Transition Metal-Based Polyoxometalates for Oxygen Electrode Bifunctional Electrocatalysis
Abstract
1. Introduction
2. Materials and Methods
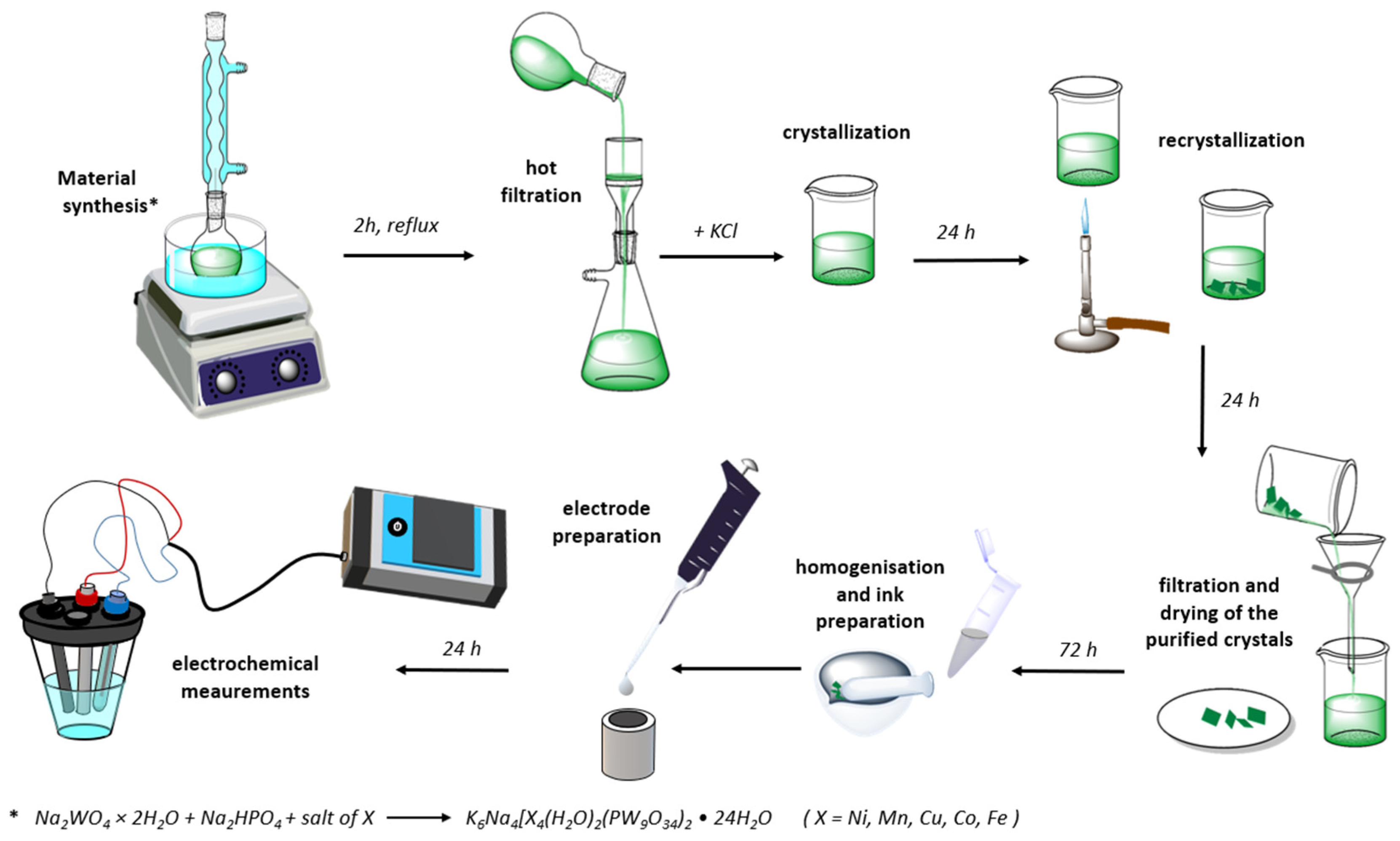
2.1. Synthesis and Characterization of Five POMs Electrocatalysts
2.2. Electrocatalysis with Transition Metal POMs
3. Results
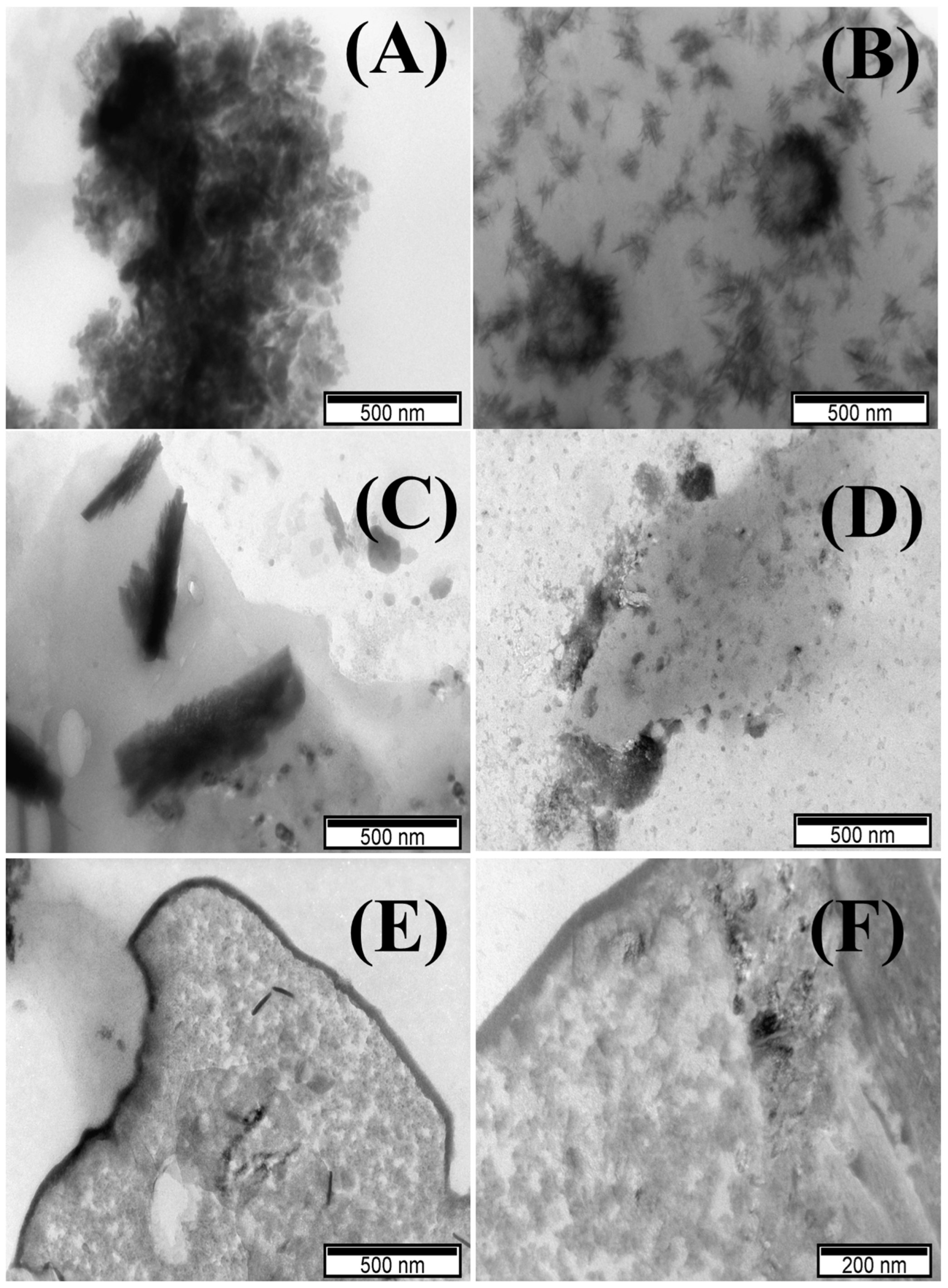
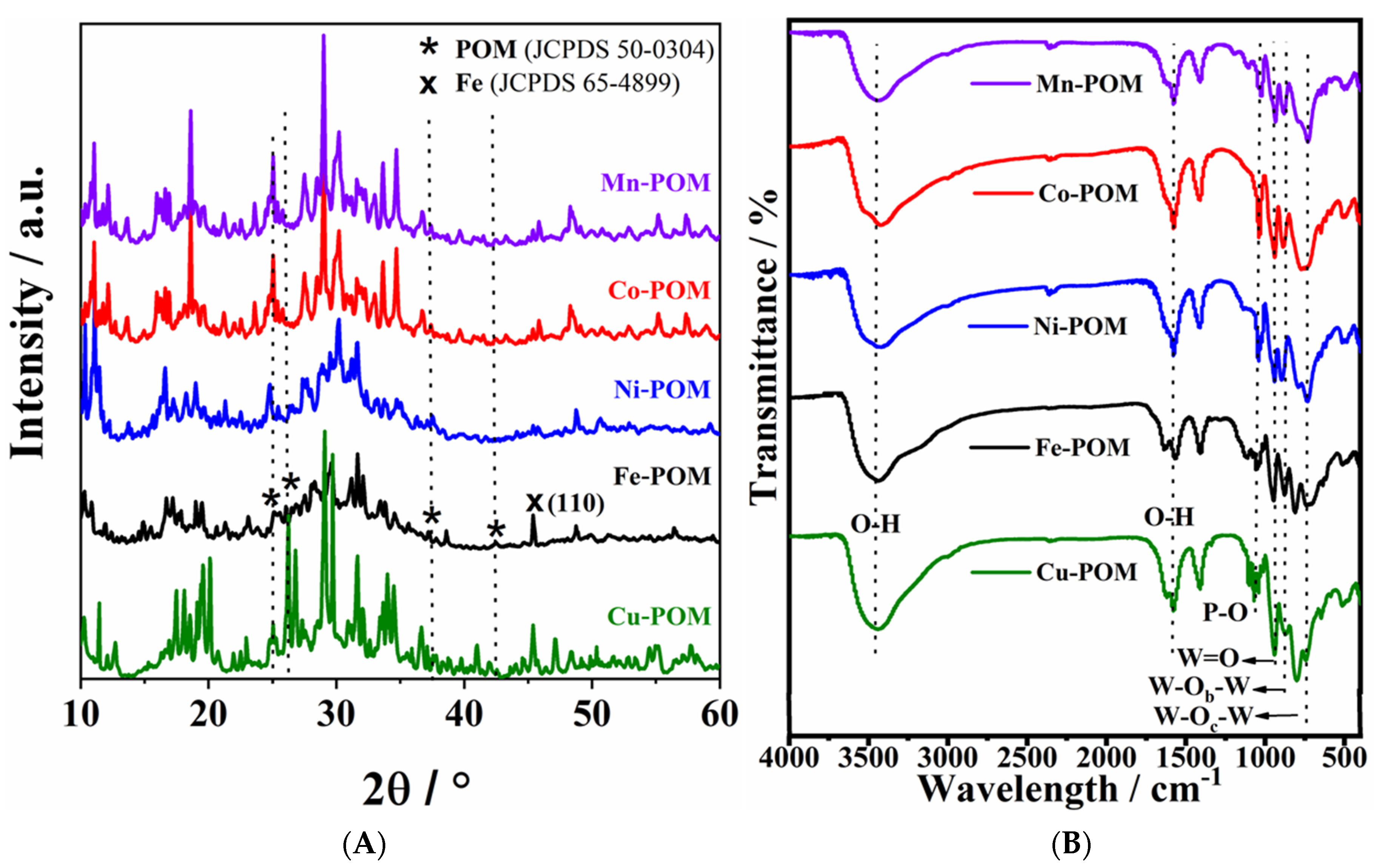
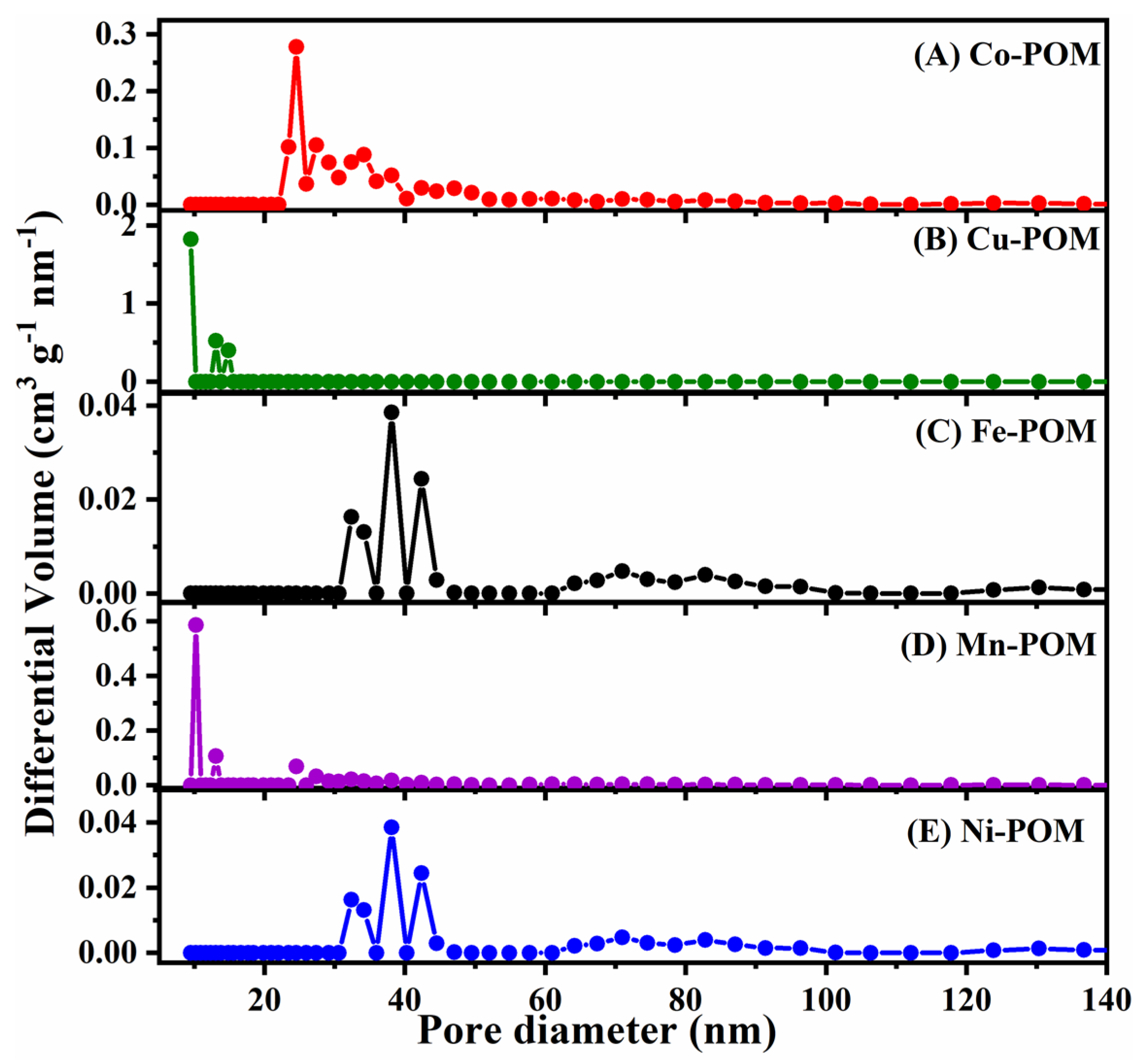
3.1. Characterization of TM-POMs
3.2. Catalysis of OER Using TM-POMs
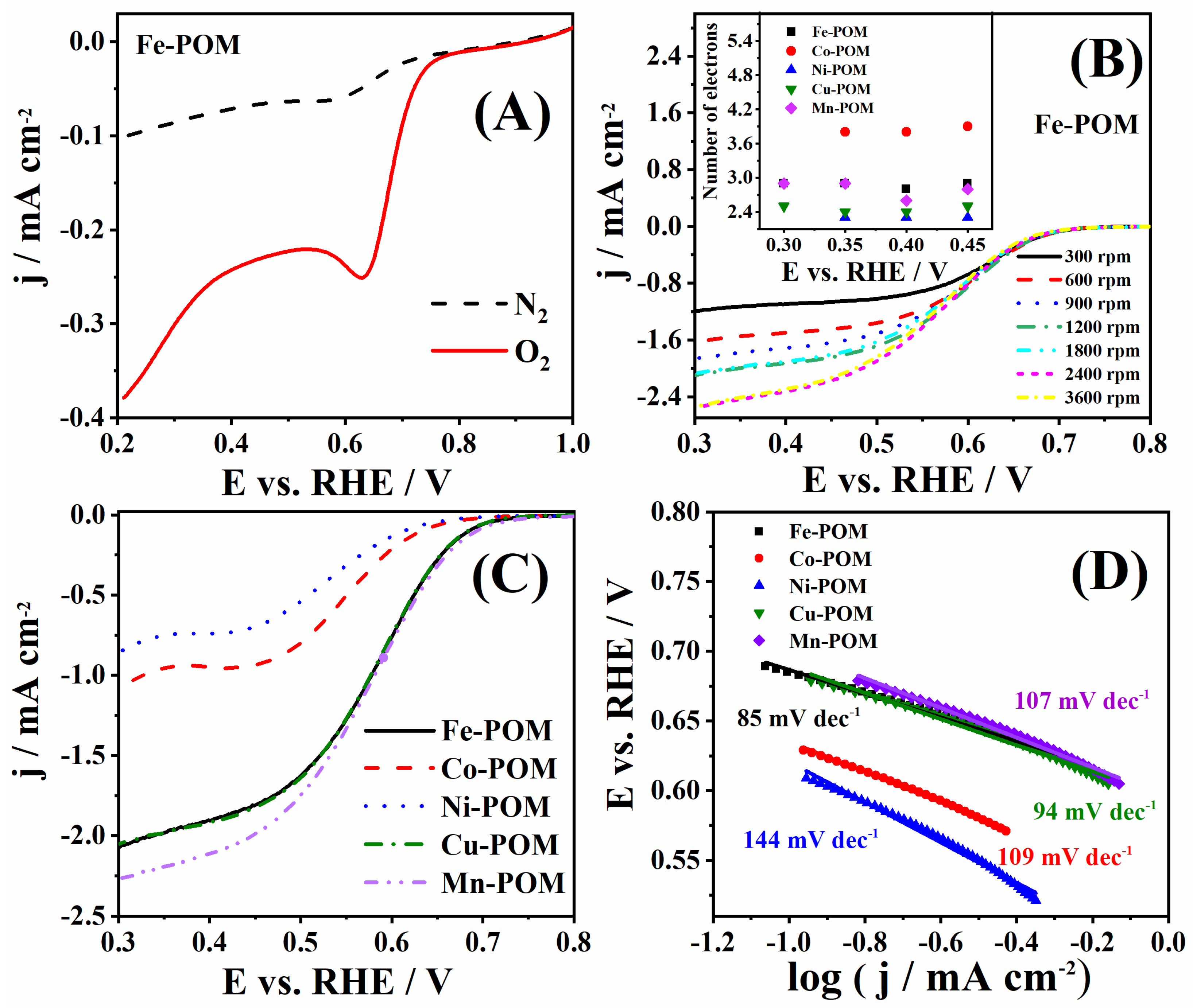
3.3. Catalysis of ORR Using TM-POMs
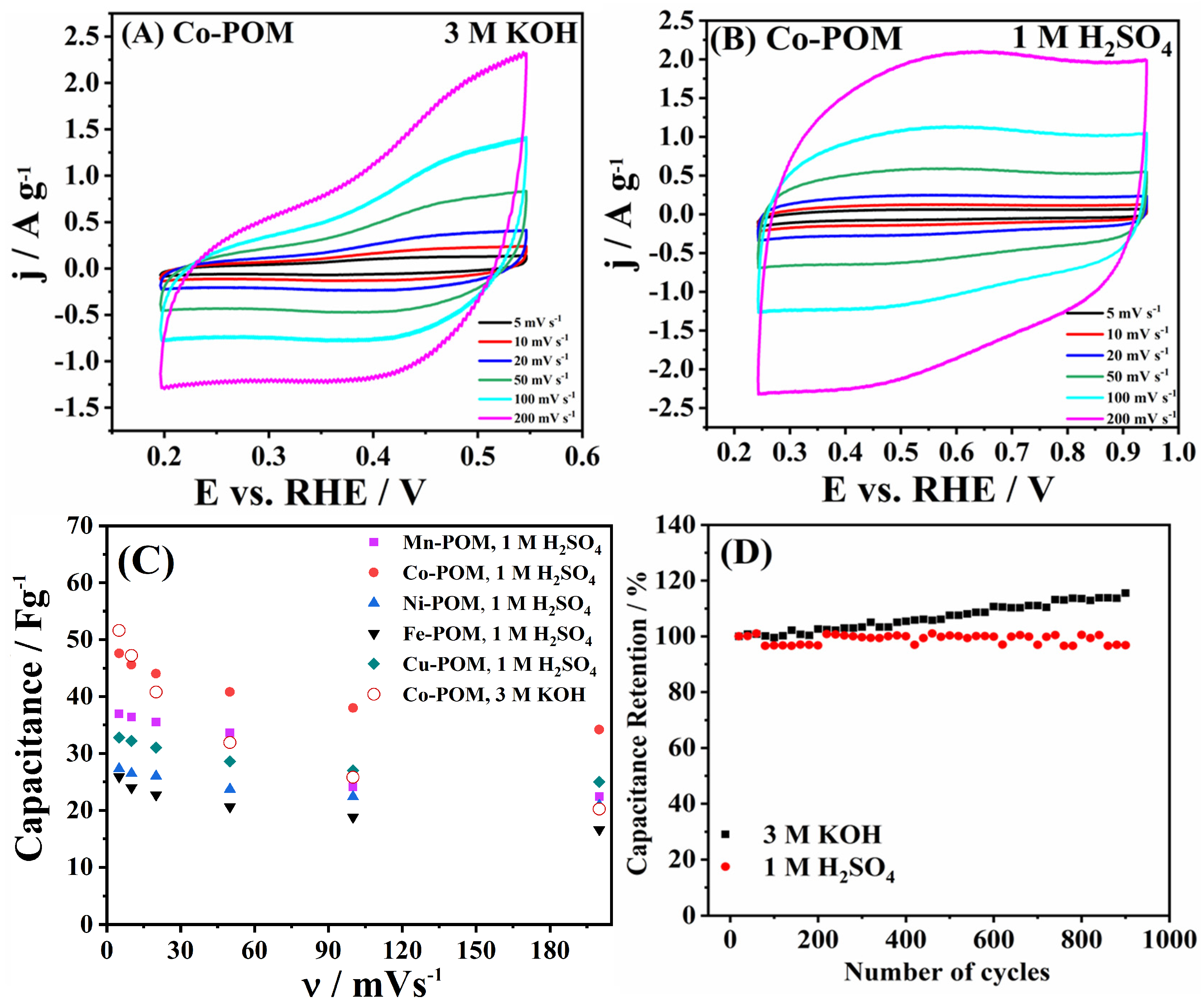
3.4. Capacitance and Impedance Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ling, W.; Wang, H.; Chen, Z.; Ji, Z.; Wang, J.; Wei, J.; Huang, Y. Intrinsic Structure Modification of Electrode Materials for Aqueous Metal-Ion and Metal-Air Batteries. Adv. Funct. Mater. 2021, 31, 2006855. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, Z.; Wang, E.; An, L.; Sun, G. Aqueous Metal-Air Batteries: Fundamentals and Applications. Energy Storage Mater. 2020, 27, 478–505. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Liu, B. Coordination Engineering of Single-Atom Catalysts for the Oxygen Reduction Reaction: A Review. Adv. Energy Mater. 2021, 11, 2002473. [Google Scholar] [CrossRef]
- Zhou, W.; Su, H.; Cheng, W.; Li, Y.; Jiang, J.; Liu, M.; Yu, F.; Wang, W.; Wei, S.; Liu, Q. Regulating the Scaling Relationship for High Catalytic Kinetics and Selectivity of the Oxygen Reduction Reaction. Nat. Commun. 2022, 13, 6414. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Du, L.; Yan, L.; Park, S.; Qiu, Y.; Sokolowski, J.; Wang, W.; Shao, Y. Oxygen Evolution Reaction in Alkaline Environment: Material Challenges and Solutions. Adv. Funct. Mater. 2022, 32, 2110036. [Google Scholar] [CrossRef]
