation to compensate for the lack of structural stability. Development of the Co3O4 spinel phase within NCM cathodes plays a critical role in mitigating severe thermal runaway propagation.
- NCM with high-nickel content is less thermally stable than low-nickel materials, and NCM with low-nickel content does not have the high capacity of high-nickel materials. Balancing thermal stability and high capacity, and exploring the optimal ratio between nickel, cobalt, and manganese chemical elements is a better method than adjusting the content of a single nickel element.
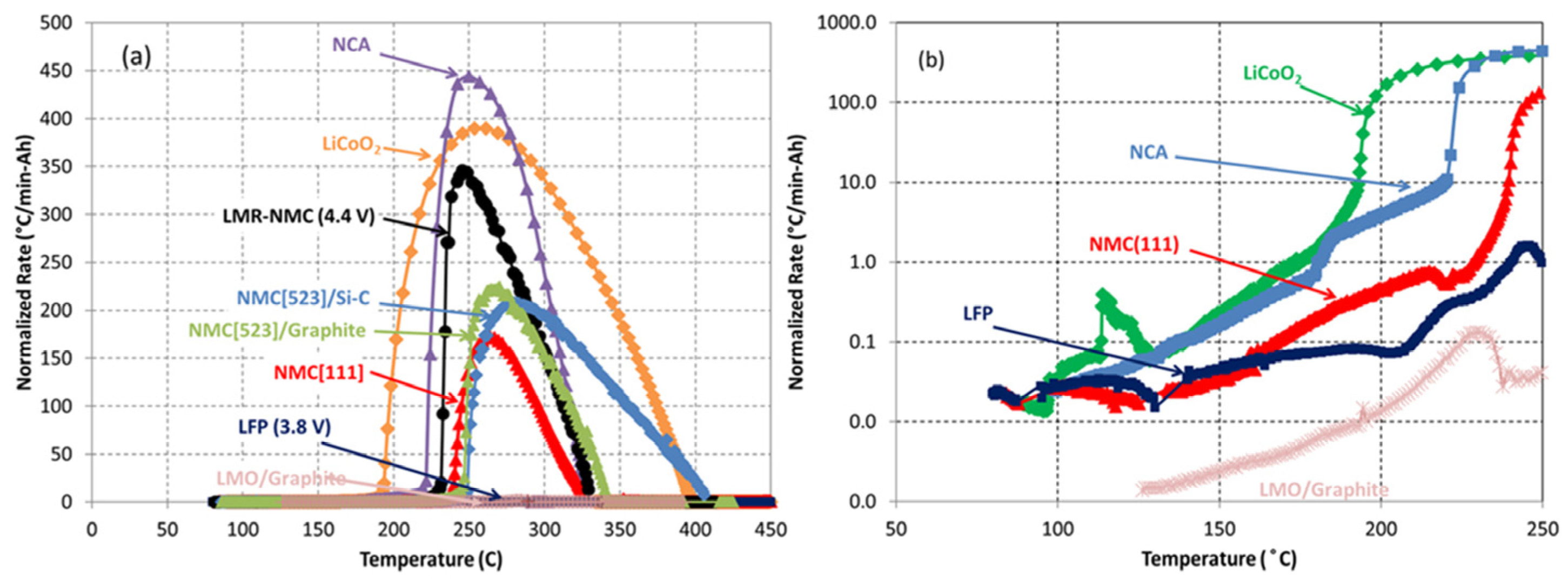
- Cathode materials exhibit an inherent thermal resilience hierarchy: LFP > LMO > NCM111 > NCM811 > LCO > NCA. However, this hierarchy is substantially modulated by state-of-charge (SOC) levels and operational environmental conditions.
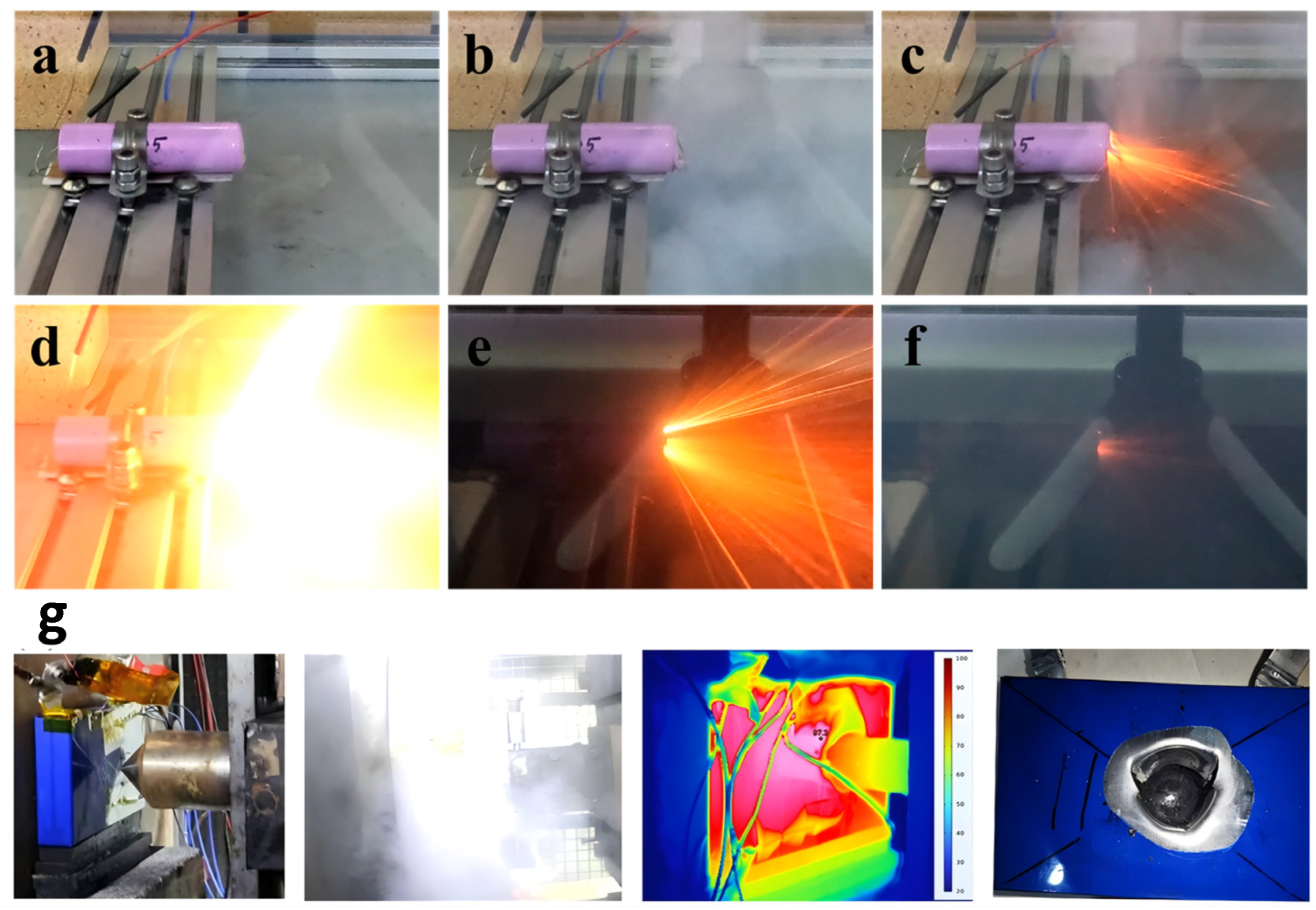
- Under three types of abuse conditions, the inherent risks of cathode materials are activated. LFP with an olivine structure demonstrates the highest safety performance under mechanical, electrical, and thermal abuse, typically exhibiting no open flames or only weak flames during thermal runaway. In contrast, layered structure materials exhibit lower onset temperatures of thermal runaway than LFP under all three abuse conditions, along with higher maximum temperatures during the process. They are also accompanied by intense jetting flames and the generation of substantial amounts of flammable gases, resulting in a significantly higher fire risk.
- The influence of cathode materials spans multiple scales. At the module level, highly reactive layered materials significantly accelerate thermal runaway propagation. At the system level, the released reactive oxygen species and substantial amounts of flammable gases increase fire suppression difficulty and the risk of gas cloud explosions, respectively, thereby dictating the safety design strategies and costs of battery systems.
6.2. Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LIB | Lithium-ion battery |
| TR | Thermal runaway |
| SEI | Solid-electrolyte interphase |
| ARC | Accelerating rate calorimetry |
| LiFePO4(LFP) | Lithium iron phosphate |
| LiMn2O4(LMO) | Lithium manganate |
| LiCoO2(LCO) | Lithium cobaltate |
| NMC | Lithium-nickel-manganese-cobalt oxide |
| NCA | Lithium-nickel-cobalt-aluminum oxide |
| Ni | Nickel |
| ISC | Internal short circuit |
| SEI | Solid electrolyte interphase |
| CO2 | Carbon dioxide |
| O2 | Oxygen |
| C2H4 | Ethylene |
| DSC | Differential scanning calorimetry |
| XRD | X-ray diffraction |
| RT | Room temperature |
| Co | Cobalt |
| Mn | Manganese |
| T-onset | Reaction onset temperature |
| △H | Total thermal runaway heat release |
| dT/dt | Temperature rise rate |
| VSP2 | Vent sizing package 2 |
| SOC | State of charge |
| HWS | Heating-waiting-search |
| Tmax | Maximum temperature reached during TR |
| Fmax | Peak expansion force |
| AEC | Adiabatic explosion chamber |
| T0 | Initial exothermic temperature |
References
- Ouyang, D.X.; Chen, M.Y.; Liu, J.H.; Wei, R.C.; Weng, J.W.; Wang, J. Investigation of a commercial lithium-ion battery under overcharge/over-discharge failure conditions. RSC Adv. 2018, 8, 33414–33424. [Google Scholar] [CrossRef]
- Ouyang, D.; Weng, J.; Chen, M.; Wang, J. What a role does the safety vent play in the safety of 18650-size lithium-ion batteries? Process Saf. Environ. Prot. 2022, 159, 433–441. [Google Scholar] [CrossRef]
- Jia, Z.; Qin, P.; Li, Z.; Wei, Z.; Jin, K.; Jiang, L.; Wang, Q. Analysis of gas release during the process of thermal runaway of lithium-ion batteries with three different cathode materials. J. Energy Storage 2022, 50, 104302. [Google Scholar] [CrossRef]
- Jhu, C.-Y.; Wang, Y.-W.; Shu, C.-M.; Chang, J.-C.; Wu, H.-C. Thermal explosion hazards on 18650 lithium ion batteries with a VSP2 adiabatic calorimeter. J. Hazard. Mater. 2011, 192, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Stefanopoulou, A.G.; Siegel, J.B. Modeling Li-Ion Battery Temperature and Expansion Force during the Early Stages of Thermal Runaway Triggered by Internal Shorts. J. Electrochem. Soc. 2019, 166, A2431. [Google Scholar] [CrossRef]
