Investigation of Pr3+ and Nd3+ Doping Effects on Sodium Gadolinium Silicate Ceramics as Fast Na+ Conductors
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Silicates
2.2. Structural and Sample Analysis
2.3. Electrochemical Preparation and Measurements
2.3.1. Electrochemical Impedance Spectroscopy (EIS)
2.3.2. Direct Current (DC) Polarization Experiment
2.3.3. Galvanostatic Sodium Symmetric Cell Experiments
3. Results and Discussion
3.1. Synthesis of Pr and Nd-Doped Sodium Gadolinium Silicates (NGPS and NGNS)
3.2. Phase Evolution of Pr and Nd-Doped Sodium Gadolinium Silicates (NGPS and NGNS)
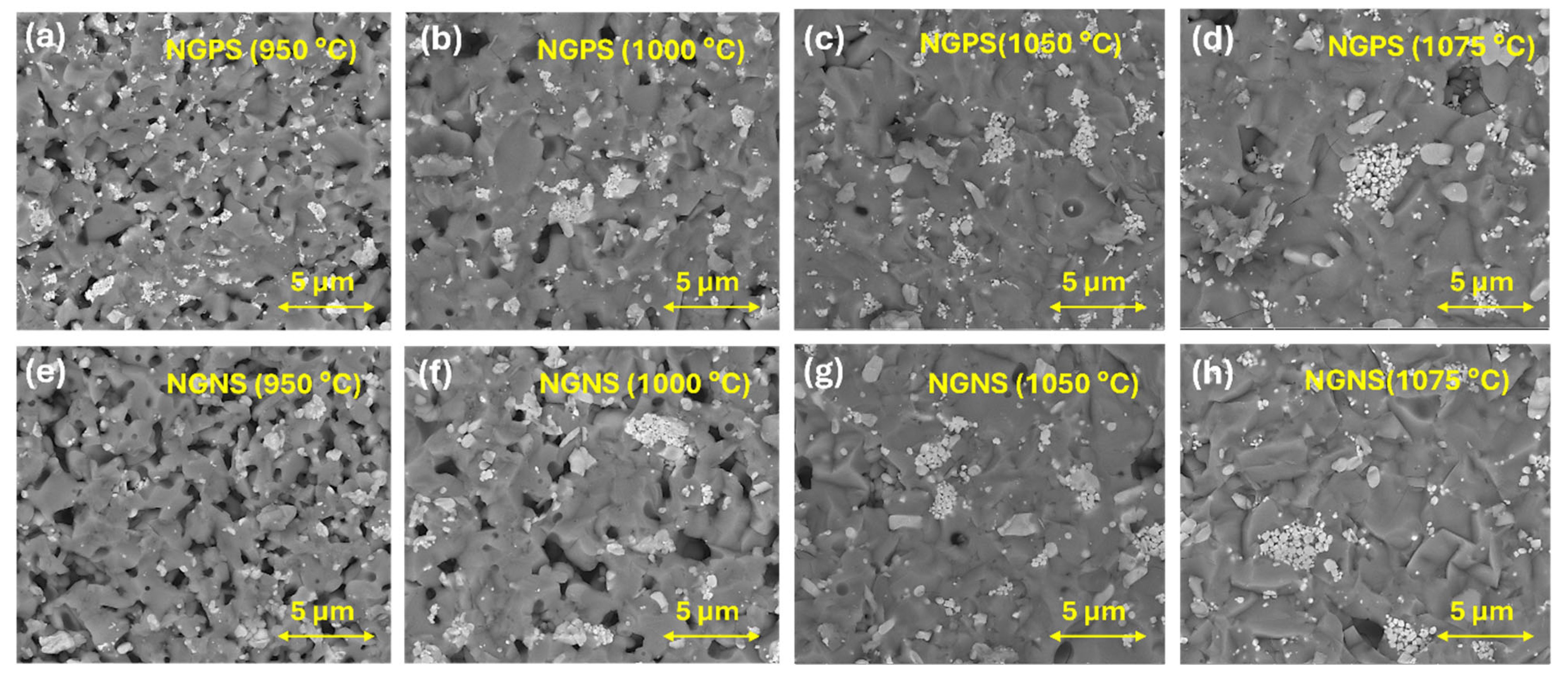
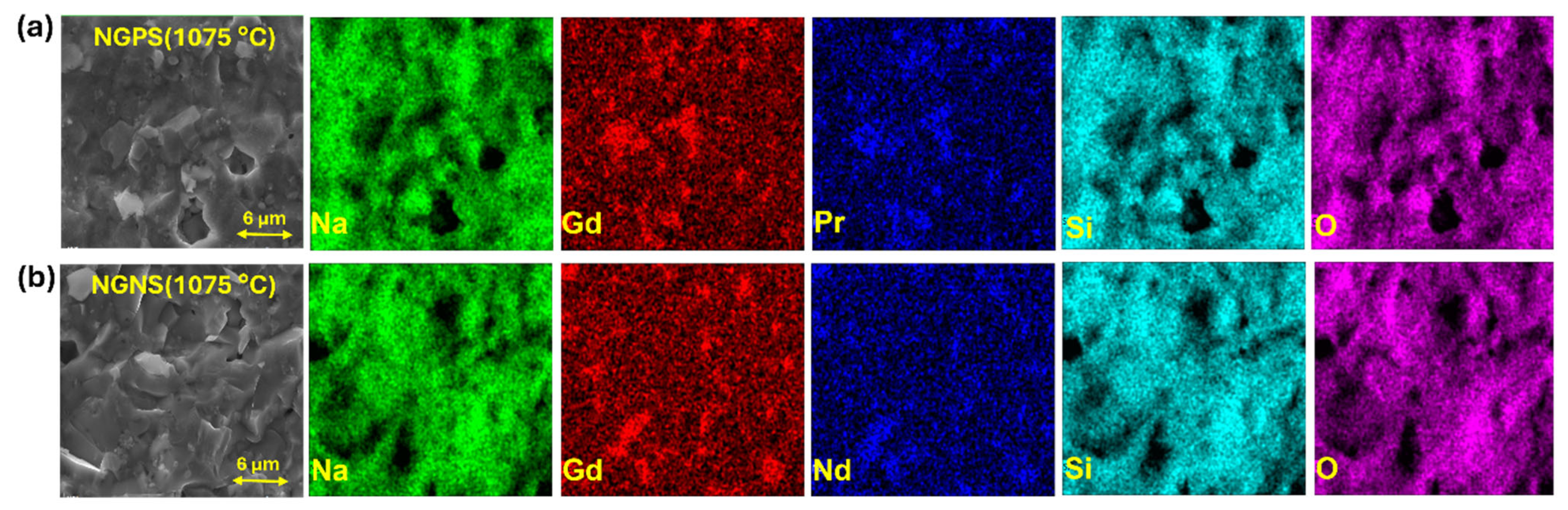
3.3. Morphological Analysis—Cross-Sectional SEM Images of NGPS and NGNS Ceramics
3.4. Electrochemical Impedance Spectroscopy (EIS) Results
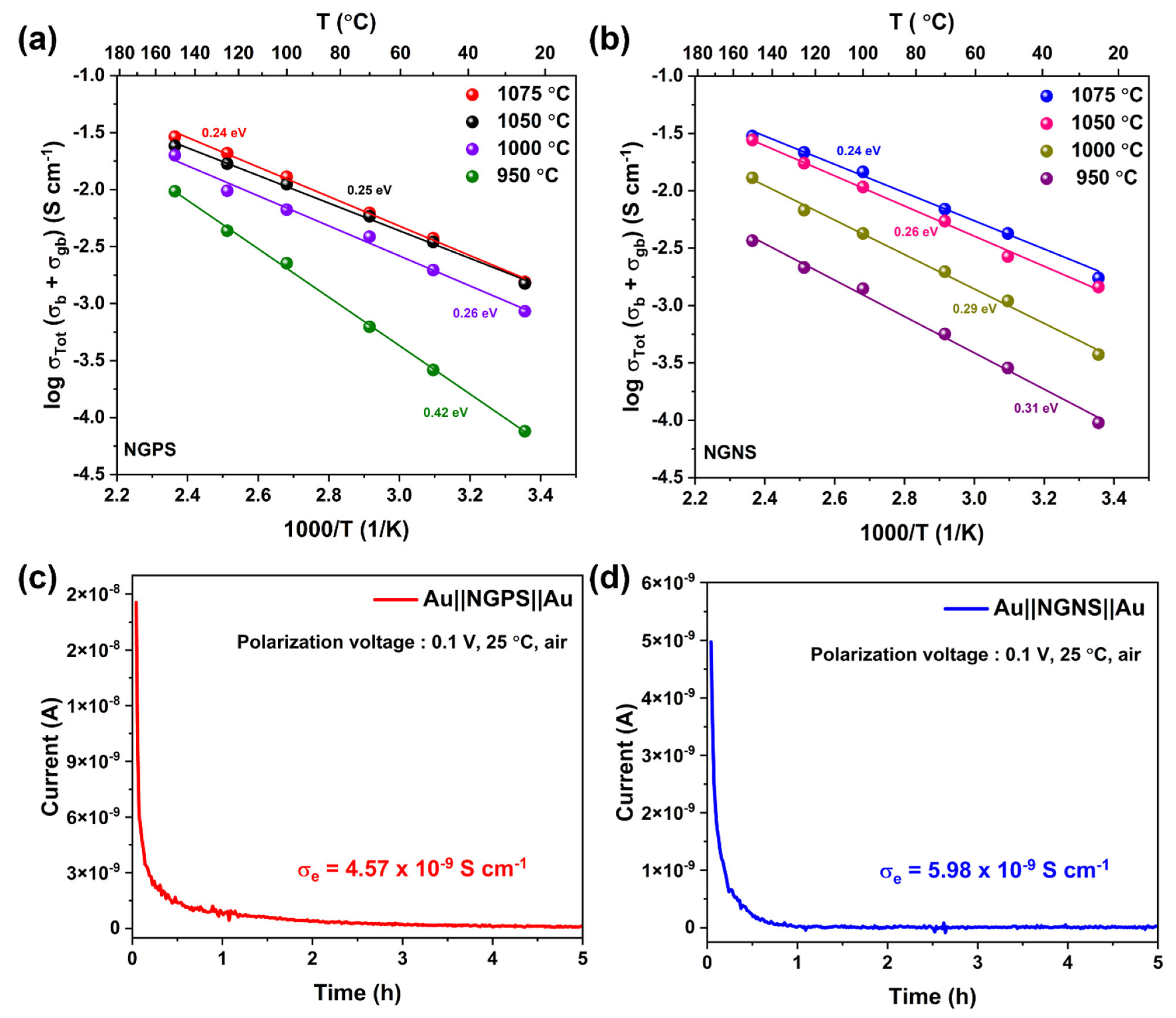
3.5. Electrochemical Performance
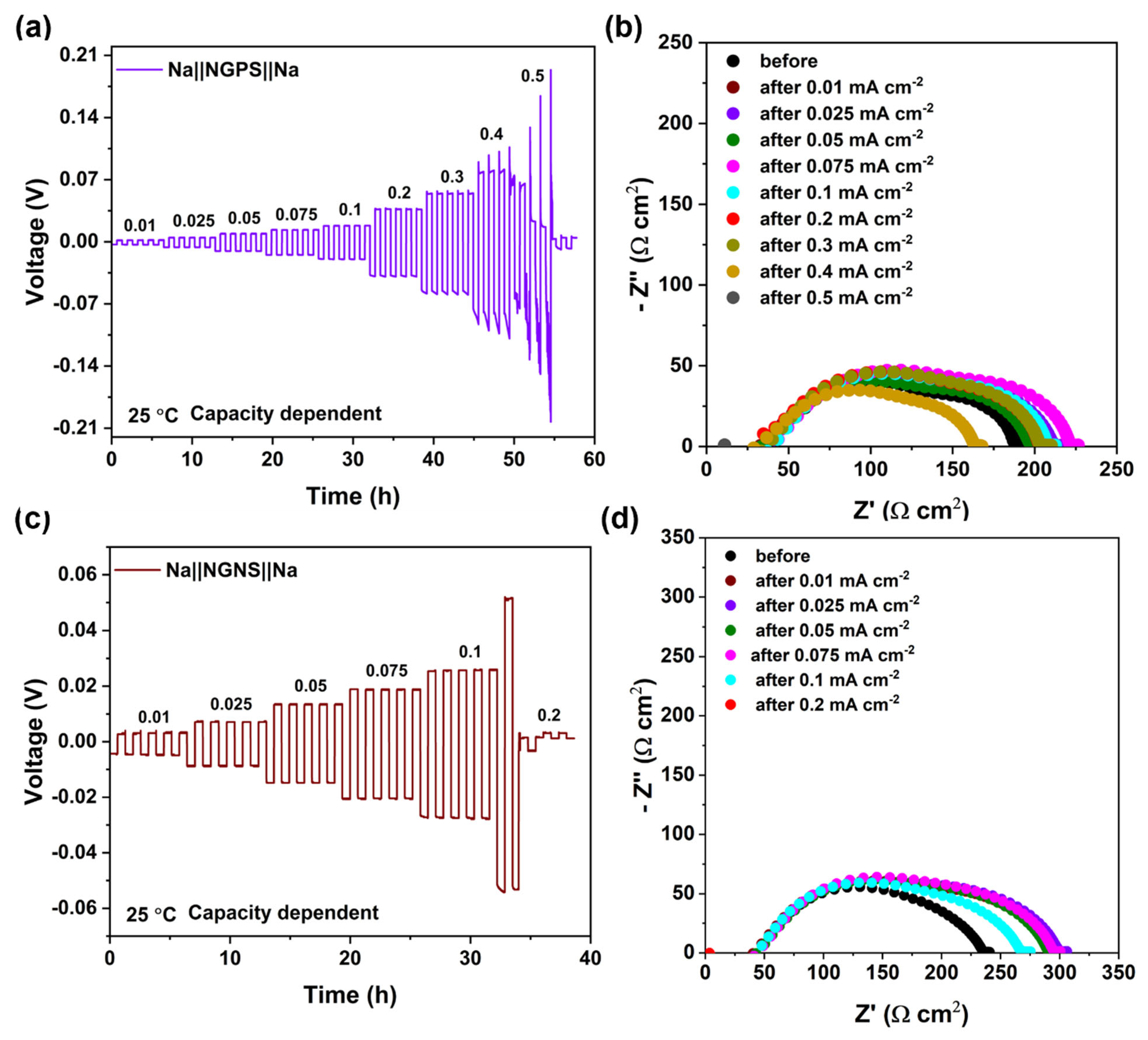
3.5.1. NGPS/NGNS Ceramic Interface Compatibility with Na Anode
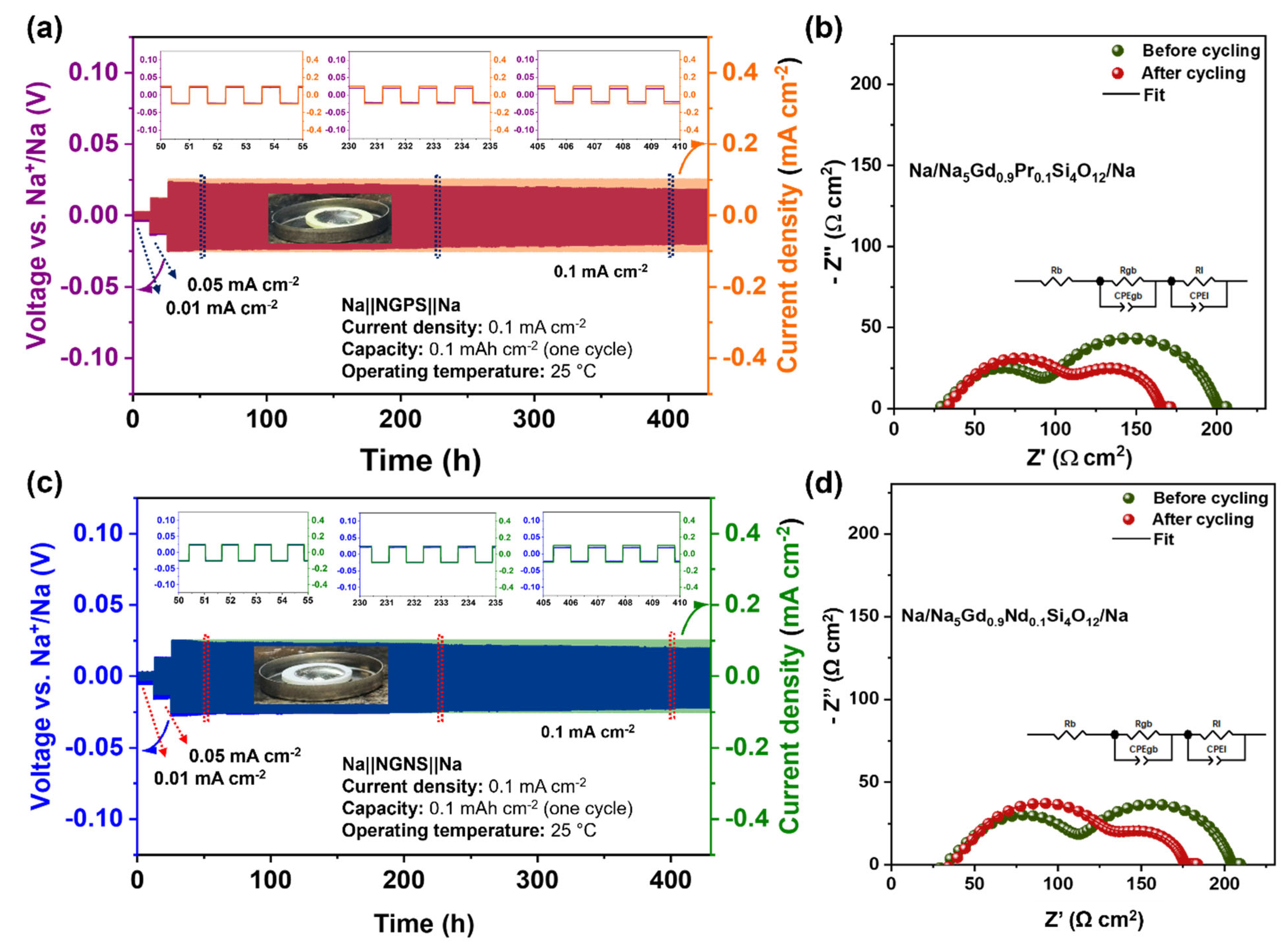
3.5.2. Long-Term Stability Tests Between Sodium Metal and Silicate Electrolytes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMB | Sodium metal batteries |
| SIB | Sodium-ion batteries |
| ASSSB | All-solid-state-sodium batteries |
| LIB | Lithium-ion batteries |
| NASICON | Sodium superionic conductor |
| NGPS | Na5Gd0.9Pr0.1Si4O12 |
| NGNS | Na5Gd0.9Nd0.1Si4O12 |
| NGS | Sodium gadolinium silicate |
| ASR | Area-specific resistance |
| PXRD | Powder X-ray |
| SEM | Scanning electron microscopy |
| EDX | Energy dispersive X-ray |
