Swelling Mechanisms, Diagnostic Applications, and Mitigation Strategies in Lithium-Ion Batteries
Abstract
1. Introduction
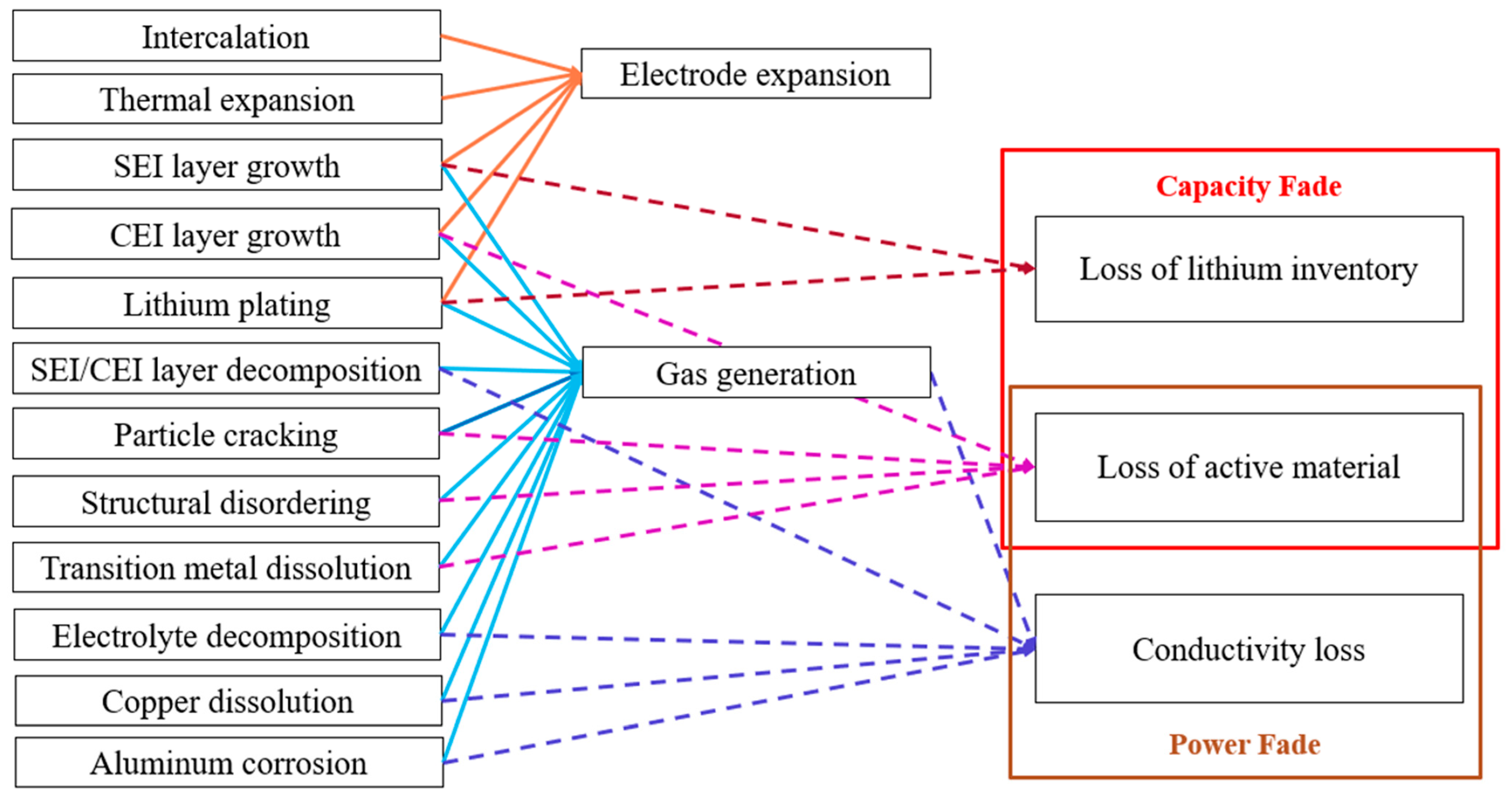
2. Causes of Battery Swelling
2.1. Electrode Expansion
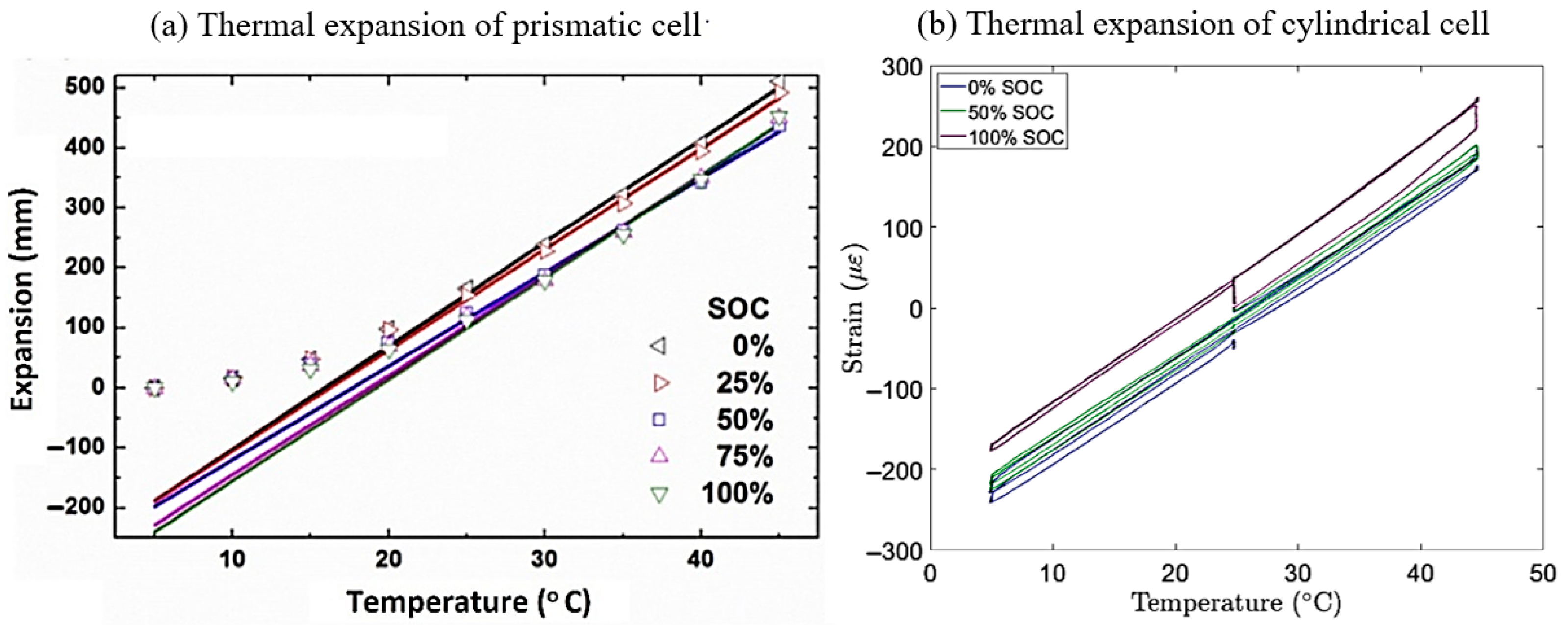
2.1.1. Thermal Expansion
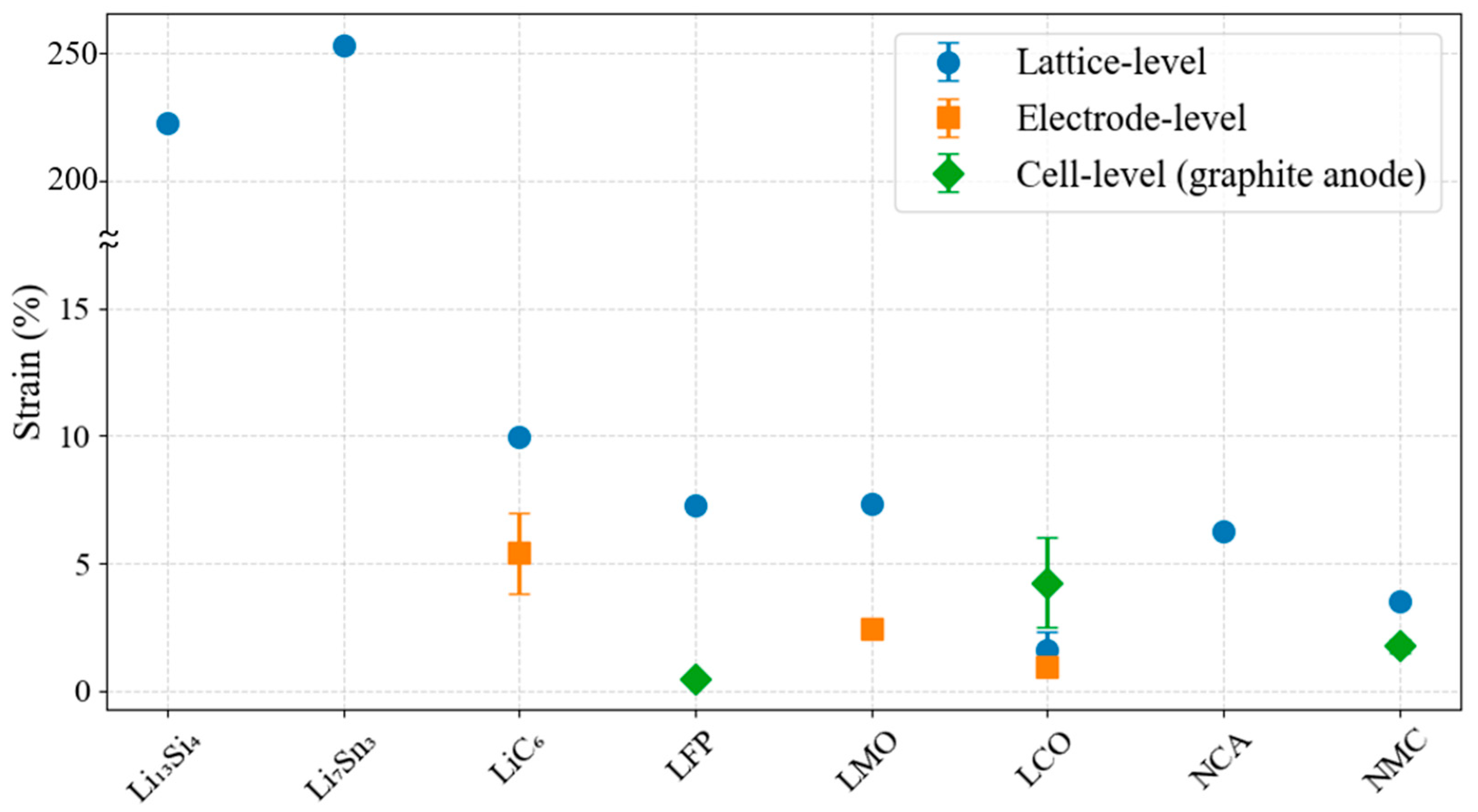
2.1.2. Intercalation-Induced Expansion
2.1.3. Electrode-Electrolyte Interphase Layer Formation and Growth
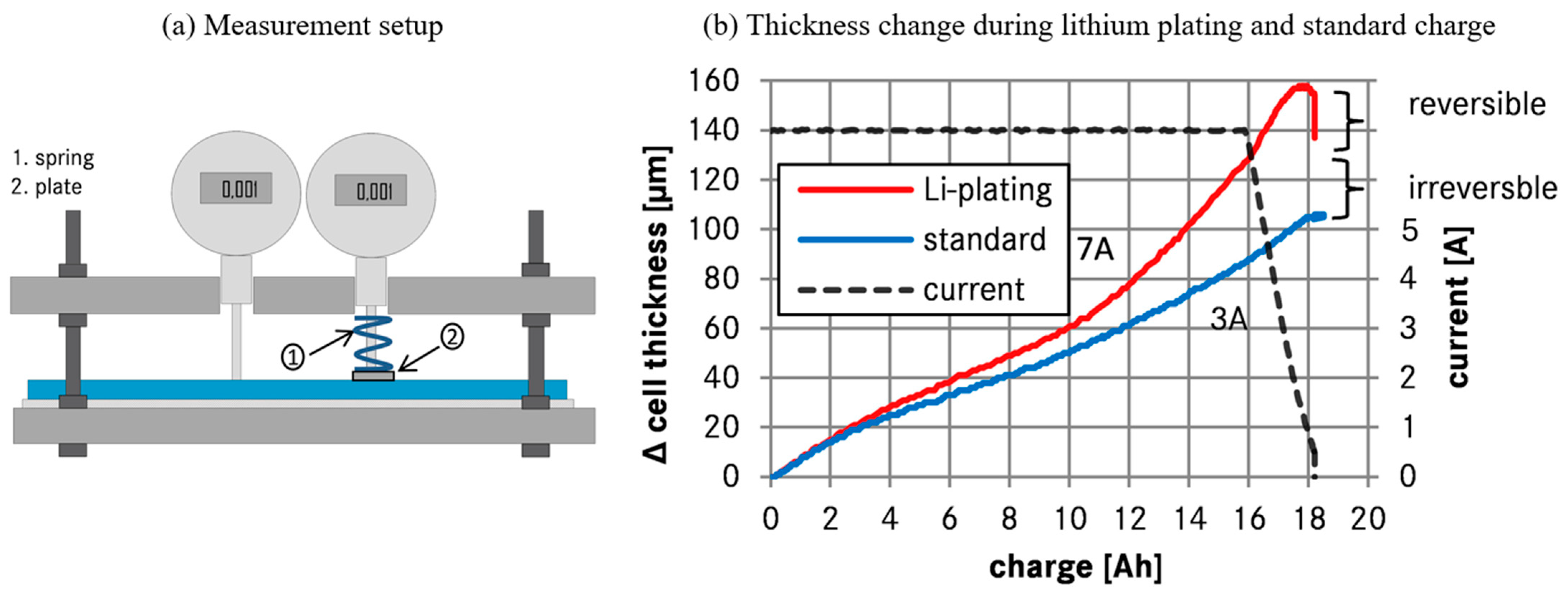
2.1.4. Lithium Plating
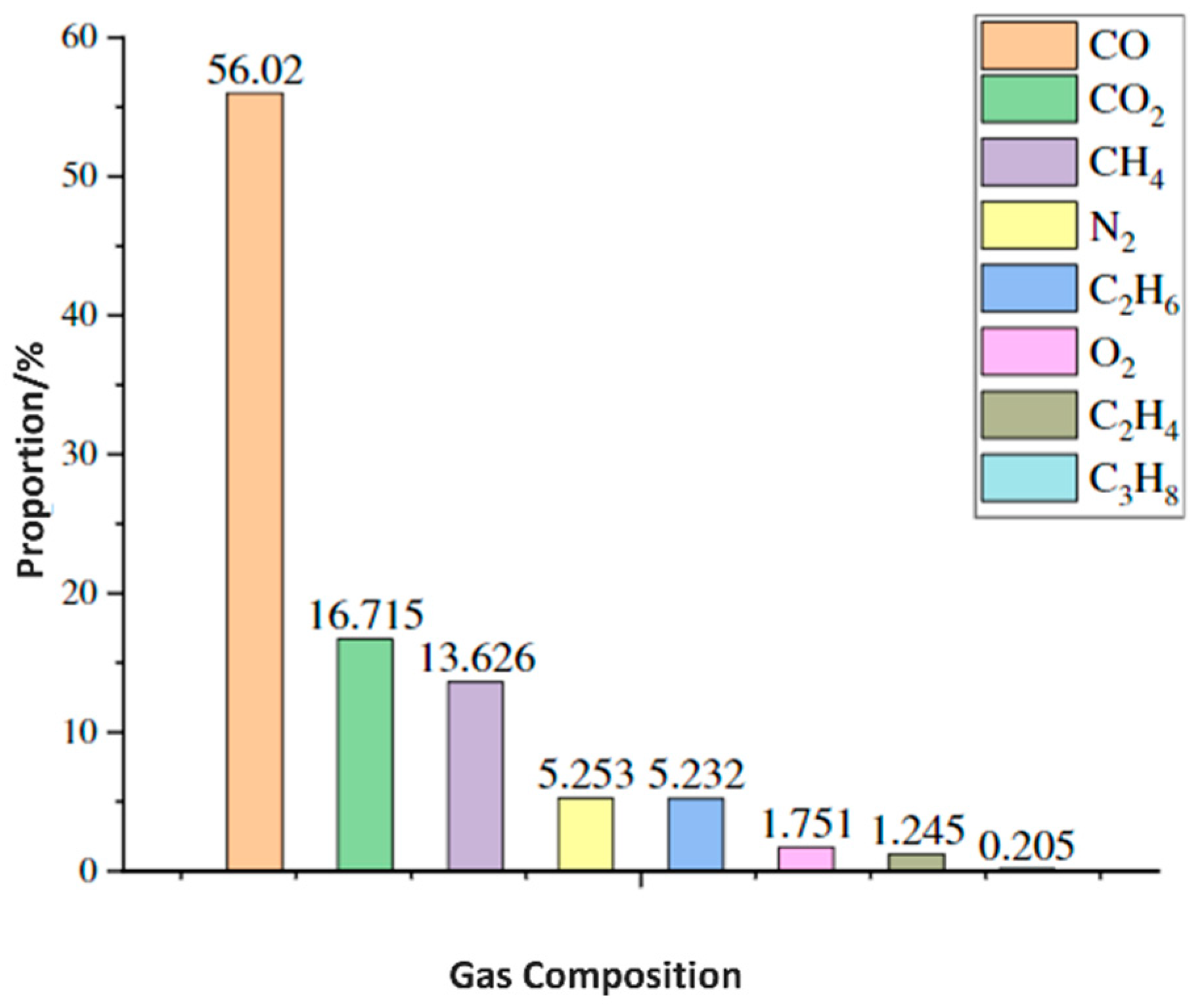
2.2. Gas Generation
2.2.1. Gases Generated at the Anode
2.2.2. Gases Generated at the Cathode
2.2.3. Electrolyte Decomposition
2.2.4. Crosstalk and Gas Consumption
3. Factors Affecting Swelling
3.1. Effect of C-Rate on Swelling
3.2. Effect of Temperature on Swelling
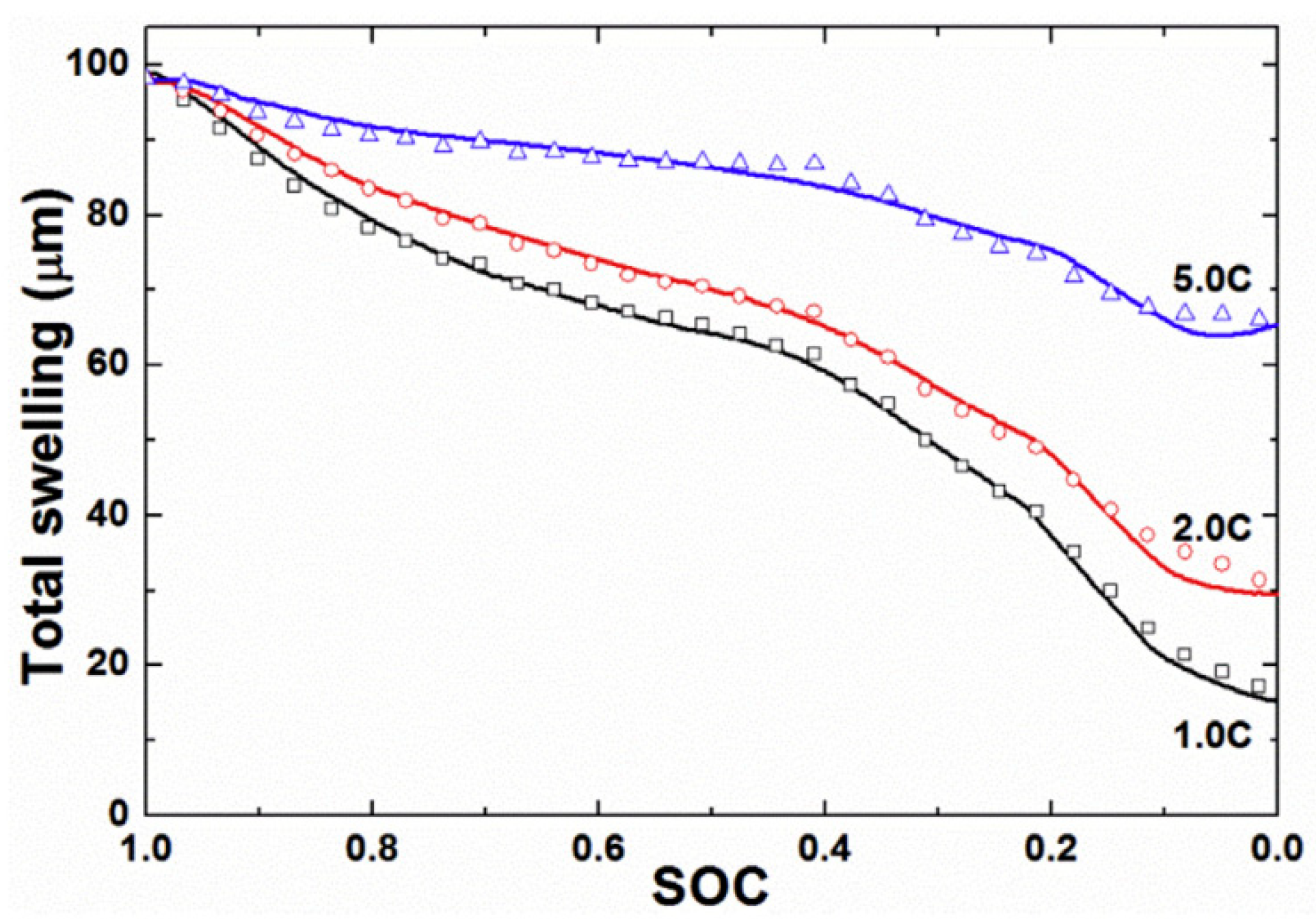
3.3. Effect of Voltage and SoC on Swelling
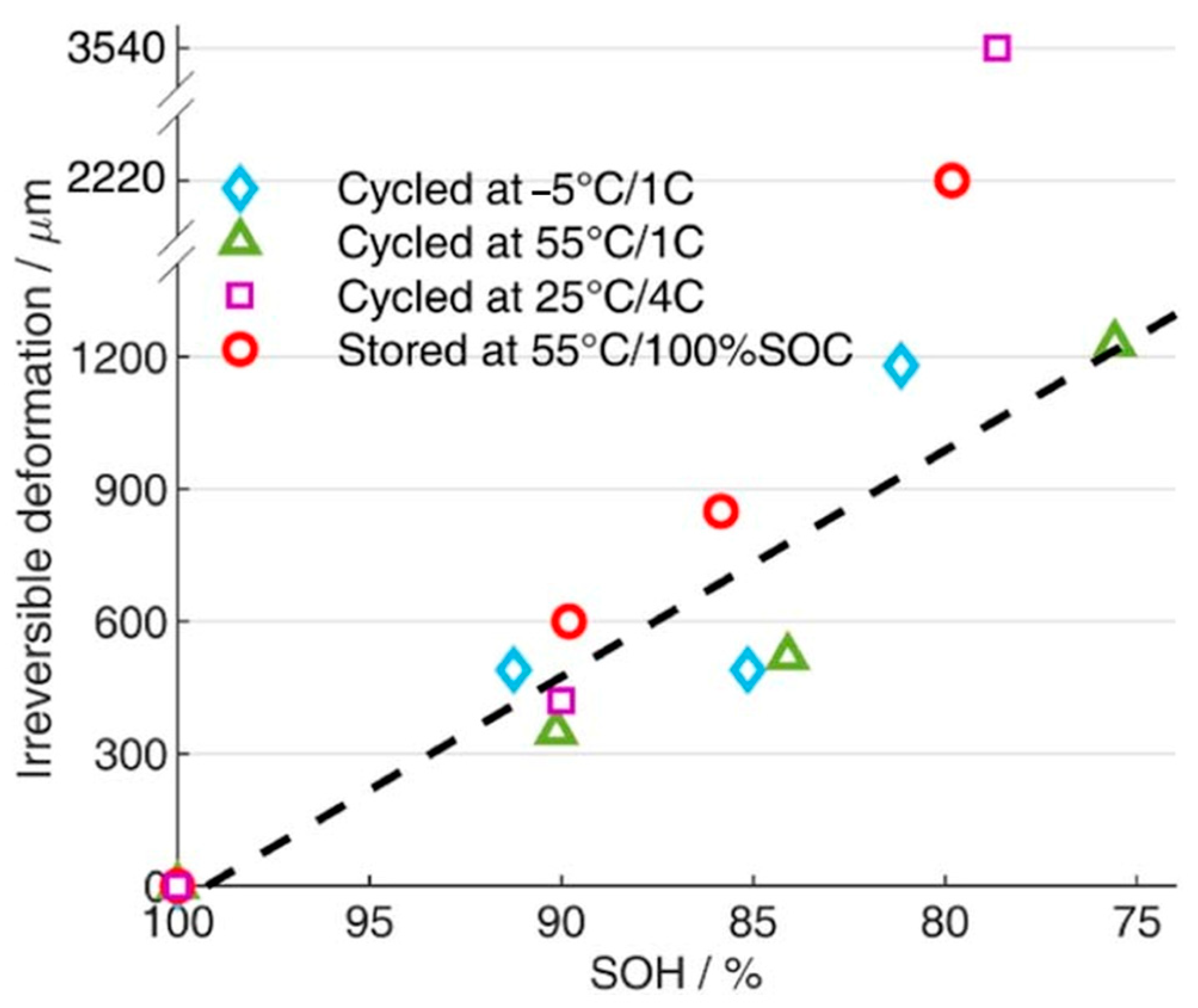
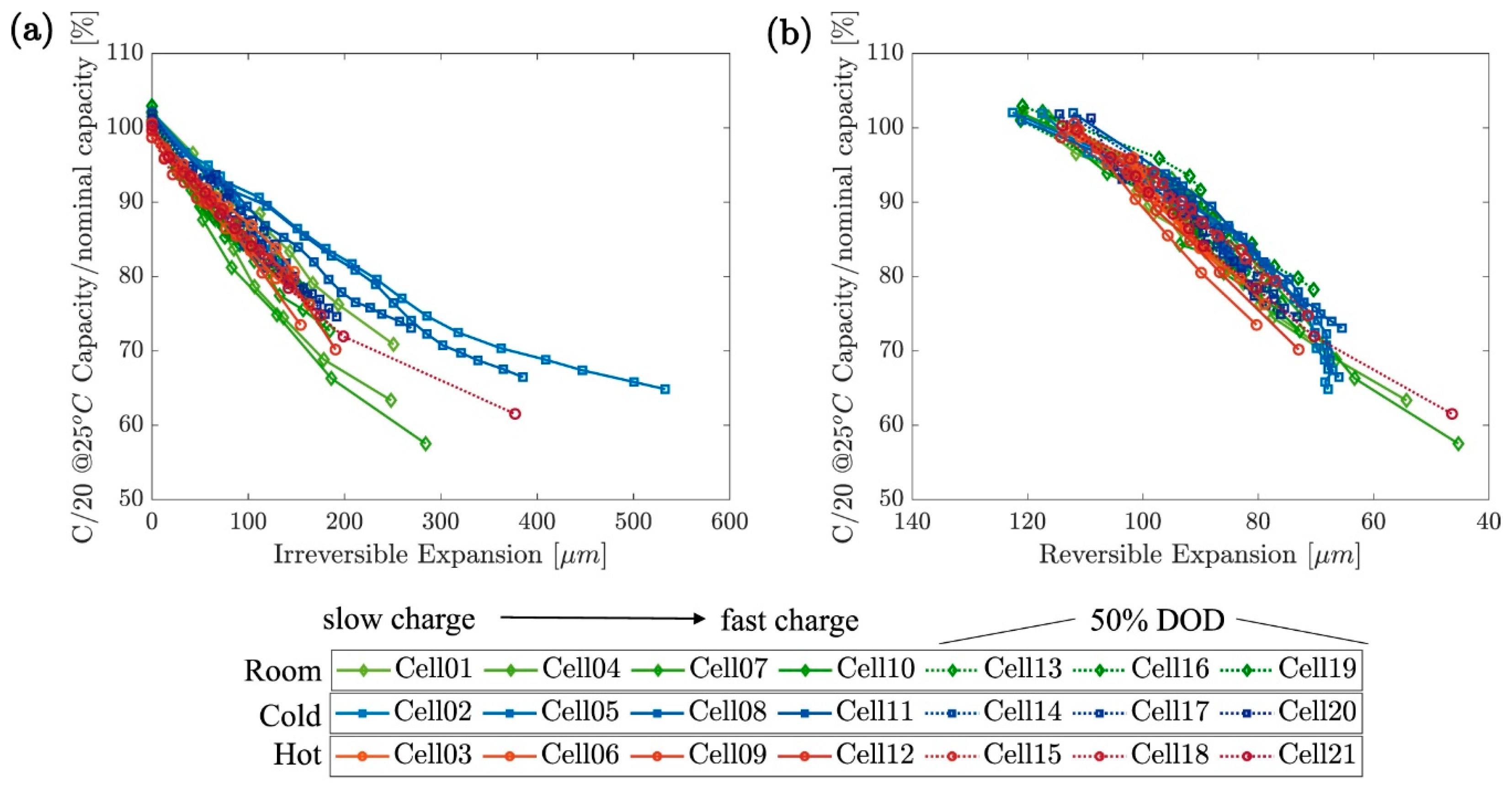
4. Relationship Between Swelling and Cell Performance
5. Swelling Models
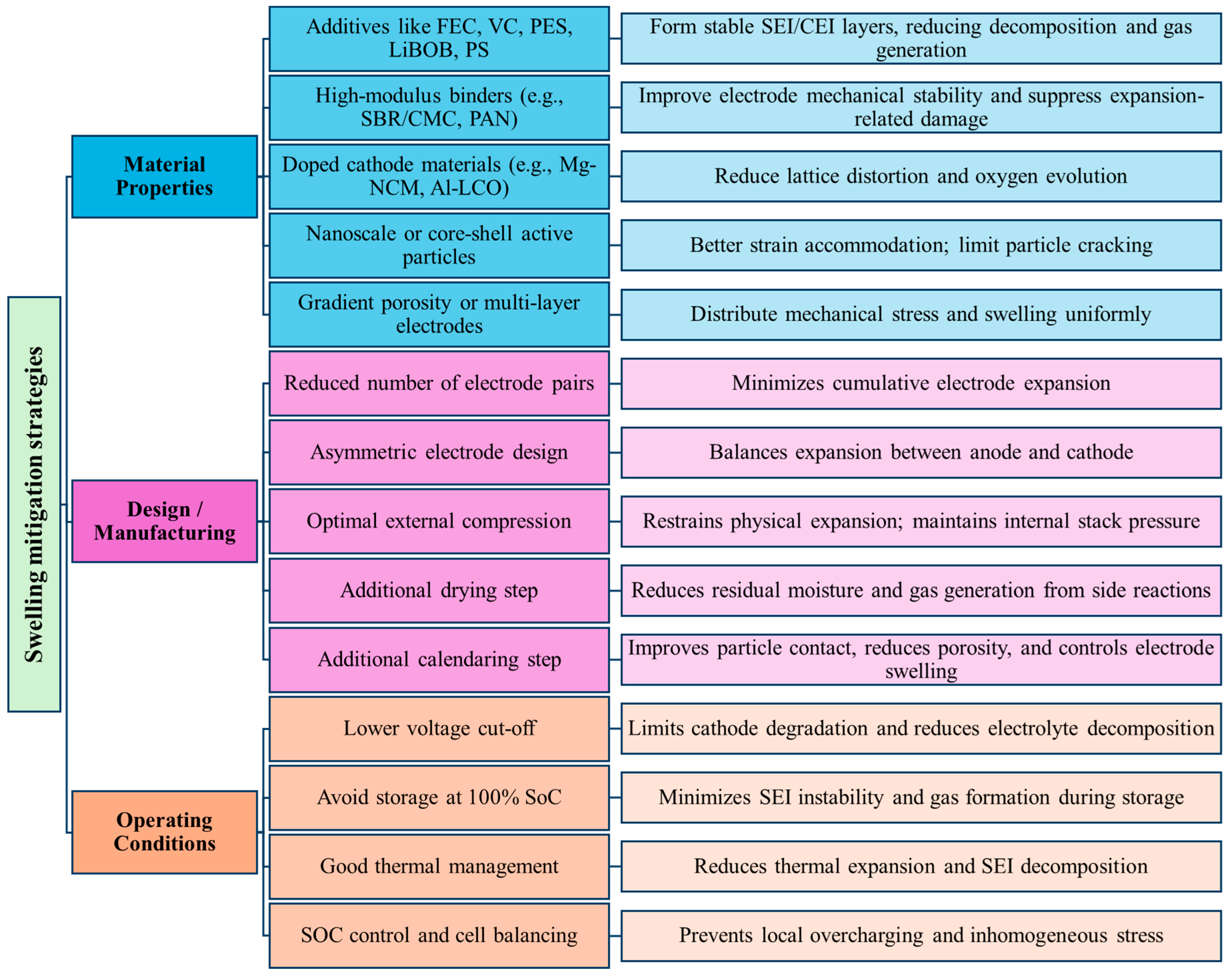
6. Methods to Mitigate Swelling
7. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LIB | Lithium-ion battery |
| SEI | Solid electrolyte interphase |
| CEI | Cathode electrolyte interphase |
| LMO | Lithium manganese oxide |
| LFP | Lithium iron phosphate |
| LCO | Lithium cobalt oxide |
| NMC | Lithium nickel manganese cobalt oxide |
| NCA | Lithium nickel cobalt aluminum oxide |
| CTE | Coefficient of thermal expansion |
| SoC | State of charge |
| SoH | State of health |
| PE | Polyethylene |
| PP | Polypropylene |
| XRD | X-ray diffraction |
| LTO | Lithium titanate |
| FBG | Fiber Bragg grating |
| LiBOB | Lithium bis-(oxalate)borate |