- Talele, V.; Moralı, U.; Patil, M.S.; Panchal, S.; Fraser, R.; Fowler, M.; Thorat, P.; Gokhale, Y.P. Computational modelling and statistical evaluation of thermal runaway safety regime response on lithium-ion battery with different cathodic chemistry and varying ambient condition. Int. Commun. Heat Mass Transf. 2023, 146, 106907. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.; Zhai, H.; Wang, Q. Experimental investigation on the characteristics of thermal runaway and its propagation of large-format lithium ion batteries under overcharging and overheating conditions. Energy 2021, 233, 121103. [Google Scholar] [CrossRef]
- Zhu, Q.; Liang, K.F.; Zhou, X. Research on Thermal Runaway Characteristics of High-Capacity Lithium Iron Phosphate Batteries for Electric Vehicles. World Electr. Veh. J. 2024, 15, 147. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Mao, B.; Chen, M.; Huang, Z.; Wang, Q. Experimental study on thermal runaway and fire behaviors of large format lithium iron phosphate battery. Appl. Therm. Eng. 2021, 192, 116949. [Google Scholar] [CrossRef]
- Lei, B.; Zhao, W.; Ziebert, C.; Uhlmann, N.; Rohde, M.; Seifert, H.J. Experimental Analysis of Thermal Runaway in 18650 Cylindrical Li-Ion Cells Using an Accelerating Rate Calorimeter. Batteries 2017, 3, 14. [Google Scholar] [CrossRef]
- Maleki, H.; Howard, J.N. Role of the cathode and anode in heat generation of Li-ion cells as a function of state of charge. J. Power Sources 2004, 137, 117–127. [Google Scholar] [CrossRef]
- Liu, X.; Ren, D.S.; Hsu, H.J.; Feng, X.N.; Xu, G.L.; Zhuang, M.H.; Gao, H.; Lu, L.G.; Han, X.B.; Chu, Z.Y.; et al. Thermal Runaway of Lithium-Ion Batteries without Internal Short Circuit. Joule 2018, 2, 2047–2064. [Google Scholar] [CrossRef]
- Jhu, C.-Y.; Wang, Y.-W.; Wen, C.-Y.; Shu, C.-M. Thermal runaway potential of LiCoO2 and Li(Ni1/3Co1/3Mn1/3)O2 batteries determined with adiabatic calorimetry methodology. Appl. Energy 2012, 100, 127–131. [Google Scholar] [CrossRef]
- Tian, Z.C.; Yu, H.; Zhang, Z.; Xu, X.J. Performance Improvements of Cobalt Oxide Cathodes for Rechargeable Lithium Batteries. ChemBioEng Rev. 2018, 5, 111–118. [Google Scholar] [CrossRef]
- Rodrigues, M.T.F.; Babu, G.; Gullapalli, H.; Kalaga, K.; Sayed, F.N.; Kato, K.; Joyner, J.; Ajayan, P.M. A materials perspective on Li-ion batteries at extreme temperatures. Nat. Energy 2017, 2, 17108. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Causes and mechanism of thermal runaway in lithium-ion batteries, contradictions in the generally accepted mechanism. J. Energy Storage 2024, 86, 111372. [Google Scholar] [CrossRef]
- Manthiram, A.; Knight, J.C.; Myung, S.T.; Oh, S.M.; Sun, Y.K. Nickel-Rich and Lithium-Rich Layered Oxide Cathodes: Progress and Perspectives. Adv. Energy Mater. 2016, 6, 1501010. [Google Scholar] [CrossRef]
- Wang, Q.S.; Sun, J.H.; Chen, X.F.; Chu, G.Q.; Chen, C.H. Effects of solvents and salt on the thermal stability of charged LiCoO2. Mater. Res. Bull. 2009, 44, 543–548. [Google Scholar] [CrossRef]
- Lamb, J.; Torres-Castro, L.; Hewson, J.C.; Shurtz, R.C.; Preger, Y. Investigating the Role of Energy Density in Thermal Runaway of Lithium-Ion Batteries with Accelerating Rate Calorimetry. J. Electrochem. Soc. 2021, 168, 060516. [Google Scholar] [CrossRef]
- Ouyang, D.; Chung, Y.-H.; Liu, J.; Bai, J.; Zhou, Y.; Chen, S.; Wang, Z.; Shu, C.-M. Characteristics and mechanisms of as well as evaluation methods and countermeasures for thermal runaway propagation in lithium-ion batteries. Prog. Energy Combust. Sci. 2025, 108, 101209. [Google Scholar] [CrossRef]
- Ouyang, D.; Liu, X.; Liu, B.; Wang, Z. An experimental investigation on thermal runaway features of lithium-ion battery modules under tunnel scenarios. Int. Commun. Heat Mass Transf. 2025, 164, 108922. [Google Scholar] [CrossRef]