| EIS | Electrochemical impedance spectroscopy |
| CCD | Critical current density |
References
- Yang, P.; Wu, Z.; Liang, Y.; Chen, H.; Lin, C.; Qiu, J.; Meng, J.; He, Y.; Zhang, S. Engineering Ion Transport in All-Solid-State Sodium-Ion Batteries: Fundamentals, Strategies, and Perspectives. Prog. Mater. Sci. 2025, 154, 101503. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, R.; Fu, Q.; Zhang, J.; Zhou, X.; Gao, P.; Xu, C.; Liu, J.; Guo, X. Inorganic Solid Electrolytes for All-Solid-State Sodium Batteries: Fundamentals and Strategies for Battery Optimization. Adv. Funct. Mater. 2021, 31, 2008165. [Google Scholar] [CrossRef]
- Zhou, C.; Bag, S.; Thangadurai, V. Engineering Materials for Progressive All-Solid-State Na Batteries. ACS Energy Lett. 2018, 3, 2181–2198. [Google Scholar] [CrossRef]
- Sivakumaran, A.; Samson, A.J.; Thangadurai, V. Progress in Sodium Silicates for All-Solid-State Sodium Batteries—A Review. Energy Technol. 2023, 11, 2201323. [Google Scholar] [CrossRef]
- Yi, B.; Wei, Z.; Jia, W.; Sun, G.; Si, W.; Yao, S.; Chen, G.; Du, F. Nonstoichiometry Induced Amorphous Grain Boundary of Na5SmSi4O12 Solid-State Electrolyte for Long-Life Dendrite-Free Sodium Metal Battery. Nano Lett. 2024, 24, 8911–8919. [Google Scholar] [CrossRef]
- Yang, A.; Yao, K.; Schaller, M.; Dashjav, E.; Li, H.; Zhao, S.; Zhang, Q.; Etter, M.; Shen, X.; Song, H.; et al. Enhanced Room-Temperature Na+ Ionic Conductivity in Na4.92Y0.92Zr0.08Si4O12. eScience 2023, 3, 100175. [Google Scholar] [CrossRef]
- Hou, W.; Guo, X.; Shen, X.; Amine, K.; Yu, H.; Lu, J. Solid Electrolytes and Interfaces in All-Solid-State Sodium Batteries: Progress and Perspective. Nano Energy 2018, 52, 279–291. [Google Scholar] [CrossRef]