- Chen, F.Y.; Wu, Z.Y.; Adler, Z.; Wang, H. Stability Challenges of Electrocatalytic Oxygen Evolution Reaction: From Mechanistic Understanding to Reactor Design. Joule 2021, 5, 1704–1731. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.; Dong, B.X.; Lan, Y.Q. Polyoxometalate-Based Compounds for Photo- and Electrocatalytic Applications. Angew. Chem.-Int. Ed. 2020, 59, 20779–20793. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, F.M.B.; Mladenović, D.; Radinović, K.; Santos, D.M.F.; Šljukić, B. Polyoxometalates as Electrocatalysts for Electrochemical Energy Conversion and Storage. Energies 2022, 15, 9021. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, X.; Chen, J.; Wang, Q. Surface O2− Regulation on POM Electrocatalyst to Achieve Accurate 2e/4e-ORR Control for H2O2 Production and Zn-Air Battery Assemble. Appl. Catal. B 2021, 285, 119788. [Google Scholar] [CrossRef]
- Song, Y.; Peng, Y.; Yao, S.; Zhang, P.; Wang, Y.; Gu, J.; Lu, T.; Zhang, Z. Co-POM@MOF-Derivatives with Trace Cobalt Content for Highly Efficient Oxygen Reduction. Chin. Chem. Lett. 2022, 33, 1047–1050. [Google Scholar] [CrossRef]
- Yin, D.; Wang, M.L.; Cao, Y.D.; Yang, X.; Ji, S.Y.; Hao, H.P.; Gao, G.G.; Fan, L.L.; Liu, H. Polyoxometalate@ZIF Induced CoWO4/WS2@C-N Nanoflower as a Highly Efficient Catalyst for Zn-Air Batteries. ACS Appl. Energy Mater. 2021, 4, 6892–6902. [Google Scholar] [CrossRef]
- Nagaiah, T.C.; Gupta, D.; Das Adhikary, S.; Kafle, A.; Mandal, D. Tuning Polyoxometalate Composites with Carbonaceous Materials towards Oxygen Bifunctional Activity. J. Mater. Chem. A Mater. 2021, 9, 9228–9237. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, Z.; Yao, K.; Xu, X.; Wang, Y. Hydrophobic POM Electrocatalyst Achieves Low Voltage “Charge” in Zn-Air Battery Coupled with Bisphenol A Degradation. Chemistry 2021, 27, 8774–8781. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yuan, L.; Wen, T.; Yu, J.; Xu, X. Enhanced Electrocatalytic Activity of POM-Derived CoMoS/FCP Heterostructures for Overall Water Splitting in Alkaline Media. Int. J. Electrochem. Sci. 2023, 18, 100076. [Google Scholar] [CrossRef]