| EIS | Electrochemical impedance spectroscopy |
| LEDC | Lithium ethylene dicarbonate |
| P2D | Pseudo-two-dimensional |
| C@SGG | Coated silicon/graphite granules |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PCMs | Phase change materials |
| BMS | Battery management system |
References
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing Mechanisms in Lithium-Ion Batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Lee, H.; Lim, H.-S.; Ren, X.; Yu, L.; Engelhard, M.H.; Han, K.S.; Lee, J.; Kim, H.-T.; Xiao, J.; Liu, J.; et al. Detrimental Effects of Chemical Crossover from the Lithium Anode to Cathode in Rechargeable Lithium Metal Batteries. ACS Energy Lett. 2018, 3, 2921–2930. [Google Scholar] [CrossRef]
- Guo, J.; Li, P.; Piccolo, F.; Jie, Y.; Jia, H.; Cao, R.; Jiao, S.; Adelhelm, P.; Xu, Y. Degradation and Failure Mechanisms of Lithium/LiNixCoyMn1−x−yO2 Batteries. ACS Energy Lett. 2025, 10, 2318–2340. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Mechanism of Gases Generation during Lithium-Ion Batteries Cycling. J. Electrochem. Soc. 2019, 166, A897–A908. [Google Scholar] [CrossRef]
- Bitzer, B.; Gruhle, A. A New Method for Detecting Lithium Plating by Measuring the Cell Thickness. J. Power Sources 2014, 262, 297–302. [Google Scholar] [CrossRef]
- Grimsmann, F.; Brauchle, F.; Gerbert, T.; Gruhle, A.; Parisi, J.; Knipper, M. Impact of Different Aging Mechanisms on the Thickness Change and the Quick-Charge Capability of Lithium-Ion Cells. J. Energy Storage 2017, 14, 158–162. [Google Scholar] [CrossRef]
- Li, X.; Zeng, T.; Qin, H.; Huo, R.; Liu, Y.; Wei, D.; Ding, X. Investigation of Inhomogeneous Degradation in Large-Format Lithium-Ion Batteries. J. Energy Storage 2021, 42, 103113. [Google Scholar] [CrossRef]