- Rizvi, S.; Aladhyani, I.; Ding, Y.; Zhang, Q. Recent Advances in Doping Na3Zr2Si2PO12 (NASICON) Solid-State Electrolyte for Sodium-Ion Batteries. Nano Energy 2024, 129, 110009. [Google Scholar] [CrossRef]
- Luo, W.; Lin, C.; Zhao, O.; Noked, M.; Zhang, Y.; Rubloff, G.W.; Hu, L. Ultrathin Surface Coating Enables the Stable Sodium Metal Anode. Adv. Energy Mater. 2017, 7, 1601526. [Google Scholar] [CrossRef]
- Liu, G.; Yang, J.; Wu, J.; Peng, Z.; Yao, X. Inorganic Sodium Solid Electrolytes: Structure Design, Interface Engineering and Application. Adv. Mater. 2024, 36, e2311475. [Google Scholar] [CrossRef]
- Lu, X.; Xia, G.; Lemmon, J.P.; Yang, Z. Advanced Materials for Sodium-Beta Alumina Batteries: Status, Challenges and Perspectives. J. Power Sources 2010, 195, 2431–2442. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Peng, J.; Zhou, X.; Liang, D.; Zhao, L.; Su, J.; Zhang, B.; Li, S.; Zhang, N.; et al. A Niobium-Substituted Sodium Superionic Conductor with Conductivity Higher than 5.5 MS Cm−1 Prepared by Solution-Assisted Solid-State Reaction Method. J. Power Sources 2022, 518, 230765. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, F.; Qu, T.; Yao, Y.; Ma, W.; Yang, B.; Dai, Y. Effect on Ionic Conductivity of Na3+xZr2−xMxSi2PO12 (M=Y, La) by Doping Rare-Earth Elements. IOP Conf. Ser. Mater. Sci. Eng. 2018, 423, 012122. [Google Scholar] [CrossRef]