- Gautam, J.; Kannan, K.; Meshesha, M.M.; Dahal, B.; Subedi, S.; Ni, L.; Wei, Y.; Yang, B.L. Heterostructure of Polyoxometalate/Zinc-Iron-Oxide Nanoplates as an Outstanding Bifunctional Electrocatalyst for the Hydrogen and Oxygen Evolution Reaction. J. Colloid. Interface Sci. 2022, 618, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yang, D.H.; Han, B.H. Application of Polyoxometalate Derivatives in Rechargeable Batteries. J. Mater. Chem. A Mater. 2020, 8, 4593–4628. [Google Scholar] [CrossRef]
- Singh, G.; Das Adhikary, S.; Mandal, D. Physico- and Electrochemical Properties of First-Row Transition-Metal-Substituted Sandwich Polyoxometalates. Inorg. Chem. 2023, 62, 8551–8564. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.; Yadollahi, B.; Omidyan, R. Theoretical Comparative Survey on the Structure and Electronic Properties of First Row Transition Metal Substituted Keggin Type Polyoxometalates. J. Solid. State Chem. 2022, 305, 122667. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, S.; Dong, X.; Ding, C.; Mu, Y.; Cui, M.; Hu, T.; Meng, C.; Zhang, Y. Anion Structure Regulation of Cobalt Silicate Hydroxide Endowing Boosted Oxygen Evolution Reaction. Small 2024. [Google Scholar] [CrossRef]
- Mu, Y.; Wang, T.; Zhang, J.; Meng, C.; Zhang, Y.; Kou, Z. Single-Atom Catalysts: Advances and Challenges in Metal-Support Interactions for Enhanced Electrocatalysis. Electrochem. Energy Rev. 2022, 5, 145–186. [Google Scholar] [CrossRef]
- Clemente-Juan, J.M.; Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.G. Increasing the Nuclearity of Magnetic Polyoxometalates. Syntheses, Structures, and Magnetic Properties of Salts of the Heteropoly Complexes [Ni3(H2O)3(PW10O39)H2O]7−, [Ni4(H2O)2(PW9O34)2]10−, and [Ni9(OH)3(H2O)6(HPO4)2(PW9O34)3]16−. Inorg. Chem. 1999, 38, 55–63. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X. Polyoxometalate Clusters: Sub-Nanometer Building Blocks for Construction of Advanced Materials. Matter 2020, 2, 816–841. [Google Scholar] [CrossRef]