- He, X.; Hu, Z.; Restuccia, F.; Yuan, H.; Rein, G. Self-heating ignition of large ensembles of Lithium-ion batteries during storage with different states of charge and cathodes. Appl. Therm. Eng. 2021, 197, 117349. [Google Scholar] [CrossRef]
- Yan, W.H.; Huang, W.X.; Yang, Y.; Wei, Z.W.; Zhen, H.S.; Lin, Y. Research on overcharge mitigations and thermal runaway risk of 18650 lithium-ion batteries. J. Energy Storage 2025, 120, 116372. [Google Scholar] [CrossRef]
- Liu, S.T.; Li, Y.; Han, D.C.; Sun, J.L.; Wang, H.B.; Li, Y. Thermal runaway procedure and residue analysis of LiFePO4 batteries with different charging states under nail penetrating. J. Appl. Electrochem. 2024, 54, 1485–1500. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, M.; Zhou, X.; Li, L.; Ju, X.; Yang, L. Investigating thermal runaway triggering mechanism of the prismatic lithium iron phosphate battery under thermal abuse. Renew. Energy 2024, 220, 119674. [Google Scholar] [CrossRef]
- E, J.; Xiao, H.; Tian, S.; Huang, Y. A comprehensive review on thermal runaway model of a lithium-ion battery: Mechanism, thermal, mechanical, propagation, gas venting and combustion. Renew. Energy 2024, 229, 120762. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, C.; Wang, Y. Failure Mechanism and Thermal Runaway in Batteries during Micro-Overcharge Aging at Different Temperatures. Materials 2024, 17, 2125. [Google Scholar] [CrossRef]
- Adanouj, I.; Kriston, Á.; Ruiz, V.; Pfrang, A. Identification and Quantification of Decomposition Mechanisms in Lithium-Ion Batteries; Input to Heat Flow Simulation for Modeling Thermal Runaway. JoVE 2022, e62376. [Google Scholar] [CrossRef]
- Chen, A.; Sahin, R.; Ströbel, M.; Kottke, T.; Hecker, S.; Fill, A. A Comprehensive Model and Experimental Investigation of Venting Dynamics and Mass Loss in Lithium-Ion Batteries Under a Thermal Runaway. Batteries 2025, 11, 96. [Google Scholar] [CrossRef]
- Wang, Z.P.; Yuan, J.; Zhu, X.Q.; Wang, H.; Huang, W.; Wang, Y.T.; Xu, S.Q. Overcharge-to-thermal-runaway behavior and safety assessment of commercial lithium-ion cells with different cathode materials: A comparison study. J. Energy Chem. 2021, 55, 484–498. [Google Scholar] [CrossRef]
- Yuan, Y.; Ma, Q.; Zhang, X.Q.; Zhang, F.; Song, X.N.; Xin, H.C.; Zhu, G.R.; Zhang, H.Z. Influence of cathode materials on thermal characteristics of lithium-ion batteries. Front. Chem. 2024, 12, 1324840. [Google Scholar] [CrossRef]
- Li, T.; Jiao, Y. Revealing the Thermal Runaway Behavior of Lithium Iron Phosphate Power Batteries at Different States of Charge and Operating Environment. Int. J. Electrochem. Sci. 2022, 17, 221030. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, S.; Gao, Y.; Shen, C. Review of mechanisms and detection methods of internal short circuits in lithium-ion batteries. Ionics 2025, 31, 3945–3964. [Google Scholar] [CrossRef]
- Perea, A.; Paolella, A.; Dubé, J.; Champagne, D.; Mauger, A.; Zaghib, K. State of charge influence on thermal reactions and abuse tests in commercial lithium-ion cells. J. Power Sources 2018, 399, 392–397. [Google Scholar] [CrossRef]
- Chen, Y. Recent advances of overcharge investigation of lithium-ion batteries. Ionics 2022, 28, 495–514. [Google Scholar] [CrossRef]
- Zhong, H.Y.; Zhong, Q.D.; Yang, J.; Zhong, S.W. Thermal behavior and failure mechanisms of 18650 lithium ion battery induced by overcharging cycling. Energy Rep. 2022, 8, 7286–7296. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Wang, L.; Feng, X.N.; He, X.M. Probing the heat sources during thermal runaway process by thermal analysis of different battery chemistries. J. Power Sources 2018, 378, 527–536. [Google Scholar] [CrossRef]
- Mao, N.; Zhang, T.; Wang, Z.R.; Gadkari, S.; Wang, J.L.; He, T.F.; Gao, T.F.; Cai, Q. Revealing the thermal stability and component heat contribution ratio of overcharged lithium-ion batteries during thermal runaway. Energy 2023, 263, 125786. [Google Scholar] [CrossRef]