- Mohtat, P.; Lee, S.; Siegel, J.B.; Stefanopoulou, A.G. Reversible and Irreversible Expansion of Lithium-Ion Batteries Under a Wide Range of Stress Factors. J. Electrochem. Soc. 2021, 168, 100520. [Google Scholar] [CrossRef]
- Li, Y.; Wei, C.; Sheng, Y.; Jiao, F.; Wu, K. Swelling Force in Lithium-Ion Power Batteries. Ind. Eng. Chem. Res. 2020, 59, 12313–12318. [Google Scholar] [CrossRef]
- Heugel, P.; Märkle, W.; Deich, T.; Von Kessel, O.; Tübke, J. Thickness Change and Jelly Roll Deformation and Its Impact on the Aging and Lifetime of Commercial 18650 Cylindrical Li-Ion Cells with Silicon Containing Anodes and Nickel-Rich Cathodes. J. Energy Storage 2022, 53, 105101. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. Stress Evolution and Capacity Fade in Constrained Lithium-Ion Pouch Cells. J. Power Sources 2014, 245, 745–751. [Google Scholar] [CrossRef]
- Oh, K.-Y.; Epureanu, B.I. Characterization and Modeling of the Thermal Mechanics of Lithium-Ion Battery Cells. Appl. Energy 2016, 178, 633–646. [Google Scholar] [CrossRef]
- Ellis, L.D.; Allen, J.P.; Thompson, L.M.; Harlow, J.E.; Stone, W.J.; Hill, I.G.; Dahn, J.R. Quantifying, Understanding and Evaluating the Effects of Gas Consumption in Lithium-Ion Cells. J. Electrochem. Soc. 2017, 164, A3518–A3528. [Google Scholar] [CrossRef]
- Aalund, R.; Endreddy, B.; Pecht, M. How Gas Generates in Pouch Cells and Affects Consumer Products. Front. Chem. Eng. 2022, 4, 828375. [Google Scholar] [CrossRef]
- Apple Now Offering Free Repairs of 42 mm Apple Watch Series 2 Models with Swollen Batteries. Available online: https://www.macrumors.com/2018/04/14/apple-watch-s2-swollen-battery-service-policy/ (accessed on 6 April 2025).