- Lafuente, M.; Pellejero, I.; Clemente, A.; Urbiztondo, M.A.; Mallada, R.; Reinoso, S.; Pina, M.P.; Gandía, L.M. In Situ Synthesis of SERS-Active Au@POM Nanostructures in a Microfluidic Device for Real-Time Detection of Water Pollutants. ACS Appl. Mater. Interfaces 2020, 12, 36458–36467. [Google Scholar] [CrossRef] [PubMed]
- Yokuş, Ö.A.; Kardaş, F.; Akyildirim, O.; Eren, T.; Atar, N.; Yola, M.L. Sensitive Voltammetric Sensor Based on Polyoxometalate/Reduced Graphene Oxide Nanomaterial: Application to the Simultaneous Determination of l-Tyrosine and l-Tryptophan. Sens. Actuators B Chem. 2016, 233, 47–54. [Google Scholar] [CrossRef]
- Rajkumar, T.; Rao, G.R. Investigation of Hybrid Molecular Material Prepared by Ionic Liquid and Polyoxometalate Anion. J. Chem. Sci. 2008, 120, 587–594. [Google Scholar] [CrossRef]
- Karimi, Z.; Mahjoub, A.R.; Harati, S.M. Polyoxometalate-Based Hybrid Mesostructured Catalysts for Green Epoxidation of Olefins. Inorg. Chim. Acta 2011, 376, 1–9. [Google Scholar] [CrossRef]
- Cuentas-Gallegos, A.K.; Martínez-Rosales, R.; Rincón, M.E.; Hirata, G.A.; Orozco, G. Design of Hybrid Materials Based on Carbon Nanotubes and Polyoxometalates. Opt. Mater. 2006, 29, 126–133. [Google Scholar] [CrossRef]
- Wu, C.; Pei, Z.; Lv, M.; Huang, D.; Wang, Y.; Yuan, S. Polypyrrole-Coated Low-Crystallinity Iron Oxide Grown on Carbon Cloth Enabling Enhanced Electrochemical Supercapacitor Performance. Molecules 2023, 28, 434. [Google Scholar] [CrossRef] [PubMed]
- Tarlani, A.; Abedini, M.; Nemati, A.; Khabaz, M.; Amini, M.M. Immobilization of Keggin and Preyssler Tungsten Heteropolyacids on Various Functionalized Silica. J. Colloid. Interface Sci. 2006, 303, 32–38. [Google Scholar] [CrossRef]