- Huang, T.; Xiong, W.; Ye, X.; Huang, Z.; Feng, Y.; Liang, J.; Ye, S.; Piao, J.; Wang, X.; Li, Y.; et al. A Cerium-Doped NASICON Chemically Coupled Poly(Vinylidene Fluoride-Hexafluoropropylene)-Based Polymer Electrolyte for High-Rate and High-Voltage Quasi-Solid-State Lithium Metal Batteries. J. Energy Chem. 2022, 73, 311–321. [Google Scholar] [CrossRef]
- Pal, S.K.; Saha, R.; Kumar, G.V.; Omar, S. Designing High Ionic Conducting NASICON-Type Na3 Zr2 Si2 PO12 Solid-Electrolytes for Na-Ion Batteries. J. Phys. Chem. C 2020, 124, 9161–9169. [Google Scholar] [CrossRef]
- Ran, L.; Baktash, A.; Li, M.; Yin, Y.; Demir, B.; Lin, T.; Li, M.; Rana, M.; Gentle, I.; Wang, L.; et al. Sc, Ge Co-Doping NASICON Boosts Solid-State Sodium Ion Batteries’ Performance. Energy Storage Mater. 2021, 40, 282–291. [Google Scholar] [CrossRef]
- Pratiwi, V.M.; Noerochim, L.; Purwaningsih, H.; Wibowo, A.A.; Maulana, F.A. Study of Addition Metal (Ti, Zn) Dopan on the Structure of NASICON as Solid Electrolyte Batteries. Eng. Chem. 2024, 5, 43–48. [Google Scholar] [CrossRef]
- Yu, Z.; Shang, S.; Seo, J.; Wang, D.; Luo, X.; Huang, Q.; Chen, S.; Lu, J.; Li, X.; Liu, Z.; et al. Exceptionally High Ionic Conductivity in Na3P0.62As0.38S4 with Improved Moisture Stability for Solid-State Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1605561. [Google Scholar] [CrossRef]
- He, S.; Xu, Y.; Chen, Y.; Ma, X. Enhanced Ionic Conductivity of an F−-Assisted Na3 Zr2 Si2 PO12 Solid Electrolyte for Solid-State Sodium Batteries. J. Mater. Chem. A 2020, 8, 12594–12602. [Google Scholar] [CrossRef]
- Zhao, S.; Che, H.; Chen, S.; Tao, H.; Liao, J.; Liao, X.-Z.; Ma, Z.-F. Research Progress on the Solid Electrolyte of Solid-State Sodium-Ion Batteries. Electrochem. Energy Rev. 2024, 7, 3. [Google Scholar] [CrossRef]