- fergie434. Macbook Battery Swelling Up. r/techsupportgore. 2013. Available online: https://www.reddit.com/r/techsupportgore/comments/1on9h2/macbook_battery_swelling_up/ (accessed on 6 April 2025).
- Ivan Apple Looking into iPhone 8 and 8 Plus Swelling Batteries Reports. Available online: https://www.gsmarena.com/does_the_iphone_8_and_8_plus_have_a_swelling_battery-news-27594.php (accessed on 6 April 2025).
- Krause, T.; Nusko, D.; Pitta Bauermann, L.; Vetter, M.; Schäfer, M.; Holly, C. Methods for Quantifying Expansion in Lithium-Ion Battery Cells Resulting from Cycling: A Review. Energies 2024, 17, 1566. [Google Scholar] [CrossRef]
- Maddipatla, S.; Kong, L.; Pecht, M. Safety Analysis of Lithium-Ion Cylindrical Batteries Using Design and Process Failure Mode and Effect Analysis. Batteries 2024, 10, 76. [Google Scholar] [CrossRef]
- Li, J.; Fleetwood, J.; Hawley, W.B.; Kays, W. From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing. Chem. Rev. 2022, 122, 903–956. [Google Scholar] [CrossRef]
- Wang, J.; Hao, F. Experimental Investigations on the Chemo-Mechanical Coupling in Solid-State Batteries and Electrode Materials. Energies 2023, 16, 1180. [Google Scholar] [CrossRef]
- Rieger, B.; Schlueter, S.; Erhard, S.V.; Schmalz, J.; Reinhart, G.; Jossen, A. Multi-Scale Investigation of Thickness Changes in a Commercial Pouch Type Lithium-Ion Battery. J. Energy Storage 2016, 6, 213–221. [Google Scholar] [CrossRef]
- Oh, K.-Y.; Siegel, J.B.; Secondo, L.; Kim, S.U.; Samad, N.A.; Qin, J.; Anderson, D.; Garikipati, K.; Knobloch, A.; Epureanu, B.I.; et al. Rate Dependence of Swelling in Lithium-Ion Cells. J. Power Sources 2014, 267, 197–202. [Google Scholar] [CrossRef]
- Shen, K.; Cao, X.; Huang, Z.-H.; Shen, W.; Kang, F. Microstructure and Thermal Expansion Behavior of Natural Microcrystalline Graphite. Carbon 2021, 177, 90–96. [Google Scholar] [CrossRef]
- Shi, D.; Xiao, X.; Huang, X.; Kia, H. Modeling Stresses in the Separator of a Pouch Lithium-Ion Cell. J. Power Sources 2011, 196, 8129–8139. [Google Scholar] [CrossRef]
- Valentin, O.; Thivel, P.-X.; Kareemulla, T.; Cadiou, F.; Bultel, Y. Modeling of Thermo-Mechanical Stresses in Li-Ion Battery. J. Energy Storage 2017, 13, 184–192. [Google Scholar] [CrossRef]
- Qu, M.; Woodford, W.H.; Maloney, J.M.; Carter, W.C.; Chiang, Y.; Van Vliet, K.J. Nanomechanical Quantification of Elastic, Plastic, and Fracture Properties of LiCoO2. Adv. Energy Mater. 2012, 2, 940–944. [Google Scholar] [CrossRef]
- Cheng, E.J.; Hong, K.; Taylor, N.J.; Choe, H.; Wolfenstine, J.; Sakamoto, J. Mechanical and Physical Properties of LiNi0.33Mn0.33Co0.33O2 (NMC). J. Eur. Ceram. Soc. 2017, 37, 3213–3217. [Google Scholar] [CrossRef]
- Shackelford, J.F.; Alexander, W. (Eds.) CRC Materials Science and Engineering Handbook; CRC Press: Boca Raton, FL, USA, 2000; ISBN 978-0-429-11786-2. [Google Scholar]
- Yan, S.; Deng, J.; Bae, C.; Xiao, X. Thermal Expansion/Shrinkage Measurement of Battery Separators Using a Dynamic Mechanical Analyzer. Polym. Test. 2018, 71, 65–71. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.; Huang, X.; Yan, S. A Multiphysics Model for the in Situ Stress Analysis of the Separator in a Lithium-Ion Battery Cell. Comput. Mater. Sci. 2014, 83, 127–136. [Google Scholar] [CrossRef]
- Abdullah, M.A.; Albarody, T.M.B.; Hussein, A.R. Graphite Thermal Expansion Coefficient Measured by in-Situ x-Ray Diffraction. Nanotechnology 2020, 31, 285709. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature Effect and Thermal Impact in Lithium-Ion Batteries: A Review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Oh, K.-Y.; Epureanu, B.I. A Novel Thermal Swelling Model for a Rechargeable Lithium-Ion Battery Cell. J. Power Sources 2016, 303, 86–96. [Google Scholar] [CrossRef]
- Bezsonov, I.I.; Waller, G.H.; Ko, J.; Nadimpalli, S.P.V. In Operando Measurement of Surface Strain of 18650 Li-Ion Cells during Cycling. J. Power Sources 2024, 592, 233915. [Google Scholar] [CrossRef]
- Liu, C.; Neale, Z.G.; Cao, G. Understanding Electrochemical Potentials of Cathode Materials in Rechargeable Batteries. Mater. Today 2016, 19, 109–123. [Google Scholar] [CrossRef]
- Yoon, W.-S.; Chung, K.Y.; McBreen, J.; Yang, X.-Q. A Comparative Study on Structural Changes of LiCo1/3Ni1/3Mn1/3O2 and LiNi0.8Co0.15Al0.05O2 during First Charge Using in Situ XRD. Electrochem. Commun. 2006, 8, 1257–1262. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Sheldon, B.W. Deformation and Stress in Electrode Materials for Li-Ion Batteries. Prog. Mater. Sci. 2014, 63, 58–116. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, H.; Hector, L.G.; Timmons, A. Threefold Increase in the Young’s Modulus of Graphite Negative Electrode during Lithium Intercalation. J. Electrochem. Soc. 2010, 157, A558. [Google Scholar] [CrossRef]
- Winter, M.; Wrodnigg, G.H.; Besenhard, J.O.; Biberacher, W.; Novák, P. Dilatometric Investigations of Graphite Electrodes in Nonaqueous Lithium Battery Electrolytes. J. Electrochem. Soc. 2000, 147, 2427. [Google Scholar] [CrossRef]
- Massoudi, A.; Nangir, M.; Moghadami, M. Characterization of Battery Materials by Mechanical Measurements. In Nanostructured Materials Engineering and Characterization for Battery Applications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 373–437. ISBN 978-0-323-91304-1. [Google Scholar]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The Success Story of Graphite as a Lithium-Ion Anode Material–Fundamentals, Remaining Challenges, and Recent Developments Including Silicon (Oxide) Composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as Anode Materials: Fundamental Mechanism, Recent Progress and Advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Hahn, M.; Buqa, H.; Ruch, P.W.; Goers, D.; Spahr, M.E.; Ufheil, J.; Novák, P.; Kötz, R. A Dilatometric Study of Lithium Intercalation into Powder-Type Graphite Electrodes. Electrochem. Solid-State Lett. 2008, 11, A151. [Google Scholar] [CrossRef]
- Liu, X.H.; Zheng, H.; Zhong, L.; Huang, S.; Karki, K.; Zhang, L.Q.; Liu, Y.; Kushima, A.; Liang, W.T.; Wang, J.W.; et al. Anisotropic Swelling and Fracture of Silicon Nanowires during Lithiation. Nano Lett. 2011, 11, 3312–3318. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Li, Y.; Chen, H.; Xie, L.; Liu, T.; Yang, N.; Song, Y.; Lin, C.; Cheng, J.; Nakashima, N.; et al. Revealing the Aging Process of Solid Electrolyte Interphase on SiOx Anode. Nat. Commun. 2023, 14, 6048. [Google Scholar] [CrossRef]
- Zhao, P.-Y.; Zhang, S.; Choy, K.-L.; Song, Y.; Zhang, S.; Guo, D.; Yang, C. Silicon Negative Electrodes for Lithium-Ion Batteries: Challenges, Advances, and Future Prospects. Mater. Horiz. 2025, 12, 6440–6484. [Google Scholar] [CrossRef]
- Cheng, H.; Shapter, J.G.; Li, Y.; Gao, G. Recent Progress of Advanced Anode Materials of Lithium-Ion Batteries. J. Energy Chem. 2021, 57, 451–468. [Google Scholar] [CrossRef]
- Sandhya, C.P.; John, B.; Gouri, C. Lithium Titanate as Anode Material for Lithium-Ion Cells: A Review. Ionics 2014, 20, 601–620. [Google Scholar] [CrossRef]
- Julien, C.; Mauger, A.; Zaghib, K.; Groult, H. Comparative Issues of Cathode Materials for Li-Ion Batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, K.; Zhang, J.; Sun, B.; Wang, G. Reaction Mechanisms of Layered Lithium-Rich Cathode Materials for High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 2208–2220. [Google Scholar] [CrossRef]
- He, P.; Yu, H.; Li, D.; Zhou, H. Layered Lithium Transition Metal Oxide Cathodes towards High Energy Lithium-Ion Batteries. J. Mater. Chem. 2012, 22, 3680. [Google Scholar] [CrossRef]
- Rieger, B.; Schlueter, S.; Erhard, S.V.; Jossen, A. Strain Propagation in Lithium-Ion Batteries from the Crystal Structure to the Electrode Level. J. Electrochem. Soc. 2016, 163, A1595–A1606. [Google Scholar] [CrossRef]
- Reimers, J.N.; Dahn, J.R. Electrochemical and In Situ X-Ray Diffraction Studies of Lithium Intercalation in LixCoO2. J. Electrochem. Soc. 1992, 139, 2091–2097. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Zhou, J.; Li, X.; Zhou, W.; Xie, Y.; Mao, J.; Dai, K. Research Development on Spinel Lithium Manganese Oxides Cathode Materials for Lithium-Ion Batteries. J. Electrochem. Soc. 2023, 170, 090532. [Google Scholar] [CrossRef]
- Arabolla Rodríguez, R.; González Montiel, M.; Della Santina Mohallem, N.; Mosqueda Laffita, Y.; Andrey Montoro, L.; Avila Santos, M.; León Ramírez, H.; Pérez-Cappe, E.L. The Role of Defects on the Jahn-Teller Effect and Electrochemical Charge Storage in Nanometric LiMn2O4 Material. Solid State Ion. 2021, 369, 115707. [Google Scholar] [CrossRef]
- Stallard, J.C.; Wheatcroft, L.; Booth, S.G.; Boston, R.; Corr, S.A.; De Volder, M.F.L.; Inkson, B.J.; Fleck, N.A. Mechanical Properties of Cathode Materials for Lithium-Ion Batteries. Joule 2022, 6, 984–1007. [Google Scholar] [CrossRef]
- Wang, X.; Sone, Y.; Kuwajima, S. In Situ Investigation of the Volume Change in Li-Ion Cell with Charging and Discharging. J. Electrochem. Soc. 2004, 151, A273. [Google Scholar] [CrossRef]
- Sauerteig, D.; Hanselmann, N.; Arzberger, A.; Reinshagen, H.; Ivanov, S.; Bund, A. Electrochemical-Mechanical Coupled Modeling and Parameterization of Swelling and Ionic Transport in Lithium-Ion Batteries. J. Power Sources 2018, 378, 235–247. [Google Scholar] [CrossRef]
- Wünsch, M.; Kaufman, J.; Sauer, D.U. Investigation of the Influence of Different Bracing of Automotive Pouch Cells on Cyclic Liefetime and Impedance Spectra. J. Energy Storage 2019, 21, 149–155. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, Y.; Hu, Y.; Choe, S.-Y. Electrochemical-Thermal Modeling of Lithium Plating/Stripping of Li(Ni0.6Mn0.2Co0.2)O2/Carbon Lithium-Ion Batteries at Subzero Ambient Temperatures. J. Power Sources 2019, 418, 61–73. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.M.; Ahn, S. Battery Dimensional Changes Occurring during Charge/Discharge Cycles—Thin Rectangular Lithium Ion and Polymer Cells. J. Power Sources 2003, 119–121, 833–837. [Google Scholar] [CrossRef]