- Lee, K.-S.; Myung, S.-T.; Kim, D.-W.; Sun, Y.-K. AlF3-coated LiCoO2 and Li[Ni1/3Co1/3Mn1/3]O2 blend composite cathode for lithium ion batteries. J. Power Sources 2011, 196, 6974–6977. [Google Scholar] [CrossRef]
- Barkholtz, H.M.; Preger, Y.; Ivanov, S.; Langendorf, J.; Torres-Castro, L.; Lamb, J.; Chalamala, B.; Ferreira, S.R. Multi-scale thermal stability study of commercial lithium-ion batteries as a function of cathode chemistry and state-of-charge. J. Power Sources 2019, 435, 226777. [Google Scholar] [CrossRef]
- Mendoza-Hernandez, O.S.; Ishikawa, H.; Nishikawa, Y.; Maruyama, Y.; Umeda, M. Cathode material comparison of thermal runaway behavior of Li-ion cells at different state of charges including over charge. J. Power Sources 2015, 280, 499–504. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wang, L.; Feng, X.N.; Ren, D.S.; Wu, Y.; Xu, G.L.; Lu, L.G.; Hou, J.X.; Zhang, W.F.; et al. Thermal runaway mechanism of lithium-ion battery with LiNi0.8Mn0.1Co0.1O2 cathode materials. Nano Energy 2021, 85, 105878. [Google Scholar] [CrossRef]
- Hou, X.Y.; Kimura, Y.; Tamenori, Y.; Nitta, K.; Yamagishi, H.; Amezawa, K.; Nakamura, T. Thermodynamic Analysis Enables Quantitative Evaluation of Lattice Oxygen Stability in Li-Ion Battery Cathodes. ACS Energy Lett. 2022, 7, 1687–1693. [Google Scholar] [CrossRef]
- Röder, P.; Stiaszny, B.; Ziegler, J.C.; Baba, N.; Lagaly, P.; Wiemhöfer, H.-D. The impact of calendar aging on the thermal stability of a LiMn2O4–Li(Ni1/3Mn1/3Co1/3)O2/graphite lithium-ion cell. J. Power Sources 2014, 268, 315–325. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Xu, R.Y.; Han, X.; Huang, K.; Ke, X.X.; Wang, B.; Sui, M.L.; Yan, P.F. Cross-scale deciphering thermal failure process of Ni-rich layered cathode. Nano Energy 2024, 126, 109685. [Google Scholar] [CrossRef]
- Röder, P.; Baba, N.; Wiemhöfer, H.D. A detailed thermal study of a Li[Ni0.33Co0.33Mn0.33]O2/LiMn2O4-based lithium ion cell by accelerating rate and differential scanning calorimetry. J. Power Sources 2014, 248, 978–987. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Z.; Sun, J.; Wang, Q. Heat generation and thermal runaway of lithium-ion battery induced by slight overcharging cycling. J. Power Sources 2022, 526, 231136. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; He, X.M. Trends in a study on thermal runaway mechanism of lithium-ion battery with LiNixMnyCo1-x-yO2 cathode materials. Battery Energy 2022, 1, 20210011. [Google Scholar] [CrossRef]
- Belharouak, I.; Lu, W.; Vissers, D.; Amine, K. Safety characteristics of Li(Ni0.8Co0.15Al0.05)O2 and Li(Ni1/3Co1/3Mn1/3)O2. Electrochem. Commun. 2006, 8, 329–335. [Google Scholar] [CrossRef]
- Monteiro, I.F.; Pinto, R.S.; Silva, M.M.; Fidalgo-Marijuan, A.; Costa, C.M.; Lanceros-Méndez, S.; Gonçalves, R. Lithium-ion battery high performance cathode electrode based on LiFePO4 and thermal sensitive microspheres with thermal shutdown properties. J. Power Sources 2024, 614, 234956. [Google Scholar] [CrossRef]
- Ping, P.; Wang, Q.S.; Huang, P.F.; Li, K.; Sun, J.H.; Kong, D.P.; Chen, C.H. Study of the fire behavior of high-energy lithium-ion batteries with full-scale burning test. J. Power Sources 2015, 285, 80–89. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Liu, F.; Fang, Z.; Gu, N.; Chen, B.; Yang, N.; Jia, Y. A comparative study of overcharge thermal runaway force-electrical-thermal characteristics and safety assessment of lithium batteries with different cathode materials. Appl. Therm. Eng. 2024, 256, 124092. [Google Scholar] [CrossRef]
- Faulkner, J.; Tatarchuk, B.J. The enthalpic reaction limits of Li-ion battery chemistries within various operating regimes and environments. J. Power Sources 2025, 635, 236329. [Google Scholar] [CrossRef]
- Nie, B.; Dong, Y.; Chang, L. The evolution of thermal runaway parameters of lithium-ion batteries under different abuse conditions: A review. J. Energy Storage 2024, 96, 112624. [Google Scholar] [CrossRef]