- Sun, G.; Lin, H.; Yao, S.; Wei, Z.; Chen, N.; Chen, G.; Zhao, H.; Du, F. High-Entropy Solid-State Na-Ion Conductor for Stable Sodium-Metal Batteries. Chem. Eur. J. 2023, 29, e202300413. [Google Scholar] [CrossRef]
- Abdelaal, M.M.; Wang, Z.; Shen, H.; Pan, Z.; Kotobuki, M. Electrochemical Properties of a La-Doped Na5YSi4O12 Ceramic Electrolyte. ACS Appl. Energy Mater. 2025, 8, 9656–9662. [Google Scholar] [CrossRef]
- Sivakumaran, A.; Samson, A.J.; Bristi, A.A.; Surendran, V.; Butler, S.; Reid, S.; Thangadurai, V. High Ionic Conducting Rare-Earth Silicate Electrolytes for Sodium Metal Batteries. J. Mater. Chem. A 2023, 11, 15792–15801. [Google Scholar] [CrossRef]
- Sivakumaran, A.; Surendran, V.; Butler, S.; Reid, S.; Thangadurai, V. Sodium Ion Conductivities in Na2O–Sm2O3–SiO2 Ceramics. EES Batter. 2025, 1, 287–297. [Google Scholar] [CrossRef]
- Okura, T.; Kawada, K.; Hashimoto, H.; Yamashita, K. Novel Fast Na+ Conducting Rare Earth-Free Na5YSi4O12-Type Borosilicate Glass-Ceramics. Ceram. Int. 2025, 51, 26078–26084. [Google Scholar] [CrossRef]
- Boukamp, B.A. A Linear Kronig-Kramers Transform Test for Immittance Data Validation. J. Electrochem. Soc. 1995, 142, 1885–1894. [Google Scholar] [CrossRef]
- Boukamp, B.A. Electrochemical Impedance Spectroscopy in Solid State Ionics: Recent Advances. Solid State Ionics 2004, 169, 65–73. [Google Scholar] [CrossRef]
- Park, H.; Jung, K.; Nezafati, M.; Kim, C.S.; Kang, B. Sodium Ion Diffusion in Nasicon (Na3Zr2Si2PO12) Solid Electrolytes: Effects of Excess Sodium. ACS Appl. Mater. Interfaces 2016, 8, 27814–27824. [Google Scholar] [CrossRef] [PubMed]