- Siegel, J.B.; Stefanopoulou, A.G.; Hagans, P.; Ding, Y.; Gorsich, D. Expansion of Lithium Ion Pouch Cell Batteries: Observations from Neutron Imaging. J. Electrochem. Soc. 2013, 160, A1031–A1038. [Google Scholar] [CrossRef]
- Sauerteig, D.; Ivanov, S.; Reinshagen, H.; Bund, A. Reversible and Irreversible Dilation of Lithium-Ion Battery Electrodes Investigated by in-Situ Dilatometry. J. Power Sources 2017, 342, 939–946. [Google Scholar] [CrossRef]
- Zhao, Y.; Spingler, F.B.; Patel, Y.; Offer, G.J.; Jossen, A. Localized Swelling Inhomogeneity Detection in Lithium Ion Cells Using Multi-Dimensional Laser Scanning. J. Electrochem. Soc. 2019, 166, A27–A34. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on Modeling of the Anode Solid Electrolyte Interphase (SEI) for Lithium-Ion Batteries. npj Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Bernhard, R.; Metzger, M.; Gasteiger, H.A. Gas Evolution at Graphite Anodes Depending on Electrolyte Water Content and SEI Quality Studied by On-Line Electrochemical Mass Spectrometry. J. Electrochem. Soc. 2015, 162, A1984–A1989. [Google Scholar] [CrossRef]
- Peled, E.; Patolsky, F.; Golodnitsky, D.; Freedman, K.; Davidi, G.; Schneier, D. Tissue-like Silicon Nanowires-Based Three-Dimensional Anodes for High-Capacity Lithium Ion Batteries. Nano Lett. 2015, 15, 3907–3916. [Google Scholar] [CrossRef] [PubMed]
- Zhen, H.; Meng, F.; Gao, J.; Liu, Y.; Liu, X. Asymmetric Swelling Behaviors of High-Energy-Density Lithium-Ion Batteries with a SiOx/Graphite Composite Anode. Small 2023, 19, 2300500. [Google Scholar] [CrossRef]
- Dopilka, A.; Larson, J.M.; Kostecki, R. Operando Infrared Nanospectroscopy of the Silicon/Electrolyte Interface during Initial Stages of Solid-Electrolyte-Interphase Layer Formation. ACS Energy Lett. 2025, 10, 410–419. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Xu, R.; Zhou, W.; Li, Y.; Oyakhire, S.T.; Wu, Y.; Xu, J.; Wang, H.; Yu, Z.; et al. Capturing the Swelling of Solid-Electrolyte Interphase in Lithium Metal Batteries. Science 2022, 375, 66–70. [Google Scholar] [CrossRef]
- Kim, K.; Ma, H.; Park, S.; Choi, N.-S. Electrolyte-Additive-Driven Interfacial Engineering for High-Capacity Electrodes in Lithium-Ion Batteries: Promise and Challenges. ACS Energy Lett. 2020, 5, 1537–1553. [Google Scholar] [CrossRef]
- Jaumann, T.; Balach, J.; Langklotz, U.; Sauchuk, V.; Fritsch, M.; Michaelis, A.; Teltevskij, V.; Mikhailova, D.; Oswald, S.; Klose, M.; et al. Lifetime vs. Rate Capability: Understanding the Role of FEC and VC in High-Energy Li-Ion Batteries with Nano-Silicon Anodes. Energy Storage Mater. 2017, 6, 26–35. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Haering, D.; Solchenbach, S.; Marino, C.; Tsiouvaras, N.; Stinner, C.; Gasteiger, H.A. Consumption of Fluoroethylene Carbonate (FEC) on Si-C Composite Electrodes for Li-Ion Batteries. J. Electrochem. Soc. 2016, 163, A1705–A1716. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Alpas, A.T. Micromechanisms of Solid Electrolyte Interphase Formation on Electrochemically Cycled Graphite Electrodes in Lithium-Ion Cells. Carbon 2012, 50, 5359–5371. [Google Scholar] [CrossRef]
- Agubra, V.A.; Fergus, J.W. The Formation and Stability of the Solid Electrolyte Interface on the Graphite Anode. J. Power Sources 2014, 268, 153–162. [Google Scholar] [CrossRef]
- Wood, D.L.; Li, J.; Daniel, C. Prospects for Reducing the Processing Cost of Lithium Ion Batteries. J. Power Sources 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Ota, M.; Izuo, S.; Nishikawa, K.; Fukunaka, Y.; Kusaka, E.; Ishii, R.; Selman, J.R. Measurement of Concentration Boundary Layer Thickness Development during Lithium Electrodeposition onto a Lithium Metal Cathode in Propylene Carbonate. J. Electroanal. Chem. 2003, 559, 175–183. [Google Scholar] [CrossRef]
- Jha, V.; Krishnamurthy, B. Modeling the SEI Layer Formation and Its Growth in Lithium-Ion Batteries (LiB) during Charge–Discharge Cycling. Ionics 2022, 28, 3661–3670. [Google Scholar] [CrossRef]
- Xu, J. Critical Review on Cathode–Electrolyte Interphase Toward High-Voltage Cathodes for Li-Ion Batteries. Nano-Micro Lett. 2022, 14, 166. [Google Scholar] [CrossRef]
- .Zhang, N.; Wang, B.; Jin, F.; Chen, Y.; Jiang, Y.; Bao, C.; Tian, J.; Wang, J.; Xu, R.; Li, Y.; et al. Modified Cathode-Electrolyte Interphase toward High-Performance Batteries. Cell Rep. Phys. Sci. 2022, 3, 101197. [Google Scholar] [CrossRef]
- Bonefacino, J.; Ghashghaie, S.; Zheng, T.; Lin, C.-P.; Zheng, W.; Blanquer, L.A.; Huang, J.; Gervillié, C.; Tam, H.-Y.; Tarascon, J.-M.; et al. High-Fidelity Strain and Temperature Measurements of Li-Ion Batteries Using Polymer Optical Fiber Sensors. J. Electrochem. Soc. 2022, 169, 100508. [Google Scholar] [CrossRef]
- Heidrich, B.; Pritzlaff, L.; Börner, M.; Winter, M.; Niehoff, P. Comparative X-Ray Photoelectron Spectroscopy Study of the SEI and CEI in Three Different Lithium Ion Cell Formats. J. Electrochem. Soc. 2022, 169, 030533. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Li Plating as Unwanted Side Reaction in Commercial Li-Ion Cells—A Review. J. Power Sources 2018, 384, 107–124. [Google Scholar] [CrossRef]
- Legrand, N.; Knosp, B.; Desprez, P.; Lapicque, F.; Raël, S. Physical Characterization of the Charging Process of a Li-Ion Battery and Prediction of Li Plating by Electrochemical Modelling. J. Power Sources 2014, 245, 208–216. [Google Scholar] [CrossRef]
- Arora, P.; Doyle, M.; White, R.E. Mathematical Modeling of the Lithium Deposition Overcharge Reaction in Lithium-Ion Batteries Using Carbon-Based Negative Electrodes. J. Electrochem. Soc. 1999, 146, 3543–3553. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, Q. Towards the Intercalation and Lithium Plating Mechanism for High Safety and Fast-Charging Lithium-Ion Batteries: A Review. Energy Lab 2022, 1, 220011. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, L.; He, C. Long Cycle Life Lithium Ion Battery with Lithium Nickel Cobalt Manganese Oxide (NCM) Cathode. J. Power Sources 2014, 261, 285–291. [Google Scholar] [CrossRef]
- Ebner, M.; Wood, V. Tool for Tortuosity Estimation in Lithium Ion Battery Porous Electrodes. J. Electrochem. Soc. 2015, 162, A3064–A3070. [Google Scholar] [CrossRef]
- Kehrwald, D.; Shearing, P.R.; Brandon, N.P.; Sinha, P.K.; Harris, S.J. Local Tortuosity Inhomogeneities in a Lithium Battery Composite Electrode. J. Electrochem. Soc. 2011, 158, A1393. [Google Scholar] [CrossRef]
- Daubinger, P.; Schelter, M.; Petersohn, R.; Nagler, F.; Hartmann, S.; Herrmann, M.; Giffin, G.A. Impact of Bracing on Large Format Prismatic Lithium-Ion Battery Cells during Aging. Adv. Energy Mater. 2022, 12, 2102448. [Google Scholar] [CrossRef]
- Zhang, S.S. Uncovering the Root Leading to Accelerated Capacity Fade of Li-Ion Coin Cells in Fast Charging. Energy Technol. 2023, 11, 2200370. [Google Scholar] [CrossRef]
- Cao, C.; Steinrück, H.-G.; Paul, P.P.; Dunlop, A.R.; Trask, S.E.; Jansen, A.N.; Kasse, R.M.; Thampy, V.; Yusuf, M.; Weker, J.N.; et al. Conformal Pressure and Fast-Charging Li-Ion Batteries. J. Electrochem. Soc. 2022, 169, 040540. [Google Scholar] [CrossRef]
- Bauer, M.; Wachtler, M.; Stöwe, H.; Persson, J.V.; Danzer, M.A. Understanding the Dilation and Dilation Relaxation Behavior of Graphite-Based Lithium-Ion Cells. J. Power Sources 2016, 317, 93–102. [Google Scholar] [CrossRef]
- Rieger, B.; Schuster, S.F.; Erhard, S.V.; Osswald, P.J.; Rheinfeld, A.; Willmann, C.; Jossen, A. Multi-Directional Laser Scanning as Innovative Method to Detect Local Cell Damage during Fast Charging of Lithium-Ion Cells. J. Energy Storage 2016, 8, 1–5. [Google Scholar] [CrossRef]
- Grimsmann, F.; Gerbert, T.; Brauchle, F.; Gruhle, A.; Parisi, J.; Knipper, M. Determining the Maximum Charging Currents of Lithium-Ion Cells for Small Charge Quantities. J. Power Sources 2017, 365, 12–16. [Google Scholar] [CrossRef]
- Gao, X.-L.; Liu, X.-H.; Xie, W.-L.; Zhang, L.-S.; Yang, S.-C. Multiscale Observation of Li Plating for Lithium-Ion Batteries. Rare Met. 2021, 40, 3038–3048. [Google Scholar] [CrossRef]
- Tanim, T.R.; Dufek, E.J.; Dickerson, C.C.; Wood, S.M. Electrochemical Quantification of Lithium Plating: Challenges and Considerations. J. Electrochem. Soc. 2019, 166, A2689–A2696. [Google Scholar] [CrossRef]
- Tian, Y.; Lin, C.; Li, H.; Du, J.; Xiong, R. Detecting Undesired Lithium Plating on Anodes for Lithium-Ion Batteries—A Review on the in-Situ Methods. Appl. Energy 2021, 300, 117386. [Google Scholar] [CrossRef]
- Lin, X.; Khosravinia, K.; Hu, X.; Li, J.; Lu, W. Lithium Plating Mechanism, Detection, and Mitigation in Lithium-Ion Batteries. Prog. Energy Combust. Sci. 2021, 87, 100953. [Google Scholar] [CrossRef]
- Janakiraman, U.; Garrick, T.R.; Fortier, M.E. Review—Lithium Plating Detection Methods in Li-Ion Batteries. J. Electrochem. Soc. 2020, 167, 160552. [Google Scholar] [CrossRef]
- Bommier, C.; Chang, W.; Lu, Y.; Yeung, J.; Davies, G.; Mohr, R.; Williams, M.; Steingart, D. In Operando Acoustic Detection of Lithium Metal Plating in Commercial LiCoO2/Graphite Pouch Cells. Cell Rep. Phys. Sci. 2020, 1, 100035. [Google Scholar] [CrossRef]
- Maiser, E. Battery Packaging-Technology Review. AIP Conf. Proc. 2014, 1597, 204–218. [Google Scholar]
- Leißing, M.; Horsthemke, F.; Wiemers-Meyer, S.; Winter, M.; Niehoff, P.; Nowak, S. The Impact of the C-Rate on Gassing During Formation of NMC622 II Graphite Lithium-Ion Battery Cells. Batter. Supercaps 2021, 4, 1344–1350. [Google Scholar] [CrossRef]