- Singh, G.; Adhikary, S.D.; Mandal, D. Stabilization and Activation of Polyoxometalate over Poly(Vinyl Butylimidazolium) Cations towards Electrocatalytic Water Oxidation in Alkaline Media. Chem. Commun. 2023, 59, 4774–4777. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, D.; Biskupek, J.; Kaiser, U.; Liu, R.; Streb, C. Polyoxometalate-Assisted Synthesis of Amorphous Zeolitic Imidazolate for Efficient Electrocatalytic Oxygen Evolution. Results Chem. 2022, 4, 100568. [Google Scholar] [CrossRef]
- Shang, W.; Wang, Y.; Jiang, Y.; Wu, M.; Zeng, M.; Wang, P.; Qiu, L.; Jia, Z. Nanocomposite: Co4-Substituted Polyoxometalate@β-FeOOH as High-Performance Electrocatalysts for Oxygen Evolution Reaction in Alkaline Conditions. Appl. Catal. A Gen. 2022, 644, 118810. [Google Scholar] [CrossRef]
- Kang, Q.; Lai, D.; Su, M.; Xiong, B.; Tang, W.; Lu, Q.; Gao, F. Tailored Dodecahedral Polyoxometalates Nanoframes with in Situ Encapsulated Co, N, C for Oxygen Evolution Reaction. Chem. Eng. J. 2022, 430, 133116. [Google Scholar] [CrossRef]
- Marques, I.S.; Jarrais, B.; Mbomekallé, S.M.; Teillout, A.L.; De Oliveira, P.; Freire, C.; Fernandes, D.M. Synergetic Effects of Mixed-Metal Polyoxometalates@Carbon-Based Composites as Electrocatalysts for the Oxygen Reduction and the Oxygen Evolution Reactions. Catalysts 2022, 12, 440. [Google Scholar] [CrossRef]
- Mladenović, D.; Daş, E.; Santos, D.M.F.; Yurtcan, A.B.; Miljanić, Š.; Šljukić, B. Boosting Oxygen Electrode Kinetics by Addition of Cost-Effective Transition Metals (Ni, Fe, Cu) to Platinum on Graphene Nanoplatelets. J. Alloys Compd. 2022, 905, 164156. [Google Scholar] [CrossRef]
- Milikić, J.; Stojanović, S.; Damjanović-Vasilić, L.; Vasilić, R.; Šljukić, B. Efficient Bifunctional Cerium-Zeolite Electrocatalysts for Oxygen Evolution and Oxygen Reduction Reactions in Alkaline Media. Synth. Met. 2023, 292, 117231. [Google Scholar] [CrossRef]