- Hu, E.Y.; Bak, S.M.; Senanayake, S.D.; Yang, X.Q.; Nam, K.W.; Zhang, L.L.; Shao, M.H. Thermal stability in the blended lithium manganese oxide—Lithium nickel cobalt manganese oxide cathode materials: An in situ time-resolved X-Ray diffraction and mass spectroscopy study. J. Power Sources 2015, 277, 193–197. [Google Scholar] [CrossRef]
- Qi, C.B.; Wang, H.W.; Li, M.H.; Li, C.; Li, Y.L.; Shi, C.; Wei, N.N.; Wang, Y.; Zhang, H.P. Research on the Thermal Runaway Behavior and Flammability Limits of Sodium-Ion and Lithium-Ion Batteries. Batteries 2025, 11, 24. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Zhang, P.; Zhao, J. A detailed thermal study of usual LiNi0.5Co0.2Mn0.3O2, LiMn2O4 and LiFePO4 cathode materials for lithium ion batteries. J. Energy Storage 2017, 12, 37–44. [Google Scholar] [CrossRef]
- El Moutchou, S.; Aziam, H.; Mansori, M.; Saadoune, I. Thermal stability of Lithium-ion batteries: Case study of NMC811 and LFP cathode materials. Mater. Today Proc. 2022, 51, A1–A7. [Google Scholar] [CrossRef]
- Zaghib, K.; Dubé, J.; Dallaire, A.; Galoustov, K.; Guerfi, A.; Ramanathan, M.; Benmayza, A.; Prakash, J.; Mauger, A.; Julien, C.M. Enhanced thermal safety and high power performance of carbon-coated LiFePO4 olivine cathode for Li-ion batteries. J. Power Sources 2012, 219, 36–44. [Google Scholar] [CrossRef]
- Park, J.-S.; Oh, S.-M.; Sun, Y.-K.; Myung, S.-T. Thermal properties of fully delithiated olivines. J. Power Sources 2014, 256, 479–484. [Google Scholar] [CrossRef]
- Nam, K.W.; Bak, S.M.; Hu, E.Y.; Yu, X.Q.; Zhou, Y.N.; Wang, X.J.; Wu, L.J.; Zhu, Y.M.; Chung, K.Y.; Yang, X.Q. Combining In Situ Synchrotron X-Ray Diffraction and Absorption Techniques with Transmission Electron Microscopy to Study the Origin of Thermal Instability in Overcharged Cathode Materials for Lithium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 1047–1063. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, B.; Lu, Y. Progress and perspective of high-voltage lithium cobalt oxide in lithium-ion batteries. J. Energy Chem. 2022, 74, 283–308. [Google Scholar] [CrossRef]
- Lin, C.; Li, J.Y.; Yin, Z.W.; Huang, W.Y.; Zhao, Q.H.; Weng, Q.S.; Liu, Q.; Sun, J.L.; Chen, G.H.; Pan, F. Structural Understanding for High-Voltage Stabilization of Lithium Cobalt Oxide. Adv. Mater. 2024, 36, 2307404. [Google Scholar] [CrossRef] [PubMed]
- Mao, N.; Wang, Z.R.; Chung, Y.H.; Shu, C.M. Overcharge cycling effect on the thermal behavior, structure, and material of lithium-ion batteries. Appl. Therm. Eng. 2019, 163, 114147. [Google Scholar] [CrossRef]
- Wu, C.J.; Wu, Y.; Feng, X.N.; Wang, H.B.; Zhang, F.K.; Chen, S.Q.; Li, B.; Deng, T.; Ouyang, M.G. Ultra-high temperature reaction mechanism of LiNi0.8Co0.1Mn0.1O2 electrode. J. Energy Storage 2022, 52, 104870. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, D.S.; Liu, G.J.; Mu, D.B.; Wang, L.; Wu, B.R.; Liu, J.H.; Wu, N.N.; He, X.M. Correlation between thermal stabilities of nickel-rich cathode materials and battery thermal runaway. Int. J. Energy Res. 2021, 45, 20867–20877. [Google Scholar] [CrossRef]
- Liu, S.; Ma, T.; Wei, Z.; Bai, G.; Liu, H.; Xu, D.; Shan, Z.; Wang, F. Study about thermal runaway behavior of high specific energy density Li-ion batteries in a low state of charge. J. Energy Chem. 2021, 52, 20–27. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, L.; He, C. Long cycle life lithium ion battery with lithium nickel cobalt manganese oxide (NCM) cathode. J. Power Sources 2014, 261, 285–291. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J. An overview of modification strategies to improve LiNi0·8Co0·1Mn0·1O2 (NCM811) cathode performance for automotive lithium-ion batteries. eTransportation 2021, 7, 100105. [Google Scholar] [CrossRef]