- Wang, H.; Rus, E.; Sakuraba, T.; Kikuchi, J.; Kiya, Y.; Abruña, H.D. CO2 and O2 Evolution at High Voltage Cathode Materials of Li-Ion Batteries: A Differential Electrochemical Mass Spectrometry Study. Anal. Chem. 2014, 86, 6197–6201. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Ko, I.-H. Failure Analysis of Swelling in Prismatic Lithium-Ion Batteries During Their Cycle Life After Long-Term Storage. J. Fail. Anal. Prev. 2018, 18, 554–561. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Lei, N.; Zhou, F.; Sun, C. An Experimental Study on the Mechanical Characteristics of Li-ion Battery during Overcharge-induced Thermal Runaway. Int. J. Energy Res. 2021, 45, 19985–20000. [Google Scholar] [CrossRef]
- Rowden, B.; Garcia-Araez, N. A Review of Gas Evolution in Lithium Ion Batteries. Energy Rep. 2020, 6, 10–18. [Google Scholar] [CrossRef]
- Zheng, T.; Muneeswara, M.; Bao, H.; Huang, J.; Zhang, L.; Hall, D.S.; Boles, S.T.; Jin, W. Gas Evolution in Li-Ion Rechargeable Batteries: A Review on Operando Sensing Technologies, Gassing Mechanisms, and Emerging Trends. ChemElectroChem 2024, 11, e202400065. [Google Scholar] [CrossRef]
- Metzger, M.; Strehle, B.; Solchenbach, S.; Gasteiger, H.A. Origin of H2 Evolution in LIBs: H2O Reduction vs. Electrolyte Oxidation. J. Electrochem. Soc. 2016, 163, A798–A809. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The State of Understanding of the Lithium-Ion-Battery Graphite Solid Electrolyte Interphase (SEI) and Its Relationship to Formation Cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Xiong, D.J.; Petibon, R.; Madec, L.; Hall, D.S.; Dahn, J.R. Some Effects of Intentionally Added Water on LiCoO2/Graphite Pouch Cells. J. Electrochem. Soc. 2016, 163, A1678–A1685. [Google Scholar] [CrossRef]
- Mogi, R.; Inaba, M.; Iriyama, Y.; Abe, T.; Ogumi, Z. Study on the Decomposition Mechanism of Alkyl Carbonate on Lithium Metal by Pyrolysis-Gas Chromatography-Mass Spectroscopy. J. Power Sources 2003, 119–121, 597–603. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Ramaswamy, S.G.; Jakoby, W.B. Enzymatic Hydrolysis of Organic Cyclic Carbonates. J. Biol. Chem. 1998, 273, 7814–7817. [Google Scholar] [CrossRef]
- Rodrigues, M.-T.F.; Kalaga, K.; Trask, S.E.; Shkrob, I.A.; Abraham, D.P. Anode-Dependent Impedance Rise in Layered-Oxide Cathodes of Lithium-Ion Cells. J. Electrochem. Soc. 2018, 165, A1697–A1705. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Yamamoto, N. Zero-Strain Insertion Material of Li[Li1/3Ti5/3]O4 for Rechargeable Lithium Cells. J. Electrochem. Soc. 1995, 142, 1431–1435. [Google Scholar] [CrossRef]
- Han, C.; He, Y.-B.; Liu, M.; Li, B.; Yang, Q.-H.; Wong, C.-P.; Kang, F. A Review of Gassing Behavior in Li4Ti5O12-Based Lithium Ion Batteries. J. Mater. Chem. A 2017, 5, 6368–6381. [Google Scholar] [CrossRef]
- Wu, K.; Yang, J.; Liu, Y.; Zhang, Y.; Wang, C.; Xu, J.; Ning, F.; Wang, D. Investigation on Gas Generation of Li4Ti5O12/LiNi1/3Co1/3Mn1/3O2 Cells at Elevated Temperature. J. Power Sources 2013, 237, 285–290. [Google Scholar] [CrossRef]
- Flügel, M.; Waldmann, T.; Kasper, M.; Wohlfahrt-Mehrens, M. Detection of Copper Deposition on Anodes of Over-Discharged Lithium Ion Cells by GD-OES Depth Profiling. ChemPhysChem 2020, 21, 2047–2050. [Google Scholar] [CrossRef]
- Guo, L.; Thornton, D.B.; Koronfel, M.A.; Stephens, I.E.L.; Ryan, M.P. Degradation in Lithium Ion Battery Current Collectors. J. Phys. Energy 2021, 3, 032015. [Google Scholar] [CrossRef]
- Hatsukade, T.; Schiele, A.; Hartmann, P.; Brezesinski, T.; Janek, J. Origin of Carbon Dioxide Evolved during Cycling of Nickel-Rich Layered NCM Cathodes. ACS Appl. Mater. Interfaces 2018, 10, 38892–38899. [Google Scholar] [CrossRef]
- Renfrew, S.E.; McCloskey, B.D. Residual Lithium Carbonate Predominantly Accounts for First Cycle CO2 and CO Outgassing of Li-Stoichiometric and Li-Rich Layered Transition-Metal Oxides. J. Am. Chem. Soc. 2017, 139, 17853–17860. [Google Scholar] [CrossRef]
- Mahne, N.; Renfrew, S.E.; McCloskey, B.D.; Freunberger, S.A. Electrochemical Oxidation of Lithium Carbonate Generates Singlet Oxygen. Angew. Chem. Int. Ed. 2018, 57, 5529–5533. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen Release and Its Effect on the Cycling Stability of LiNixMnyCozO2 (NMC) Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]
- Wandt, J.; Freiberg, A.T.S.; Ogrodnik, A.; Gasteiger, H.A. Singlet Oxygen Evolution from Layered Transition Metal Oxide Cathode Materials and Its Implications for Lithium-Ion Batteries. Mater. Today 2018, 21, 825–833. [Google Scholar] [CrossRef]
- Streich, D.; Erk, C.; Guéguen, A.; Müller, P.; Chesneau, F.-F.; Berg, E.J. Operando Monitoring of Early Ni-Mediated Surface Reconstruction in Layered Lithiated Ni–Co–Mn Oxides. J. Phys. Chem. C 2017, 121, 13481–13486. [Google Scholar] [CrossRef]
- Rinkel, B.L.D.; Vivek, J.P.; Garcia-Araez, N.; Grey, C.P. Two Electrolyte Decomposition Pathways at Nickel-Rich Cathode Surfaces in Lithium-Ion Batteries. Energy Environ. Sci. 2022, 15, 3416–3438. [Google Scholar] [CrossRef]
- Onuki, M.; Kinoshita, S.; Sakata, Y.; Yanagidate, M.; Otake, Y.; Ue, M.; Deguchi, M. Identification of the Source of Evolved Gas in Li-Ion Batteries Using [Sup 13]C-Labeled Solvents. J. Electrochem. Soc. 2008, 155, A794. [Google Scholar] [CrossRef]
- Metzger, M.; Marino, C.; Sicklinger, J.; Haering, D.; Gasteiger, H.A. Anodic Oxidation of Conductive Carbon and Ethylene Carbonate in High-Voltage Li-Ion Batteries Quantified by On-Line Electrochemical Mass Spectrometry. J. Electrochem. Soc. 2015, 162, A1123–A1134. [Google Scholar] [CrossRef]
- Guéguen, A.; Streich, D.; He, M.; Mendez, M.; Chesneau, F.F.; Novák, P.; Berg, E.J. Decomposition of LiPF6 in High Energy Lithium-Ion Batteries Studied with Online Electrochemical Mass Spectrometry. J. Electrochem. Soc. 2016, 163, A1095–A1100. [Google Scholar] [CrossRef]
- Renfrew, S.E.; Kaufman, L.A.; McCloskey, B.D. Altering Surface Contaminants and Defects Influences the First-Cycle Outgassing and Irreversible Transformations of LiNi0.6Mn0.2Co0.2O2. ACS Appl. Mater. Interfaces 2019, 11, 34913–34921. [Google Scholar] [CrossRef]
- Kaufman, L.A.; Huang, T.-Y.; Lee, D.; McCloskey, B.D. Particle Surface Cracking Is Correlated with Gas Evolution in High-Ni Li-Ion Cathode Materials. ACS Appl. Mater. Interfaces 2022, 14, 39959–39964. [Google Scholar] [CrossRef]
- Michalak, B.; Sommer, H.; Mannes, D.; Kaestner, A.; Brezesinski, T.; Janek, J. Gas Evolution in Operating Lithium-Ion Batteries Studied In Situ by Neutron Imaging. Sci. Rep. 2015, 5, 15627. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, H.; Zhang, Y.; Liu, J.; Zhang, J.; Li, L. Corrosion and Protection of Aluminum Current Collector in Lithium-Ion Batteries. Innov. Mater. 2023, 1, 100030. [Google Scholar] [CrossRef]
- Kim, Y. Investigation of the Gas Evolution in Lithium Ion Batteries: Effect of Free Lithium Compounds in Cathode Materials. J. Solid State Electrochem. 2013, 17, 1961–1965. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Chemical versus Electrochemical Electrolyte Oxidation on NMC111, NMC622, NMC811, LNMO, and Conductive Carbon. J. Phys. Chem. Lett. 2017, 8, 4820–4825. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Cairns, E.J. Review—Recent Progress in Electrocatalysts for Oxygen Reduction Suitable for Alkaline Anion Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2015, 162, F1504–F1539. [Google Scholar] [CrossRef]
- Nie, M.; Chalasani, D.; Abraham, D.P.; Chen, Y.; Bose, A.; Lucht, B.L. Lithium Ion Battery Graphite Solid Electrolyte Interphase Revealed by Microscopy and Spectroscopy. J. Phys. Chem. C 2013, 117, 1257–1267. [Google Scholar] [CrossRef]
- Michalak, B.; Berkes, B.B.; Sommer, H.; Brezesinski, T.; Janek, J. Electrochemical Cross-Talk Leading to Gas Evolution and Capacity Fade in LiNi0.5Mn1.5O4/Graphite Full-Cells. J. Phys. Chem. C 2017, 121, 211–216. [Google Scholar] [CrossRef]
- Xiong, D.J.; Petibon, R.; Nie, M.; Ma, L.; Xia, J.; Dahn, J.R. Interactions between Positive and Negative Electrodes in Li-Ion Cells Operated at High Temperature and High Voltage. J. Electrochem. Soc. 2016, 163, A546–A551. [Google Scholar] [CrossRef]
- Wuersig, A.; Scheifele, W.; Novák, P. CO2 Gas Evolution on Cathode Materials for Lithium-Ion Batteries. J. Electrochem. Soc. 2007, 154, A449. [Google Scholar] [CrossRef]
- Sloop, S.E.; Kerr, J.B.; Kinoshita, K. The Role of Li-Ion Battery Electrolyte Reactivity in Performance Decline and Self-Discharge. J. Power Sources 2003, 119–121, 330–337. [Google Scholar] [CrossRef]
- Xiong, D.J.; Ellis, L.D.; Petibon, R.; Hynes, T.; Liu, Q.Q.; Dahn, J.R. Studies of Gas Generation, Gas Consumption and Impedance Growth in Li-Ion Cells with Carbonate or Fluorinated Electrolytes Using the Pouch Bag Method. J. Electrochem. Soc. 2017, 164, A340–A347. [Google Scholar] [CrossRef]
- Manceron, L.; Andrews, L. Infrared Spectra and Structures of Lithium-Ethylene Complexes [Li(C2H4)n (n = 1, 2, 3) and Li2C2H4] in Solid Argon. J. Phys. Chem. 1986, 90, 4514–4528. [Google Scholar] [CrossRef]
- Self, J.; Aiken, C.P.; Petibon, R.; Dahn, J.R. Survey of Gas Expansion in Li-Ion NMC Pouch Cells. J. Electrochem. Soc. 2015, 162, A796–A802. [Google Scholar] [CrossRef]
- Berkes, B.B.; Jozwiuk, A.; Sommer, H.; Brezesinski, T.; Janek, J. Simultaneous Acquisition of Differential Electrochemical Mass Spectrometry and Infrared Spectroscopy Data for in Situ Characterization of Gas Evolution Reactions in Lithium-Ion Batteries. Electrochem. Commun. 2015, 60, 64–69. [Google Scholar] [CrossRef]
- Ota, H.; Sakata, Y.; Inoue, A.; Yamaguchi, S. Analysis of Vinylene Carbonate Derived SEI Layers on Graphite Anode. J. Electrochem. Soc. 2004, 151, A1659. [Google Scholar] [CrossRef]
- Bernhard, R.; Meini, S.; Gasteiger, H.A. On-Line Electrochemical Mass Spectrometry Investigations on the Gassing Behavior of Li4Ti5O12 Electrodes and Its Origins. J. Electrochem. Soc. 2014, 161, A497–A505. [Google Scholar] [CrossRef]