- Milikić, J.; Knežević, S.; Stojadinović, S.; Alsaiari, M.; Harraz, F.A.; Santos, D.M.F.; Šljukić, B. Facile Synthesis of Low-Cost Copper-Silver and Cobalt-Silver Alloy Nanoparticles on Reduced Graphene Oxide as Efficient Electrocatalysts for Oxygen Reduction Reaction in Alkaline Media. Nanomaterials 2022, 12, 2657. [Google Scholar] [CrossRef] [PubMed]
- Milikić, J.; Knežević, S.; Ognjanović, M.; Stanković, D.; Rakočević, L.; Šljukić, B. Template-Based Synthesis of Co3O4 and Co3O4/SnO2 Bifunctional Catalysts with Enhanced Electrocatalytic Properties for Reversible Oxygen Evolution and Reduction Reaction. Int. J. Hydrogen Energy 2023, 48, 27568–27581. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Novais, H.C.; Bacsa, R.; Serp, P.; Bachiller-Baeza, B.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Freire, C. Polyoxotungstate@Carbon Nanocomposites As Oxygen Reduction Reaction (ORR) Electrocatalysts. Langmuir 2018, 34, 6376–6387. [Google Scholar] [CrossRef]
- Liu, R.; Cao, K.; Clark, A.H.; Lu, P.; Anjass, M.; Biskupek, J.; Kaiser, U.; Zhang, G.; Streb, C. Top-down Synthesis of Polyoxometalate-like Sub-Nanometer Molybdenum-Oxo Clusters as High-Performance Electrocatalysts. Chem. Sci. 2020, 11, 1043–1051. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Heydari-Soureshjani, E.; Rezaei, B. [PW11MO39]5− Decorated on Ru-Reduced Graphene Oxide Nanosheets, Characterizations and Application as a High Performance Storage Energy and Oxygen Reduction Reaction. Chem. Eng. J. 2017, 330, 1109–1118. [Google Scholar] [CrossRef]
- Gezović, A.; Mišurović, J.; Milovanović, B.; Etinski, M.; Krstić, J.; Grudić, V.; Dominko, R.; Mentus, S.; Vujković, M.J. High Al-Ion Storage of Vine Shoots-Derived Activated Carbon: New Concept for Affordable and Sustainable Supercapacitors. J. Power Sources 2022, 538, 231561. [Google Scholar] [CrossRef]