- Wang, H.; Du, Z.; Rui, X.; Wang, S.; Jin, C.; He, L.; Zhang, F.; Wang, Q.; Feng, X. A comparative analysis on thermal runaway behavior of Li (NixCoyMnz) O2 battery with different nickel contents at cell and module level. J. Hazard. Mater. 2020, 393, 122361. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wang, H.; Li, M.; Li, C.; Zhang, Y.; Li, Y.; Yang, X.; Feng, X.; Ouyang, M. Thermal Runaway Characteristics and Gas Composition Analysis of Lithium-Ion Batteries with Different LFP and NCM Cathode Materials under Inert Atmosphere. Electronics 2023, 12, 1603. [Google Scholar] [CrossRef]
- Zhao, L.; Hou, J.; Feng, X.; Xu, J.; Xu, C.; Wang, H.; Liu, H.; Hou, B.; Rui, X.; Gu, Y.; et al. The trade-off characteristic between battery thermal runaway and combustion. Energy Storage Mater. 2024, 69, 103380. [Google Scholar] [CrossRef]
- Ahmed, S.; Nelson, P.A.; Gallagher, K.G.; Susarla, N.; Dees, D.W. Cost and energy demand of producing nickel manganese cobalt cathode material for lithium ion batteries. J. Power Sources 2017, 342, 733–740. [Google Scholar] [CrossRef]
- Cui, Z.H.; Manthiram, A. Thermal Stability and Outgassing Behaviors of High-nickel Cathodes in Lithium-ion Batteries. Angew. Chem.-Int. Ed. 2023, 62, e202307243. [Google Scholar] [CrossRef]
- Jo, M.; Park, S.H.; Lee, H. Effects of a Sodium Phosphate Electrolyte Additive on Elevated Temperature Performance of Spinel Lithium Manganese Oxide Cathodes. Materials 2021, 14, 4670. [Google Scholar] [CrossRef]
- Bak, S.-M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.-J.; Kim, K.-B.; Chung, K.Y.; Yang, X.-Q.; Nam, K.-W. Structural Changes and Thermal Stability of Charged LiNixMnyCozO2 Cathode Materials Studied by Combined In Situ Time-Resolved XRD and Mass Spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef]
- Ohneseit, S.; Finster, P.; Floras, C.; Lubenau, N.; Uhlmann, N.; Seifert, H.J.; Ziebert, C. Thermal and Mechanical Safety Assessment of Type 21700 Lithium-Ion Batteries with NMC, NCA and LFP Cathodes-Investigation of Cell Abuse by Means of Accelerating Rate Calorimetry (ARC). Batteries 2023, 9, 237. [Google Scholar] [CrossRef]
- Wang, S.; Song, L.; Li, C.; Tian, J.; Jin, K.; Duan, Q.; Wang, Q. Experimental study of gas production and flame behavior induced by the thermal runaway of 280 Ah lithium iron phosphate battery. J. Energy Storage 2023, 74, 109368. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, J.R.; Yin, L.; Li, B.; Tian, Y. Staged thermal runaway behaviours of three typical lithium-ion batteries for hazard prevention. J. Therm. Anal. Calorim. 2024, 149, 10321–10333. [Google Scholar] [CrossRef]
- Jiang, J.; Dahn, J.R. ARC studies of the thermal stability of three different cathode materials: LiCoO2; Li[Ni0.1Co0.8Mn0.1]O2; and LiFePO4, in LiPF6 and LiBoB EC/DEC electrolytes. Electrochem. Commun. 2004, 6, 39–43. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, C.; Li, K.; Deng, T. A Comparative Analysis on Thermal Stability of Delithiated Nickel-Rich LiNi0.8Co0.15Al0.05O2 and LiNi0.8Co0.1Mn0.1O2 in Pouch Cells. J. Electrochem. Energy Convers. Storage 2023, 21, 011006. [Google Scholar] [CrossRef]
- Zhang, Z.; Fouchard, D.; Rea, J.R. Differential scanning calorimetry material studies: Implications for the safety of lithium-ion cells. J. Power Sources 1998, 70, 16–20. [Google Scholar] [CrossRef]
- Xiang, H.F.; Wang, H.; Chen, C.H.; Ge, X.W.; Guo, S.; Sun, J.H.; Hu, W.Q. Thermal stability of LiPF6-based electrolyte and effect of contact with various delithiated cathodes of Li-ion batteries. J. Power Sources 2009, 191, 575–581. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, W.H.; Wei, Z.S.; Wang, Z.Y.; Wang, Q.S.; Sun, J.H. Transition-metal redox evolution and its effect on thermal stability of LiNixCoyMnzO2 based on synchrotron soft X-ray absorption spectroscopy. J. Energy Chem. 2021, 59, 446–454. [Google Scholar] [CrossRef]
- Peng, P.; Jiang, F. Thermal safety of lithium-ion batteries with various cathode materials: A numerical study. Int. J. Heat Mass Transf. 2016, 103, 1008–1016. [Google Scholar] [CrossRef]
- GB 38031-2025; Safety Requirements for Traction Batteries of Electric Vehicles. Standardization Administration of the People’s Republic of China: Beijing, China, 2025.