- Lanz, P.; Sommer, H.; Schulz-Dobrick, M.; Novák, P. Oxygen Release from High-Energy xLi2MnO3·(1 − x)LiMO2 (M = Mn, Ni, Co): Electrochemical, Differential Electrochemical Mass Spectrometric, in Situ Pressure, and in Situ Temperature Characterization. Electrochim. Acta 2013, 93, 114–119. [Google Scholar] [CrossRef]
- Li, R.; Ren, D.; Guo, D.; Xu, C.; Fan, X.; Hou, Z.; Lu, L.; Feng, X.; Han, X.; Ouyang, M. Volume Deformation of Large-Format Lithium Ion Batteries under Different Degradation Paths. J. Electrochem. Soc. 2019, 166, A4106–A4114. [Google Scholar] [CrossRef]
- Belharouak, I.; Koenig, G.M.; Tan, T.; Yumoto, H.; Ota, N.; Amine, K. Performance Degradation and Gassing of Li4Ti5O12/LiMn2O4 Lithium-Ion Cells. J. Electrochem. Soc. 2012, 159, A1165–A1170. [Google Scholar] [CrossRef]
- Kong, W.; Li, H.; Huang, X.; Chen, L. Gas Evolution Behaviors for Several Cathode Materials in Lithium-Ion Batteries. J. Power Sources 2005, 142, 285–291. [Google Scholar] [CrossRef]
- Parimalam, B.S.; MacIntosh, A.D.; Kadam, R.; Lucht, B.L. Decomposition Reactions of Anode Solid Electrolyte Interphase (SEI) Components with LiPF6. J. Phys. Chem. C 2017, 121, 22733–22738. [Google Scholar] [CrossRef]
- Dose, W.M.; Li, W.; Temprano, I.; O’Keefe, C.A.; Mehdi, B.L.; De Volder, M.F.L.; Grey, C.P. Onset Potential for Electrolyte Oxidation and Ni-Rich Cathode Degradation in Lithium-Ion Batteries. ACS Energy Lett. 2022, 7, 3524–3530. [Google Scholar] [CrossRef]
- Dai, K.; Wang, Z.; Ai, G.; Zhao, H.; Yuan, W.; Song, X.; Battaglia, V.; Sun, C.; Wu, K.; Liu, G. The Transformation of Graphite Electrode Materials in Lithium-Ion Batteries after Cycling. J. Power Sources 2015, 298, 349–354. [Google Scholar] [CrossRef]
- Szurke, S.K.; Lakatos, I. The Lithium Polymer Battery Swelling Test with High-Precision Displacement Sensors. In Proceedings of the 2018 20th International Symposium on Electrical Apparatus and Technologies (SIELA), Bourgas, Bulgaria, 3–6 June 2018; pp. 1–4. [Google Scholar]
- He, M.; Castel, E.; Laumann, A.; Nuspl, G.; Novák, P.; Berg, E.J. In Situ Gas Analysis of Li4Ti5O12 Based Electrodes at Elevated Temperatures. J. Electrochem. Soc. 2015, 162, A870–A876. [Google Scholar] [CrossRef]
- Kim, Y. Mechanism of Gas Evolution from the Cathode of Lithium-Ion Batteries at the Initial Stage of High-Temperature Storage. J. Mater. Sci. 2013, 48, 8547–8551. [Google Scholar] [CrossRef]
- Chen, C.; Wei, Y.; Zhao, Z.; Zou, Y.; Luo, D. Investigation of the Swelling Failure of Lithium-Ion Battery Packs at Low Temperatures Using 2D/3D X-Ray Computed Tomography. Electrochim. Acta 2019, 305, 65–71. [Google Scholar] [CrossRef]
- Kumai, K.; Miyashiro, H.; Kobayashi, Y.; Takei, K.; Ishikawa, R. Gas Generation Mechanism Due to Electrolyte Decomposition in Commercial Lithium-Ion Cell. J. Power Sources 1999, 81–82, 715–719. [Google Scholar] [CrossRef]
- Mao, C.; Ruther, R.E.; Geng, L.; Li, Z.; Leonard, D.N.; Meyer, H.M.; Sacci, R.L.; Wood, D.L. Evaluation of Gas Formation and Consumption Driven by Crossover Effect in High-Voltage Lithium-Ion Batteries with Ni-Rich NMC Cathodes. ACS Appl. Mater. Interfaces 2019, 11, 43235–43243. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhao, F.; Wang, W.; Zhao, Y.; Liang, Z.; Yan, D. Overcharge Failure Investigation of Lithium-Ion Batteries. Electrochim. Acta 2015, 178, 682–688. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, H.; Jin, Y. Experimental and Numerical Study on Mechanical Deformation Characteristics of Lithium Iron Phosphate Pouch Battery Modules under Overcharge Conditions. Energy Fuels 2021, 35, 15172–15184. [Google Scholar] [CrossRef]
- Li, H.; Gao, J.; Zhang, S. Effect of Overdischarge on Swelling and Recharge Performance of Lithium Ion Cells. Chin. J. Chem. 2008, 26, 1585–1588. [Google Scholar] [CrossRef]
- Hashimoto, M.; Yamashiro, M.; Ichihashi, T.; Toda, A.; Miyazaki, T.; Fujieda, S. Mechanism of Gas Generation in Lithium Ion Batteries By Overdischarge. ECS Meet. Abstr. 2015, MA2015-02, 354. [Google Scholar] [CrossRef]
- Fahy, K.F.; Shafaque, H.W.; Shrestha, P.; Ouellette, D.; Ge, N.; Ikeda, N.; Kotaka, T.; Tabuchi, Y.; Bazylak, A. Tracking Battery Swelling in Uncompressed Li-Ion Cells Via in-Operando X-Ray Radiography and Micro-Tomography. ECS Meet. Abstr. 2019, MA2019-02, 338. [Google Scholar] [CrossRef]
- Read, J.; Collins, E.; Piekarski, B.; Zhang, S. LiF Formation and Cathode Swelling in the Li/CFx Battery. J. Electrochem. Soc. 2011, 158, A504–A510. [Google Scholar] [CrossRef]
- Lu, D.; Lin, S.; Cui, W.; Hu, S.; Zhang, Z.; Peng, W. Swelling Mechanism of 0%SOC Lithium Iron Phosphate Battery at High Temperature Storage. J. Energy Storage 2020, 32, 101791. [Google Scholar] [CrossRef]
- Lawder, M.T.; Northrop, P.W.C.; Subramanian, V.R. Model-Based SEI Layer Growth and Capacity Fade Analysis for EV and PHEV Batteries and Drive Cycles. J. Electrochem. Soc. 2014, 161, A2099–A2108. [Google Scholar] [CrossRef]
- Juarez-Robles, D.; Vyas, A.A.; Fear, C.; Jeevarajan, J.A.; Mukherjee, P.P. Overcharge and Aging Analytics of Li-Ion Cells. J. Electrochem. Soc. 2020, 167, 090547. [Google Scholar] [CrossRef]
- Xing, J.; Bliznakov, S.; Bonville, L.; Oljaca, M.; Maric, R. A Review of Nonaqueous Electrolytes, Binders, and Separators for Lithium-Ion Batteries. Electrochem. Energy Rev. 2022, 5, 14. [Google Scholar] [CrossRef]
- Stravova, Z.; Klvac, O.; Bana, J.; Anothumakkool, B.; Zikmund, T.; Blazek, P.; Kaiser, J.; Kazda, T. Comprehensive Study of Rapid Capacity Fade in Prismatic Li-Ion Cells with Flexible Packaging. Sci. Rep. 2024, 14, 28546. [Google Scholar] [CrossRef]
- Hendricks, C.; Sood, B.; Pecht, M. Lithium-Ion Battery Strain Gauge Monitoring and Depth of Discharge Estimation. J. Electrochem. Energy Convers. Storage 2023, 20, 011008. [Google Scholar] [CrossRef]
- Peng, J.; Jia, S.; Yang, S.; Kang, X.; Yu, H.; Yang, Y. State Estimation of Lithium-Ion Batteries Based on Strain Parameter Monitored by Fiber Bragg Grating Sensors. J. Energy Storage 2022, 52, 104950. [Google Scholar] [CrossRef]
- Zeng, X.; Berecibar, M. Emerging Sensor Technologies and Physics-Guided Methods for Monitoring Automotive Lithium-Based Batteries. Commun. Eng. 2025, 4, 44. [Google Scholar] [CrossRef]
- Cai, T.; Pannala, S.; Stefanopoulou, A.G.; Siegel, J.B. Battery Internal Short Detection Methodology Using Cell Swelling Measurements. In Proceedings of the 2020 American Control Conference (ACC), Denver, CO, USA, 1–3 July 2020; pp. 1143–1148. [Google Scholar]
- Jiang, H.; Chen, J.; Li, X.; Jin, Z.; Chen, T.; Liu, J.; Li, D. A Comprehensive Review of In Situ Measurement Techniques for Evaluating the Electro-Chemo-Mechanical Behaviors of Battery Electrodes. Molecules 2024, 29, 1873. [Google Scholar] [CrossRef]
- Jeevarajan, J.A.; Joshi, T.; Parhizi, M.; Rauhala, T.; Juarez-Robles, D. Battery Hazards for Large Energy Storage Systems. ACS Energy Lett. 2022, 7, 2725–2733. [Google Scholar] [CrossRef]
- Yin, S.; Liu, J.; Cong, B. Review of Thermal Runaway Monitoring, Warning and Protection Technologies for Lithium-Ion Batteries. Processes 2023, 11, 2345. [Google Scholar] [CrossRef]
- Chen, S.; Wei, X.; Zhang, G.; Wang, X.; Feng, X.; Dai, H.; Ouyang, M. Mechanical Strain Signal Based Early Warning for Failure of Different Prismatic Lithium-Ion Batteries. J. Power Sources 2023, 580, 233397. [Google Scholar] [CrossRef]
- Lin, C.; Mao, J.; Yang, J.; Qi, C.; Zhang, Y.; Dan, S.; Liu, X. Early Warning for Thermal Runaway of Li[Ni0.5Co0.2Mn0.3]O2 and LiFePO4 Batteries under External Heating, Penetration and Overcharging Conditions. J. Energy Storage 2025, 109, 115085. [Google Scholar] [CrossRef]
- Yuan, C.; Hahn, Y.; Lu, W.; Oancea, V.; Xu, J. Quantification of Electrochemical-Mechanical Coupling in Lithium-Ion Batteries. Cell Rep. Phys. Sci. 2022, 3, 101158. [Google Scholar] [CrossRef]
- Qian, Z.; Li, Y.; Rao, Z. Thermal Performance of Lithium-Ion Battery Thermal Management System by Using Mini-Channel Cooling. Energy Convers. Manag. 2016, 126, 622–631. [Google Scholar] [CrossRef]
- Oh, K.-Y.; Epureanu, B.I.; Siegel, J.B.; Stefanopoulou, A.G. Phenomenological Force and Swelling Models for Rechargeable Lithium-Ion Battery Cells. J. Power Sources 2016, 310, 118–129. [Google Scholar] [CrossRef]
- Köbbing, L.; Latz, A.; Horstmann, B. Growth of the Solid-Electrolyte Interphase: Electron Diffusion versus Solvent Diffusion. arXiv 2022, arXiv:2209.10854. [Google Scholar] [CrossRef]
- von Kolzenberg, L.; Latz, A.; Horstmann, B. Chemo-Mechanical Model of SEI Growth on Silicon Electrode Particles. arXiv 2021, arXiv:2108.04078. [Google Scholar] [CrossRef]
- Cai, T.; Stefanopoulou, A.G.; Siegel, J.B. Modeling Li-Ion Battery Temperature and Expansion Force during the Early Stages of Thermal Runaway Triggered by Internal Shorts. J. Electrochem. Soc. 2019, 166, A2431–A2443. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, J.; Hou, W.; Mei, X. A Stack Pressure Based Equivalent Mechanical Model of Lithium-Ion Pouch Batteries. Energy 2021, 221, 119804. [Google Scholar] [CrossRef]
- Choi, H.; Jiang, C.; Youn, B.D.; Kim, T. Uncertainty Analysis of Stack Pressure in EV Battery Module Systems Using a Phenomenological Modeling Approach. J. Energy Storage 2023, 73, 108948. [Google Scholar] [CrossRef]
- Hahn, S.; Theil, S.; Kroggel, J.; Birke, K.P. Pressure Prediction Modeling and Validation for Lithium-Ion Pouch Cells in Buffered Module Assemblies. J. Energy Storage 2021, 40, 102517. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. State of Health and Charge Measurements in Lithium-Ion Batteries Using Mechanical Stress. J. Power Sources 2014, 269, 7–14. [Google Scholar] [CrossRef]
- Perez Estevez, M.A.; Conte, F.V.; Tremonti, C.; Renzi, M. Aging Estimation of Lithium Ion Cells under Real-World Conditions through Mechanical Stress Measurements. J. Energy Storage 2023, 64, 107186. [Google Scholar] [CrossRef]