- Irvine, J.T.S.; Sinclair, D.C.; West, A.R. Electroceramics: Characterization by Impedance Spectroscopy. Adv. Mater. 1990, 2, 132–138. [Google Scholar] [CrossRef]
- Shannon, R.D.; Taylor, B.E.; Gier, T.E.; Chen, H.Y.; Berzins, T. Ionic Conductivity in Sodium Yttrium Silicon Oxide (Na5YSi4O12)-Type Silicates. Inorg. Chem. 1978, 17, 958–964. [Google Scholar] [CrossRef]
- Horiuchi, N.; Ryu, K.; Nagai, A.; Okura, T.; Yamashita, K. Sol–Gel Synthesis and Electrical Properties of Sodium Ion Conducting Solid Electrolyte with Na5YSi4O12-Type Structure. Open Ceram. 2021, 8, 100175. [Google Scholar] [CrossRef]
- Okura, T.; Yoshida, N.; Yamashita, K. Na+ Superionic Conducting Silicophosphate Glass-Ceramics—Review. Solid State Ionics 2016, 285, 143–154. [Google Scholar] [CrossRef]
- Michalak, A.; Behara, S.; Anji Reddy, M. Reinvestigation of Na5GdSi4O12: A Potentially Better Solid Electrolyte than Sodium β Alumina for Solid-State Sodium Batteries. ACS Appl. Mater. Interfaces 2024, 16, 7112–7118. [Google Scholar] [CrossRef]
- Lowack, A.; Nakum, Y.; Anton, R.; Nikolowski, K.; Partsch, M.; Michaelis, A. Quantifying Sodium Dendrite Formation in Na5SmSi4O12 Solid Electrolytes. Batter. Supercaps, 2025; early view. [Google Scholar] [CrossRef]
- Bay, M.; Wang, M.; Grissa, R.; Heinz, M.V.F.; Sakamoto, J.; Battaglia, C. Sodium Plating from Na-β″-Alumina Ceramics at Room Temperature, Paving the Way for Fast-Charging All-Solid-State Batteries. Adv. Energy Mater. 2020, 10, 1902899. [Google Scholar] [CrossRef]