- Wei, D.; Zhang, M.; Zhu, L.; Chen, H.; Huang, W.; Yao, J.; Yuan, Z.; Xu, C.; Feng, X. Study on Thermal Runaway Behavior of Li-Ion Batteries Using Different Abuse Methods. Batteries 2022, 8, 201. [Google Scholar] [CrossRef]
- An, Z.; Li, W.; Du, X.; Jia, L.; Li, Q.; Zhang, D. Experimental study on behaviors of lithium-ion cells experiencing internal short circuit and thermal runaway under nail penetration abuse condition. Appl. Therm. Eng. 2024, 247, 123058. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Zhou, L.; Han, C.; He, T.; Wang, Z. Influencing factors of lithium-ion battery thermal runaway in confined space. J. Energy Storage 2023, 73, 109125. [Google Scholar] [CrossRef]
- Mao, N.; Gadkari, S.; Wang, Z.R.; Zhang, T.; Bai, J.L.; Cai, Q. A comparative analysis of lithium-ion batteries with different cathodes under overheating and nail penetration conditions. Energy 2023, 278, 128027. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Sun, R.; Han, C.; Li, K.; Zhang, L.; Zhang, Y.; Guo, D.; Li, S.; Wang, Z.; et al. Thermal runaway and jet flame features of 314 Ah lithium iron phosphate battery: Mechanism exploration and safety assessment. Appl. Therm. Eng. 2025, 272, 126371. [Google Scholar] [CrossRef]
- Jeon, M.; Lee, E.; Park, H.; Yoon, H.; Keel, S. Effect of Thermal Abuse Conditions on Thermal Runaway of NCA 18650 Cylindrical Lithium-Ion Battery. Batteries 2022, 8, 196. [Google Scholar] [CrossRef]
- Chai, Z.X.; Li, J.Q.; Liu, Z.M.; Liu, Z.N.; Jin, X. Experimental analysis and safety assessment of thermal runaway behavior in lithium iron phosphate batteries under mechanical abuse. Sci. Rep. 2024, 14, 8673. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.P.; Sun, J.H.; Wang, Q.S. Thermal runaway hazards investigation on 18650 lithium-ion battery using extended volume accelerating rate calorimeter. J. Energy Storage 2020, 28, 101232. [Google Scholar] [CrossRef]
- Wang, G.Q.; Ping, P.; Kong, D.P.; Peng, R.Q.; He, X.; Zhang, Y.; Dai, X.Y.; Wen, J. Advances and challenges in thermal runaway modeling of lithium-ion batteries. Innovation 2024, 5, 100624. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Gayen, D. Thermal behaviour and thermal runaway propagation in lithium-ion battery systems—A critical review. J. Energy Storage 2023, 62, 106894. [Google Scholar] [CrossRef]
- Wang, H.B.; Liu, B.; Xu, C.S.; Jin, C.Y.; Li, K.J.; Du, Z.M.; Wang, Q.Z.; Ouyang, M.G.; Feng, X.N. Dynamic thermophysical modeling of thermal runaway propagation and parametric sensitivity analysis for large format lithium-ion battery modules. J. Power Sources 2022, 520, 230724. [Google Scholar] [CrossRef]
- Li, Y.T.; Wang, H.B.; Wang, S.L.; Xu, L.J.; Li, Y.; Sun, J.L.; Gao, Y. Thermal Runaway Propagation Characteristics of Lithium-Ion Batteries with Different Cathode Materials: A Comparative Study. Fire Technol. 2025, 61, 1–22. [Google Scholar] [CrossRef]
- Wang, H.B.; Xu, H.; Zhang, Z.L.; Wang, Q.Z.; Jin, C.Y.; Wu, C.J.; Xu, C.S.; Hao, J.Y.; Sun, L.; Du, Z.M.; et al. Fire and explosion characteristics of vent gas from lithium-ion batteries after thermal runaway: A comparative study. Transportation 2022, 13, 100190. [Google Scholar] [CrossRef]


| Accelerated Calorimetry (ARC) | Differential Scanning Calorimetry (DSC) | C80 Calorimeter | Vent Sizing Package 2 (VSP2) | |
|---|---|---|---|---|
| Core Principles | Measured under near-adiabatic conditions sample self-heating rate (Heat-Wait-Search mode) | Measurement of the difference in heat flow between the sample and the reference at the programmed temperature | Measurement of sample heat flow in a confined high-pressure cell using a three-dimensional wraparound thermopile | Simultaneous tracking of sample temperature and self-generated pressure changes under near-adiabatic conditions |
| Test Patterns | Main Insulation Modes | Major isochronous scanning(dynamic), Few constant temperatures (isothermal) | Isometric Scanning(dynamic), Constant temperature (isothermal), Custom programs | Main Insulation Modes |
| Thermal insulation | Excellent | Inferior | Superior | Excellent |
| Main output parameters | Temperature-time curve, self-heating rate, adiabatic temperature rise, time to maximum rate | Onset temperature, peak temperature, reaction enthalpy | Temperature-time curve, Onset temperature, peak temperature, reaction enthalpy | Temperature-time curve, Self-heating rate |
| Vantages | The closest approximation to real thermal runaway conditions provides critical safety parameters | High sensitivity, Measurable phase change | Higher sensitivity than ARC, provides reaction enthalpy data | Simultaneous acquisition of adiabatic temperature rise |
| Limitations | Relatively low sensitivity to small exotherms | Non-adiabatic results extrapolated to adiabatic conditions need to be modeled | Insulation not as good as ARC/VSP2; maximum pressure/temperature may be slightly lower than ARC/VSP2 | Relatively low sensitivity to small exotherms |
| Representative Applications in Cathode Thermal Behavior Research | Evaluating the thermal behavior of materials under near-realistic thermal runaway conditions, Evaluating the effects of different charging states | Rapid comparison of exothermic onset temperature, peak temperature, and reaction enthalpy for different materials and SOCs | Evaluation of reactions under different states of charge, electrolyte coexistence | Specialized in gas generation during thermal runaway |