- Gandara, M.; Mladenović, D.; Oliveira Martins, M.d.J.; Rakocevic, L.; Kruszynski de Assis, J.M.; Šljukić, B.; Sarmento Gonçalves, E. MAX Phase (Nb4AlC3) For Electrocatalysis Applications. Small 2024. [Google Scholar] [CrossRef] [PubMed]
- Milikić, J.; Nastasić, A.; Rakočević, L.; Radinović, K.; Stojadinović, S.; Stanković, D.; Šljukić, B. FeM/RGO (M = Ni and Cu) as Bifunctional Oxygen Electrode. Fuel 2024, 368, 131654. [Google Scholar] [CrossRef]

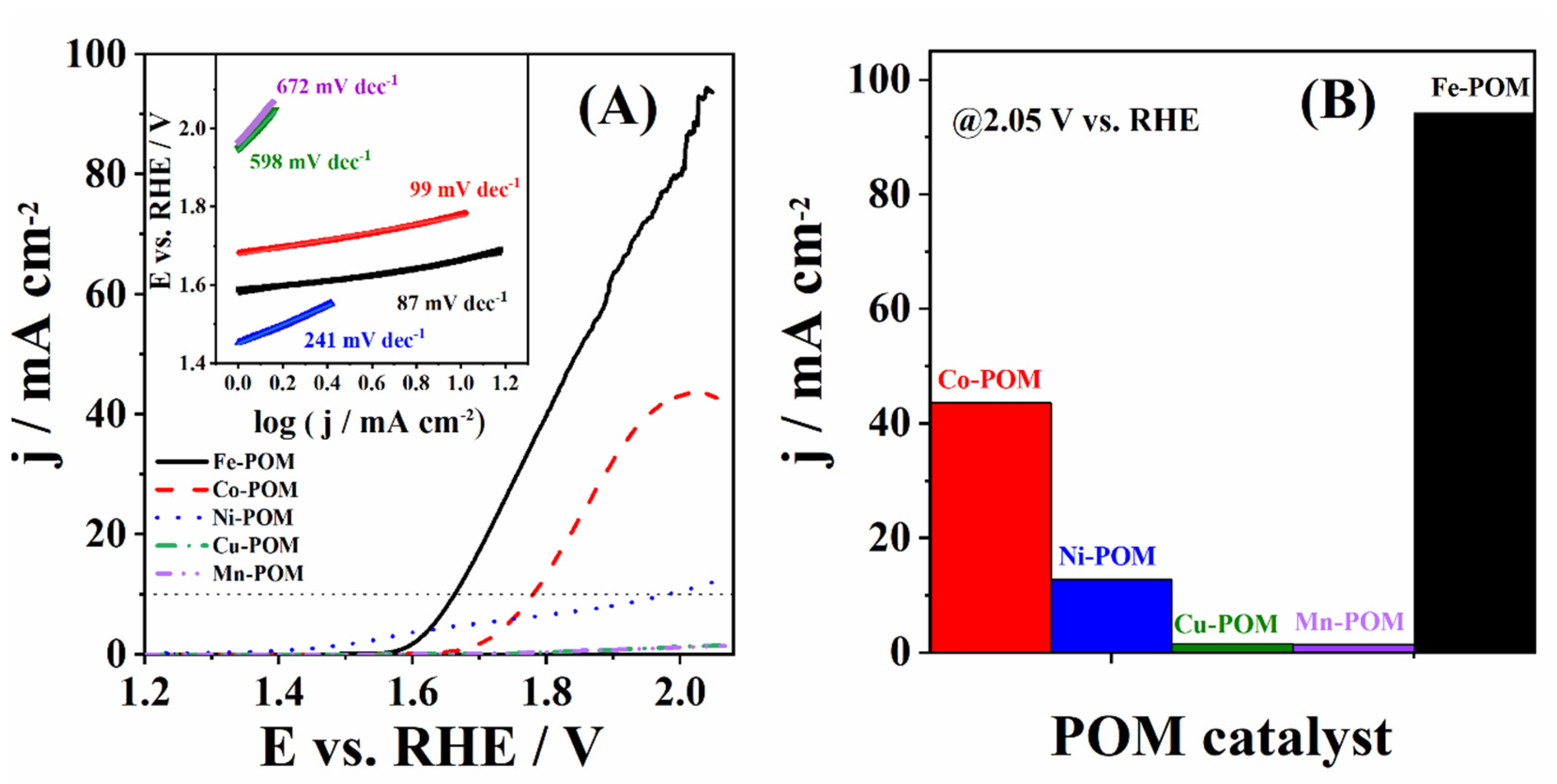

| Catalyst | Eonset/V | η10/mV | j400/mA cm−2 | b/mV dec−1 | Source |
|---|---|---|---|---|---|
| Fe-POM | 1.588 | 434 | 4.78 | 87 | This work |
| Co-POM | 1.683 | 551 | 0.14 | 99 | This work |
| Ni-POM | 1.453 | 752 | 4.13 | 241 | This work |
| Cu-POM | 1.948 | – | 0.04 | 598 | This work |
| Mn-POM | 1.966 | – | 0.03 | 672 | This work |
| ([PVIM][V-Co4]) | – | 430 | – | – | [30] |
| PNiW11@amZIF | – | 375 | – | 69 | [31] |
| PW12@amZIF | – | 423 | – | 86 | [31] |
| PW12@FeOOH-P | – | 230 | – | 79 | [32] |
| PMo12@FeOOH-P | – | 235 | – | 123 | [32] |
| SiW12@FeOOH-P | – | 260 | – | 126 | [32] |
| FeOOH-P | – | 290 | – | 202 | [32] |
| CNCP-0 | – | 310 | – | 105.8 | [33] |
| CNCP-1 | – | 293 | – | 88.2 | [33] |
| CNCP-12 | – | 269 | – | 79.5 | [33] |
| IrO2 | – | – | – | 90.2 | [33] |
| Fe4@MWCNT_N6 | – | 580 | – | 102 | [34] |
| NiP4Mo6 | – | 250 | – | 73 | [9] |
| Electrocatalysts | Eonset/V | E1/2/V | Epp/V | b/mV dec−1 | n | Source |
|---|---|---|---|---|---|---|
| Fe-POM | 0.695 | 0.580 | 0.632 | 85 | 2.8–2.9 | This work |
| Co-POM | 0.655 | 0.552 | 0.558 | 109 | 3.8–3.9 | This work |
| Ni-POM | 0.639 | 0.533 | 0.494 | 144 | 2.3 | This work |
| Cu-POM | 0.695 | 0.580 | 0.529 | 94 | 2.4–2.5 | This work |
| Mn-POM | 0.694 | 0.556 | 0.622 | 107 | 2.6–2.9 | This work |
| NiP4Mo6 | – | – | – | 106 | 3.80–3.85 | [9] |