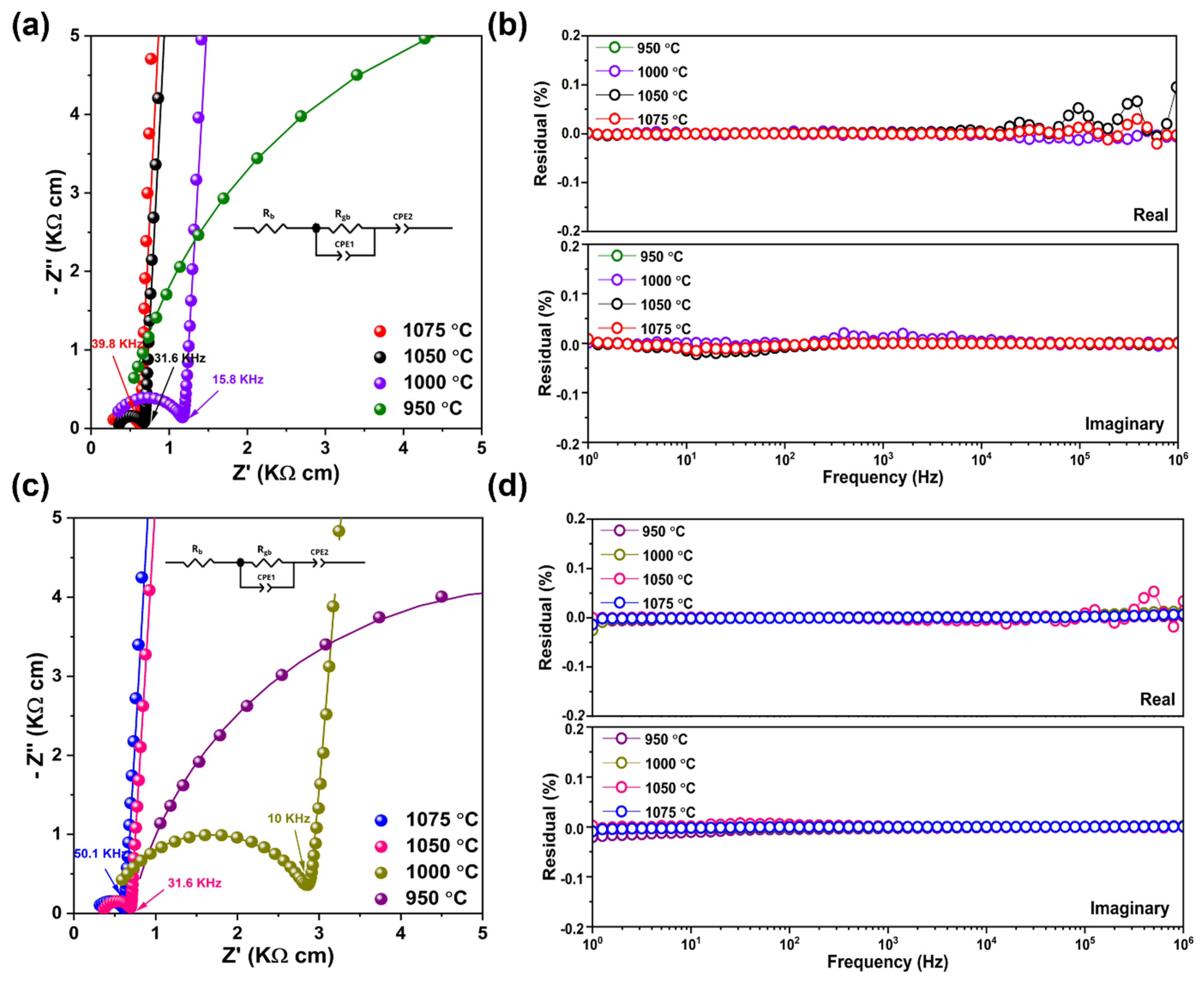

| Sample | Sintering Temperature (°C) | Total Ionic Conductivity (σi) (S cm−1) | Rgb (Ω) | ngb | CPE 1 (Fs[n−1]) (10−8) | nel | CPE 2 (Fs[n−1]) (10−6) | X2 |
|---|---|---|---|---|---|---|---|---|
| 950 | 7.61 × 10−5 | 1101 | 0.89 | 1.48 | 0.94 | 2.77 | 0.0009 | |
| 1000 | 7.93 × 10−4 | 79.94 | 0.88 | 4.34 | 0.96 | 2.39 | 0.0006 | |
| NGPS | 1050 | 1.50 × 10−3 | 32.94 | 0.94 | 5.26 | 0.97 | 1.93 | 0.0012 |
| 1075 | 1.64 × 10−3 | 33.41 | 0.94 | 3.19 | 0.97 | 1.76 | 0.0002 | |
| 950 | 9.52 × 10−5 | 835.1 | 0.87 | 1.09 | 0.90 | 2.41 | 0.0045 | |
| 1000 | 3.74 × 10−4 | 207.9 | 0.85 | 3.64 | 0.95 | 2.23 | 0.0019 | |
| NGNS | 1050 | 1.45 × 10−3 | 30.04 | 0.96 | 3.23 | 0.96 | 2.26 | 0.0010 |
| 1075 | 1.74 × 10−3 | 31.29 | 0.96 | 2.35 | 0.96 | 2.01 | 0.0015 |
| Composition | Ionic Conductivity (σi) (S cm−1) | Temperature (°C) | References |
|---|---|---|---|
| Na5GdSi3.75Ge0.25O0.2 | 3 × 10−1 | 300 | [30] |
| Na5Gd0.8La0.2Si4O12 | 3 × 10−1 | 300 | [30] |
| Na4.9Gd0.9Zr0.1Si4O12 | 4 × 10−1 | 300 | [30] |
| Na4.0Y0.6P0.2Si2.8O9 | 3.7 × 10−2 | 300 | [31] |
| Na3.9Y0.6P0.3Si2.7O9 | 6.6 × 10−3 | 300 | [32] |
| Na3.9Sm0.6P0.3Si2.7O9 | 1.3 × 10−2 | 300 | [32] |
| Na3.9Gd0.6P0.3Si2.7O9 | 6.3 × 10−3 | 300 | [32] |
| Na4.92Y0.92Zr0.08Si4O12 | 3.3 × 10−3 | 25 | [6] |
| Na4.9Sm0.3Y0.2Gd0.2La0.1Al0.1Zr0.1Si4O12 | 6.7 × 10−4 | 25 | [21] |
| Na4.9Gd0.9Zr0.1Si4O12 | 1.9 × 10−3 | 25 | [33] |
| Na5.2FeBa0.2Si0.8O12 | 3.05 × 10−3 | 300 | [25] |
| Na5SmSi4O12 | 1.5 × 10−3 | 30 | [34] |
| Na5La0.1Y0.9Si4O12 | 4.3 × 10−4 | 25 | [22] |
| Na5Gd0.9Pr0.1Si4O12 | 1.64 × 10−3 | 25 | This work |