| References | Abuse of Conditions | Cathode Materials | Key Parameters | Notes | ||||
|---|---|---|---|---|---|---|---|---|
| Wei [88] | Tmax (°C) | Mass loss (g) | Mass loss ratio (%) | TR trigger time (s) | Time of ejection & fire (s) | Tmax represents the maximum temperature reached during TR | ||
| Mechanical abuse (nail piercing) | NCM 523 | 964.3 | 331.3 | 35.6 | 1 | 6 | ||
| Thermal abuse (heating) | NCM 523 | 1020 | 372.1 | 39.9 | 180 | 136 | ||
| Electrical abuse (overcharge) | NCM 523 | \ | 601.1 | 64.3 | 4700 | 165 | ||
| Ohneseit [78] | Mechanical abuse (nail piercing) | LFP 21700 | Thermal runaway does not occur at SOC of 30%, 50%, and 100% The maximum temperature measured at different locations is 101.9 °C at SOC of 100% | ARC | ||||
| Thermal abuse (HWS) | LFP 21700 | The onset temperature at 100% SOC is about 124.4 °C Startup thermal runaway temperature of about 256.3 °C, maximum temperature of about 498.6 °C | ||||||
| Mechanical abuse (nail piercing) | NCM 21700 (high-nickel) | No thermal runaway at 0% SOC, thermal runaway at 30%, 50%, 100% Maximum temperature at different locations at 100% SOC: 760.3 °C | ||||||
| Thermal abuse (HWS) | NCM 21700 (high-nickel) | The SOC is 100%, thermal runaway onset temperature is about 85.5 °C Startup thermal runaway temperature is about 198.0 °C, maximum temperature is about 591.6 °C | ||||||
| Mechanical abuse (nail piercing) | NCA 21700 (high-nickel) | No thermal runaway at 0% SOC, thermal runaway at 30%, 50%, 100% Maximum temperature at different locations at 100% SOC: 840.7 °C | ||||||
| Thermal abuse (HWS) | NCA 21700 (high-nickel) | The onset temperature at 100% SOC is about 95.3 °C Startup thermal runaway temperature is about 203.1 °C, maximum temperature is about 644.3 °C | ||||||
| An [89] | Mass loss (g) | Mass loss ratio (%) | Tmax (°C) | (dT/dt) max (°C/s) | Under 100% SOC 8 mm nail penetration | |||
| Mechanical abuse (nail piercing) | LCO | 9.55 | 23.01 | About 800 | 443.9 | |||
| LFP | 0 | 0 | 54.9 | 0.124 | ||||
| NCM | 12.22 | 26.92 | About700 | 213.2 | ||||
| LMO | 13.96 | 22.84 | About520 | 127.7 | ||||
| Wang [30] | Mass loss (g) | Mass loss ratio (%) | T onset (°C) | Tmax (°C) | Arrival at the point in time when the TR occurs (S) | ARC | ||
| Electrical abuse (overcharge) | LFP | 162.8 | 17.7 | 112 | 308 | 1001 | ||
| NCM 111 | 309.6 | 38.7 | 93 | 589 | 1982 | |||
| NCM 622 | 332.3 | 42.6 | 92 | 629 | 1450 | |||
| NCM 811 | 318.9 | 44.3 | 93 | 695 | 1229 | |||
| Wang [52] | Mass loss (g) | Mass loss ratio (%) | Tmax (°C) | Fmax (N) | Fmax: peak expansion force | |||
| Electrical abuse (overcharge) | LCO | Charging Rate0.5C | 82.9 | 65.8 | 798.2 | 615 | ||
| Charging Rate1C | 84.8 | 67.3 | 774.6 | 609 | ||||
| Charging Rate3C | 86.6 | 68.8 | 917.2 | 521 | ||||
| NCM 523 | Charging Rate0.5C | 85.7 | 47.4 | 679.8 | 4039 | |||
| Charging Rate1C | 98.3 | 54.4 | 680.3 | 2931 | ||||
| Charging Rate3C | 103.7 | 57.4 | 683.2 | 2021 | ||||
| LFP | Charging Rate0.5C | 32.6 | 11.4 | 55.8 | 7385 | |||
| Charging Rate1C | 36.5 | 12.8 | 127.2 | 7330 | ||||
| Charging Rate3C | 39.1 | 13.7 | 252.7 | 4243 | ||||
| Shen [72] | T onset (°C) | T1: Peak temperature at the battery side surface during thermal runaway (°C) | T2: Maximum temperature recorded on the external battery surface during the thermal runaway event (°C) | T3: Maximum temperature observed on the battery heating surface throughout thermal runaway (°C) | T4: Peak average temperature attained during the thermal runaway process (°C) | adiabatic explosion chamber (AEC) T onset: TR onset temperature. | ||
| Thermal abuse | LFP | 184.0 | 170.9 | 306.6 | 559.2 | 302.1 | ||
| NCM 523 | 142.7 | 370.6 | 589.3 | 695.5 | 549.3 | |||
| NCM 622 | 140.8 | 555.9 | 504.8 | 600.6 | 597.1 | |||
| NCM 811 | 135.6 | 564.2 | 767.6 | 826.1 | 762.8 | |||
| NCM 9 0.5 0.5 | 130.6 | 843.5 | 903.7 | 943.9 | 842.1 | |||
| Jhu [13] | T0 (°C) | Tmax (°C) | (dT/dt) max (°C/min) | ∆H (KJ) | VSP2 | |||
| Thermal abuse | LCO | 131.5 | 708.8 | 54,090.7 | 18.9 | |||
| NCM 111 | 175.4 | 665.6 | 15,213.4 | 14.9 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Gao, Y.; Miao, Y.; Liang, Y.; Ren, X. Research Progress on the Influence of Cathode Materials on Thermal Runaway Behavior of Lithium-Ion Batteries. Batteries 2025, 11, 373. https://doi.org/10.3390/batteries11100373
Yang Y, Gao Y, Miao Y, Liang Y, Ren X. Research Progress on the Influence of Cathode Materials on Thermal Runaway Behavior of Lithium-Ion Batteries. Batteries. 2025; 11(10):373. https://doi.org/10.3390/batteries11100373
Chicago/Turabian StyleYang, Yanru, Yang Gao, Yu Miao, Yuan Liang, and Xiaoqiang Ren. 2025. "Research Progress on the Influence of Cathode Materials on Thermal Runaway Behavior of Lithium-Ion Batteries" Batteries 11, no. 10: 373. https://doi.org/10.3390/batteries11100373
APA StyleYang, Y., Gao, Y., Miao, Y., Liang, Y., & Ren, X. (2025). Research Progress on the Influence of Cathode Materials on Thermal Runaway Behavior of Lithium-Ion Batteries. Batteries, 11(10), 373. https://doi.org/10.3390/batteries11100373