- Ebert, F.; Sextl, G.; Adermann, J.; Reiter, C.; Lienkamp, M. Detection of Cell-Stack Inhomogeneities via Mechanical SOC and SOH Measurement. In Proceedings of the 2017 IEEE Transportation Electrification Conference and Expo (ITEC), Chicago, IL, USA, 22–24 June 2017; pp. 545–549. [Google Scholar]
- Li, R.; Li, W.; Singh, A.; Ren, D.; Hou, Z.; Ouyang, M. Effect of External Pressure and Internal Stress on Battery Performance and Lifespan. Energy Storage Mater. 2022, 52, 395–429. [Google Scholar] [CrossRef]
- De Vasconcelos, L.S.; Xu, R.; Xu, Z.; Zhang, J.; Sharma, N.; Shah, S.R.; Han, J.; He, X.; Wu, X.; Sun, H.; et al. Chemomechanics of Rechargeable Batteries: Status, Theories, and Perspectives. Chem. Rev. 2022, 122, 13043–13107. [Google Scholar] [CrossRef] [PubMed]
- Boyce, A.M.; Martínez-Pañeda, E.; Shearing, P.R. The Role of Chemo-Mechanical Modelling in the Development of Battery Technology—A Perspective. J. Phys. Energy 2024, 6, 021001. [Google Scholar] [CrossRef]
- Müllner, S.; Michlik, T.; Reichel, M.; Held, T.; Moos, R.; Roth, C. Effect of Water-Soluble CMC/SBR Binder Ratios on Si-rGO Composites Using Μm- and Nm-Sized Silicon as Anode Materials for Lithium-Ion Batteries. Batteries 2023, 9, 248. [Google Scholar] [CrossRef]
- Wang, E.; Ye, X.; Zhang, B.; Qu, B.; Guo, J.; Zheng, S. Enhancing the Stability of 4.6 V LiCoO2 Cathode Material via Gradient Doping. Nanomaterials 2024, 14, 147. [Google Scholar] [CrossRef]
- Lee, D.; Kondo, A.; Lee, S.; Myeong, S.; Sun, S.; Hwang, I.; Song, T.; Naito, M.; Paik, U. Controlled Swelling Behavior and Stable Cycling of Silicon/Graphite Granular Composite for High Energy Density in Lithium Ion Batteries. J. Power Sources 2020, 457, 228021. [Google Scholar] [CrossRef]
- Zybert, M.; Ronduda, H.; Ostrowski, A.; Sobczak, K.; Moszyński, D.; Raróg-Pilecka, W.; Hamankiewicz, B.; Wieczorek, W. Structural Analysis and Electrochemical Investigation of Dual-Doped NMC622 Cathode Material: Effect of Sodium and Neodymium on the Performance in Li-Ion Batteries. Energy Rep. 2023, 10, 1238–1248. [Google Scholar] [CrossRef]
- Zybert, M.; Ronduda, H.; Dąbrowska, K.; Ostrowski, A.; Sobczak, K.; Moszyński, D.; Hamankiewicz, B.; Rogulski, Z.; Raróg-Pilecka, W.; Wieczorek, W. Suppressing Ni/Li Disordering in LiNi0.6Mn0.2Co0.2O2 Cathode Material for Li-Ion Batteries by Rare Earth Element Doping. Energy Rep. 2022, 8, 3995–4005. [Google Scholar] [CrossRef]
- Su, L.; Choi, P.; Nakamura, N.; Charalambous, H.; Litster, S.; Ilavsky, J.; Reeja-Jayan, B. Multiscale Operando X-Ray Investigations Provide Insights into Electro-Chemo-Mechanical Behavior of Lithium Intercalation Cathodes. Appl. Energy 2021, 299, 117315. [Google Scholar] [CrossRef]
- Li, X.; Yan, P.; Xiao, X.; Woo, J.H.; Wang, C.; Liu, J.; Zhang, J.-G. Design of Porous Si/C–Graphite Electrodes with Long Cycle Stability and Controlled Swelling. Energy Environ. Sci. 2017, 10, 1427–1434. [Google Scholar] [CrossRef]
- Jain, R.; Lakhnot, A.S.; Bhimani, K.; Sharma, S.; Mahajani, V.; Panchal, R.A.; Kamble, M.; Han, F.; Wang, C.; Koratkar, N. Nanostructuring versus Microstructuring in Battery Electrodes. Nat. Rev. Mater. 2022, 7, 736–746. [Google Scholar] [CrossRef]
- Niu, C.; Lee, H.; Chen, S.; Li, Q.; Du, J.; Xu, W.; Zhang, J.-G.; Whittingham, M.S.; Xiao, J.; Liu, J. High-Energy Lithium Metal Pouch Cells with Limited Anode Swelling and Long Stable Cycles. Nat. Energy 2019, 4, 551–559. [Google Scholar] [CrossRef]
- Park, K.; Myeong, S.; Shin, D.; Cho, C.-W.; Kim, S.C.; Song, T. Improved Swelling Behavior of Li Ion Batteries by Microstructural Engineering of Anode. J. Ind. Eng. Chem. 2019, 71, 270–276. [Google Scholar] [CrossRef]
- Zhang, N.; Tang, H.; Zhang, L.; Trifonova, A. Asymmetric Electrode for Suppressing Cell Swelling in Commercial Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A2152–A2156. [Google Scholar] [CrossRef]
- Spielbauer, M.; Steinhardt, M.; Singer, J.; Aufschläger, A.; Bohlen, O.; Jossen, A. Influence of Breathing and Swelling on the Jelly-Roll Case Gap of Cylindrical Lithium-Ion Battery Cells. Batteries 2022, 9, 6. [Google Scholar] [CrossRef]
- Ecker, M.; Nieto, N.; Käbitz, S.; Schmalstieg, J.; Blanke, H.; Warnecke, A.; Sauer, D.U. Calendar and Cycle Life Study of Li(NiMnCo)O2-Based 18650 Lithium-Ion Batteries. J. Power Sources 2014, 248, 839–851. [Google Scholar] [CrossRef]
- Mao, Z.; Farkhondeh, M.; Pritzker, M.; Fowler, M.; Chen, Z. Calendar Aging and Gas Generation in Commercial Graphite/NMC-LMO Lithium-Ion Pouch Cell. J. Electrochem. Soc. 2017, 164, A3469–A3483. [Google Scholar] [CrossRef]
- Yang, S.; Ling, C.; Fan, Y.; Yang, Y.; Tan, X.; Dong, H. A Review of Lithium-Ion Battery Thermal Management System Strategies and the Evaluate Criteria. Int. J. Electrochem. Sci. 2019, 14, 6077–6107. [Google Scholar] [CrossRef]
- Ghaeminezhad, N.; Wang, Z.; Ouyang, Q. A Review on Lithium-Ion Battery Thermal Management System Techniques: A Control-Oriented Analysis. Appl. Therm. Eng. 2023, 219, 119497. [Google Scholar] [CrossRef]
- Anwajler, B. Potential of 3D Printing for Heat Exchanger Heat Transfer Optimization—Sustainability Perspective. Inventions 2024, 9, 60. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, D.; Jiang, L.; Zhang, G.; Wu, H.; Day, R.; Jiang, W. Advanced Thermal Management System Driven by Phase Change Materials for Power Lithium-Ion Batteries: A Review. Renew. Sustain. Energy Rev. 2022, 159, 112207. [Google Scholar] [CrossRef]
- Ge, X.; Yuan, B.; Xu, S.; Xu, P.; Shi, Y.; Liu, Y.; Li, Z.; Sun, Q.; Guo, J.; Liang, E.; et al. Anodic Lithium Ion Battery Material with Negative Thermal Expansion. Ceram. Int. 2020, 46, 19127–19134. [Google Scholar] [CrossRef]
- Hao, H.; Xu, S.; Jing, N.; Wang, M.; Wang, Z.; Yang, L.; Chen, J.; Wang, G.; Wang, G. Negative Thermal Expansion Material: Promising for Improving Electrochemical Performance and Safety of Lithium-Ion Batteries. J. Phys. Chem. Lett. 2021, 12, 6134–6142. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, M.; Liu, J.; Wei, R.; Weng, J.; Wang, J. Investigation of a Commercial Lithium-Ion Battery under Overcharge/over-Discharge Failure Conditions. RSC Adv. 2018, 8, 33414–33424. [Google Scholar] [CrossRef]
- Qi, J.; Dah-Chuan Lu, D. Review of Battery Cell Balancing Techniques. In Proceedings of the 2014 Australasian Universities Power Engineering Conference (AUPEC), Perth, Australia, 28 September–1 October 2014; pp. 1–6. [Google Scholar]

| Component | Material | CTE (×10−6/°C) | Temperature Range | Ref. |
|---|---|---|---|---|
| Anode active material | Graphite IG-110 | 3.9–4.2 | 25–500 °C | [24] |
| Mitsubishi coke | 4.0–4.3 | 25–500 °C | [24] | |
| Microcrystalline graphite | 2.1–2.2 | 25–500 °C | [24] | |
| Cathode active material | Lithium manganese oxide (LMO) | 8.6 | - | [25] |
| Lithium iron phosphate (LFP) | 15.0 | - | [26] | |
| Lithium cobalt oxide (LCO) | 12.0–15.0 | 75–400 °C | [27] | |
| Lithium nickel manganese cobalt oxide (NMC) | 12.0–13.0 | 75–400 °C | [28] | |
| Current collector | Copper | 17.0 | 20–200 °C | [29] |
| Aluminum | 23.6 | 20–200 °C | [29] | |
| Separator | Polypropylene (PP) | 44.0 | 30–65 °C | [30] |
| 130.0 | - | [29] | ||
| PP-PE-PP tri-layer | 30.0 | 30–60 °C | [30] | |
| Ceramic-coated PP-PE-PP tri-layer | −1.2 T + 39.0 | 30–160 °C | [30] | |
| Can/casing | Nickel-plated steel | 20.6 | - | [31] |
| Location | Cell/Materials | Gas Generated | Chemical Equation | Ref. |
|---|---|---|---|---|
| Anode | Graphite, LTO; Electrolyte with H2O | H2 | H2O + e− → OH− + ½ H2 (g) | [151] |
| LTO/LFP | H2 | Li7Ti5O12 + 3 H2O → Li4Ti5O12 + 1.5 H2 (g) + 3 Li+ + 3 OH− | [148] | |
| Electrolyte solvents (ROH) | H2 | ROH + e− → RO− + ½ H2 (g) | [110] | |
| Graphite; EC-derived SEI | C2H4, CO2, O2 | (CH2OCO2Li)2 → Li2CO3 + C2H4 (g) + CO2 + ½ O2 | [110] | |
| EC + O2 (e.g., from NMC) | CO, CO2, H2O | (CH2O)2CO + O2 → CO (g) + 2 CO2 + 2 H2O | [124] | |
| EC/DMC electrolyte | CO | DMC + 2 Li+ + 2 e− → CH3OLi + CO (g) | [44] | |
| EC/EMC electrolyte | CO | EMC + 2 Li+ + 2 e− → CH3OLi + EtOLi + CO (g) | [44] | |
| Lithiated graphite | CO | CO2 + 2 Li+ + 2 e− → CO (g) + Li2CO3 | [44] | |
| Cathode | NMC cathodes; EC solvent | CO, CO2, H2O | (CH2O)2CO + O2 → CO (g) + 2 CO2 + 2 H2O | [124] |
| Carbon black (additive) + H2O | CO | C + H2O → CO (g) + 2 H+ + 2 e− | [44] | |
| Carbon black + H2O | CO2 | C + 2 H2O → CO2 + 4 H+ + 4 e− | [4] | |
| Organic carbonate (RCO3R) | CO2 | RCO3R → ROR + CO2 (g) | [152] | |
| EC + H2O | CO2 | (CH2O)2CO + H2O → (CH2OH)2 + CO2 (g) | [114] | |
| LiPF6 + Li2CO3 | CO2, POF3 | LiPF6 + Li2CO3 → CO2 (g) + 3 LiF + POF3 (g) | [153] | |
| LiPF6 + LMC (SEI component) | CO2, CH3OCH3 | LiPF6 + 3 LMC → 3 CO2 (g) + 4 LiF + OPF2OCH3 + CH3OCH3 | [153] | |
| Ni-rich NMC (e.g., NMC811) | O2 | MO2 → MO2–δ + ½ O2 (g) (M = Ni, Mn, Co) | [154] | |
| Li2CO3 | 1O2 | Li2CO2 → CO2 + ½ O2 (g) | [123] | |
| EC (oxidized by O2) | CO, CO2, H2O | (CH2O)2CO + O2 → CO (g) + 2 CO2 + 2 H2O | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maddipatla, S.; Rauf, H.; Osterman, M.; Arshad, N.; Pecht, M. Swelling Mechanisms, Diagnostic Applications, and Mitigation Strategies in Lithium-Ion Batteries. Batteries 2025, 11, 356. https://doi.org/10.3390/batteries11100356
Maddipatla S, Rauf H, Osterman M, Arshad N, Pecht M. Swelling Mechanisms, Diagnostic Applications, and Mitigation Strategies in Lithium-Ion Batteries. Batteries. 2025; 11(10):356. https://doi.org/10.3390/batteries11100356
Chicago/Turabian StyleMaddipatla, Sahithi, Huzaifa Rauf, Michael Osterman, Naveed Arshad, and Michael Pecht. 2025. "Swelling Mechanisms, Diagnostic Applications, and Mitigation Strategies in Lithium-Ion Batteries" Batteries 11, no. 10: 356. https://doi.org/10.3390/batteries11100356
APA StyleMaddipatla, S., Rauf, H., Osterman, M., Arshad, N., & Pecht, M. (2025). Swelling Mechanisms, Diagnostic Applications, and Mitigation Strategies in Lithium-Ion Batteries. Batteries, 11(10), 356. https://doi.org/10.3390/batteries11100356