| Na5Gd0.9Nd0.1Si4O12 | 1.74 × 10−3 | 25 | This work |
| Applied Current Density (mA cm−2) | Expected Voltage (mV) | Observed Voltage (mV) | Overvoltage (mV) | Total Interfacial Resistance (Ω) | One Side Interfacial Resistance (Ω) | Area Specific Resistance (Ω cm2) |
|---|---|---|---|---|---|---|
| NGPS (0.1 mA cm−2) | 4.2 | 25 | 21 | 141.7 (before cycling) | 70.85 | 55.65 |
| 75.03 (after cycling) | 37.52 | 29.47 | ||||
| NGNS (0.1 mA cm−2) | 4.6 | 25 | 20 | 122.7 (before cycling) | 61.35 | 48.18 |
| 58.26 (after cycling) | 29.13 | 22.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivakumaran, A.; Butler, S.; Reid, S.; Thangadurai, V. Investigation of Pr3+ and Nd3+ Doping Effects on Sodium Gadolinium Silicate Ceramics as Fast Na+ Conductors. Batteries 2025, 11, 354. https://doi.org/10.3390/batteries11100354
Sivakumaran A, Butler S, Reid S, Thangadurai V. Investigation of Pr3+ and Nd3+ Doping Effects on Sodium Gadolinium Silicate Ceramics as Fast Na+ Conductors. Batteries. 2025; 11(10):354. https://doi.org/10.3390/batteries11100354
Chicago/Turabian StyleSivakumaran, Abinaya, Shantel Butler, Samuel Reid, and Venkataraman Thangadurai. 2025. "Investigation of Pr3+ and Nd3+ Doping Effects on Sodium Gadolinium Silicate Ceramics as Fast Na+ Conductors" Batteries 11, no. 10: 354. https://doi.org/10.3390/batteries11100354
APA StyleSivakumaran, A., Butler, S., Reid, S., & Thangadurai, V. (2025). Investigation of Pr3+ and Nd3+ Doping Effects on Sodium Gadolinium Silicate Ceramics as Fast Na+ Conductors. Batteries, 11(10), 354. https://doi.org/10.3390/batteries11100354