| S-NiP4Mo6 | – | – | – | 98 | 2.08–2.15 | [9] |
| Co4(PW9)2@N-CNT | 0.90 | – | – | 89 | 2.73 | [39] |
| [Mo-oxo]n clusters | 0.75 | – | 0.62 | 109 | ~1.5 | [40] |
| Fe4@MWCNT_N6 | 0.81 | – | – | 35.4 | 2.9 | [34] |
| Ni4@MWCNT_N6 | 0.80 | – | – | 34.7 | 2.7 | [34] |
| Fe2Ni2@MWCNT_N6 | 0.80 | – | – | 37.9 | 3.2 | [34] |
| [PW11MO39]5@Ru-rGO (M = Co, Ni, Cu) | – | – | – | – | 3.9–4.2 | [41] |
| Electrocatalyst | Rs/Ω | Re/Ω | Rct/Ω | Qe/mF | Qdl/mF |
|---|---|---|---|---|---|
| Fe-POM | 5.27 | 0.15 | 3.35 | 3.20 × 10−3 | 7.40 × 10−4 |
| Co-POM | 50.49 | 1.36 | 15.04 | 3.25 × 10−4 | 1.93 × 10−2 |
| Ni-POM | 184.80 | 44.78 | 137.00 | 8.08 × 10−4 | 1.94 × 10−4 |
| Cu-POM | 31.49 | 22.08 | 67.28 | 4.72 × 10−4 | 6.57 × 10−4 |
| Mn-POM | 9.25 | 2.68 | 239.00 | 8.22 × 10−2 | 1.65 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milikić, J.; Gusmão, F.; Knežević, S.; Gavrilov, N.; Paul, A.; Santos, D.M.F.; Šljukić, B. Transition Metal-Based Polyoxometalates for Oxygen Electrode Bifunctional Electrocatalysis. Batteries 2024, 10, 197. https://doi.org/10.3390/batteries10060197
Milikić J, Gusmão F, Knežević S, Gavrilov N, Paul A, Santos DMF, Šljukić B. Transition Metal-Based Polyoxometalates for Oxygen Electrode Bifunctional Electrocatalysis. Batteries. 2024; 10(6):197. https://doi.org/10.3390/batteries10060197
Chicago/Turabian StyleMilikić, Jadranka, Filipe Gusmão, Sara Knežević, Nemanja Gavrilov, Anup Paul, Diogo M. F. Santos, and Biljana Šljukić. 2024. "Transition Metal-Based Polyoxometalates for Oxygen Electrode Bifunctional Electrocatalysis" Batteries 10, no. 6: 197. https://doi.org/10.3390/batteries10060197
APA StyleMilikić, J., Gusmão, F., Knežević, S., Gavrilov, N., Paul, A., Santos, D. M. F., & Šljukić, B. (2024). Transition Metal-Based Polyoxometalates for Oxygen Electrode Bifunctional Electrocatalysis. Batteries, 10(6), 197. https://doi.org/10.3390/batteries10060197









