1. Introduction
Lithium-ion batteries (LIBs) are widely used in portable electronic devices, electric vehicles, energy storage systems, and other fields. However, during their usage, especially under abuse conditions such as high temperatures, overcharging, over-discharging, and mechanical damage, there is a risk of thermal runaway (TR) [
1,
2,
3]. This refers to the potential scenario where a lithium-ion battery (LIB) may experience a violent exothermic reaction internally, leading to a loss of control and, in severe cases, causing fires or explosions. Therefore, the study of TR characteristics and gas generation in LIBs holds significant background and importance [
4,
5]. It is conducive to formulating more scientifically sound safety designs and usage strategies, thereby enhancing the safety performance of LIBs [
6,
7].
Firstly, the TR of LIBs poses a potential threat to the environment. Large-scale applications of LIBs in electric vehicles and energy storage systems may lead to significant environmental and ecological impacts in the event of a TR incident [
8]. This could result in air and water pollution, presenting a potential threat to ecosystems and human health. Therefore, conducting in-depth research on the characteristics and gas generation of tTR in LIBs is beneficial for mitigating potential harm to the environment and promoting the development of sustainable energy storage technologies.
Moreover, research on the TR of LIBs offers valuable insights for addressing the challenges of energy transition and sustainable development. LIBs, as a critical energy storage technology, find widespread applications in electric vehicles and renewable energy systems and play a pivotal role in driving the adoption of clean energy. However, the safety and reliability of LIBs are essential considerations for achieving a sustainable energy transition. Investigating the TR issues associated with LIBs can help mitigate potential safety risks, providing more reliable and secure solutions for the widespread adoption of LIBs in sustainable energy systems [
9].
The triggering conditions for battery TR primarily involve three aspects: electrical abuse, thermal abuse, and mechanical abuse [
10]. These three aspects share a common TR trigger: an internal short circuit, which is also a primary cause of battery failure. During TR, batteries experience a phenomenon known as capacity degradation before internal heat generation, referred to as high-temperature self-discharge [
11], following this, the initial heat generation occurs due to the decomposition of the Solid Electrolyte Interphase (SEI) [
12]; as a result of SEI decomposition, the negative electrode comes into direct contact with the electrolyte, initiating a reaction and forming an irregular SEI [
13], this leads to a continuous reaction between the negative electrode and the electrolyte, causing the internal temperature of the battery to rise until it reaches the melting point of the separator [
14], the melting of the separator induces an endothermic reaction, slowing down or even reducing the rate of temperature increase within the battery. Following the melting of the separator, an internal short circuit occurs, leading to jelly roll failure and the release of a significant amount of heat. At this point, various chemical reactions occur simultaneously, such as positive electrode decomposition and electrolyte decomposition. The electrolyte decomposition generates a substantial amount of gas [
15], resulting in an increase in internal gas pressure. The battery swells, and upon reaching a certain threshold, the safety valve opens, releasing high-pressure gas [
16], the expelled gas contains combustible components. In the presence of oxygen in the environment and encountering an ignition source, it can lead to a severe combustion reaction [
17]. In recent research, it has been discovered that TR can occur even in the absence of internal short circuits. The positively charged electrode material undergoes a phase transition at around 250 °C, releasing reactive oxygen species that react with the negative electrode [
18,
19]. Therefore, the significant heat generated by the oxidation–reduction reactions of the positive and negative electrodes could potentially be a direct cause of TR, not solely due to internal short circuits [
20,
21].
In summary, the research background and significance of TR in LIBs primarily lie in enhancing the safety of LIBs, reducing potential environmental hazards, advancing battery technology, and promoting sustainable energy transition. An in-depth study of the mechanisms and patterns of TR in LIBs can provide a scientific basis for battery design, production, usage, and management [
22]. This, in turn, enables the optimal utilization of LIBs as a crucial energy storage technology, expanding their applications across various domains and facilitating the realization of sustainable energy and sustainable development goals.
Feng X. et al. provided an overview of the mechanisms of TR in LIBs used in electric vehicles, summarizing the abusive conditions that may lead to TR [
23]. N.E. Galushkin analyzed the mechanism of TR in LIBs and found that the cause of TR in LIBs is the strong exothermic reaction of atomic hydrogen accumulated in the anode graphite during the battery cycling process [
24]. Shen H. et al. compared the TR characteristics of LIBs with different cathode materials and analyzed their gas generation characteristics. They found that the gas composition of high-nickel LiNi
0.9Co
0.05Mn
0.05O
2 batteries is the most hazardous compared to other systems [
25]. JH Kim compared the TR characteristics between NCM and LFP batteries and found that, compared to NCM, LFP batteries exhibit a delayed time to reach the maximum temperature during TR due to their higher thermal stability [
26]. Sascha K. and his team conducted TR experiments on 51 batteries in a gas-tight container and analyzed their mass loss characteristics as well as gas compositions [
27]. Yuan L. et al. conducted multiple TR temperature and gas generation characteristic tests on four batteries using ARC and found that the main gas concentrations in the emitted gases depend mainly on the chemical properties of the battery [
28]. Zhang Q. et al. analyzed the effect of different state-of-charges (SOCs) on the gas composition generated by the battery and found that batteries at 100% (state-of-charge) SOC generate a large amount of unsaturated hydrocarbons, which pose a higher thermal hazard [
29].
High-nickel layered ternary materials, as positive electrode materials for lithium-ion batteries possess numerous advantages, such as high energy density, excellent cycling stability, outstanding power performance, high resource utilization efficiency, and environmental friendliness. For batteries, the typical hazards resulting from TR are often associated with the nickel content in the positive electrode material [
30,
31] and the initial SOC [
32], pouch cells and cylindrical cells without safety valves pose increased risks in the event of TR [
33]. A higher Ni content leads to an increase in capacity, as Ni serves as the primary redox material in the core structure; the thermal stability of Li[Ni
1−x−yCo
xMn
y]O
2 is directly related to the Ni content; Mn ensures excellent cycle life and safety and Co increases electronic conductivity [
31]. For the same positive electrode material at different SOCs, as the SOC increases, the onset temperature of self-heating reactions shifts towards lower temperatures. Additionally, the self-heating rate exponentially increases, especially beyond 50% SOC, significantly affecting the thermal instability [
34].
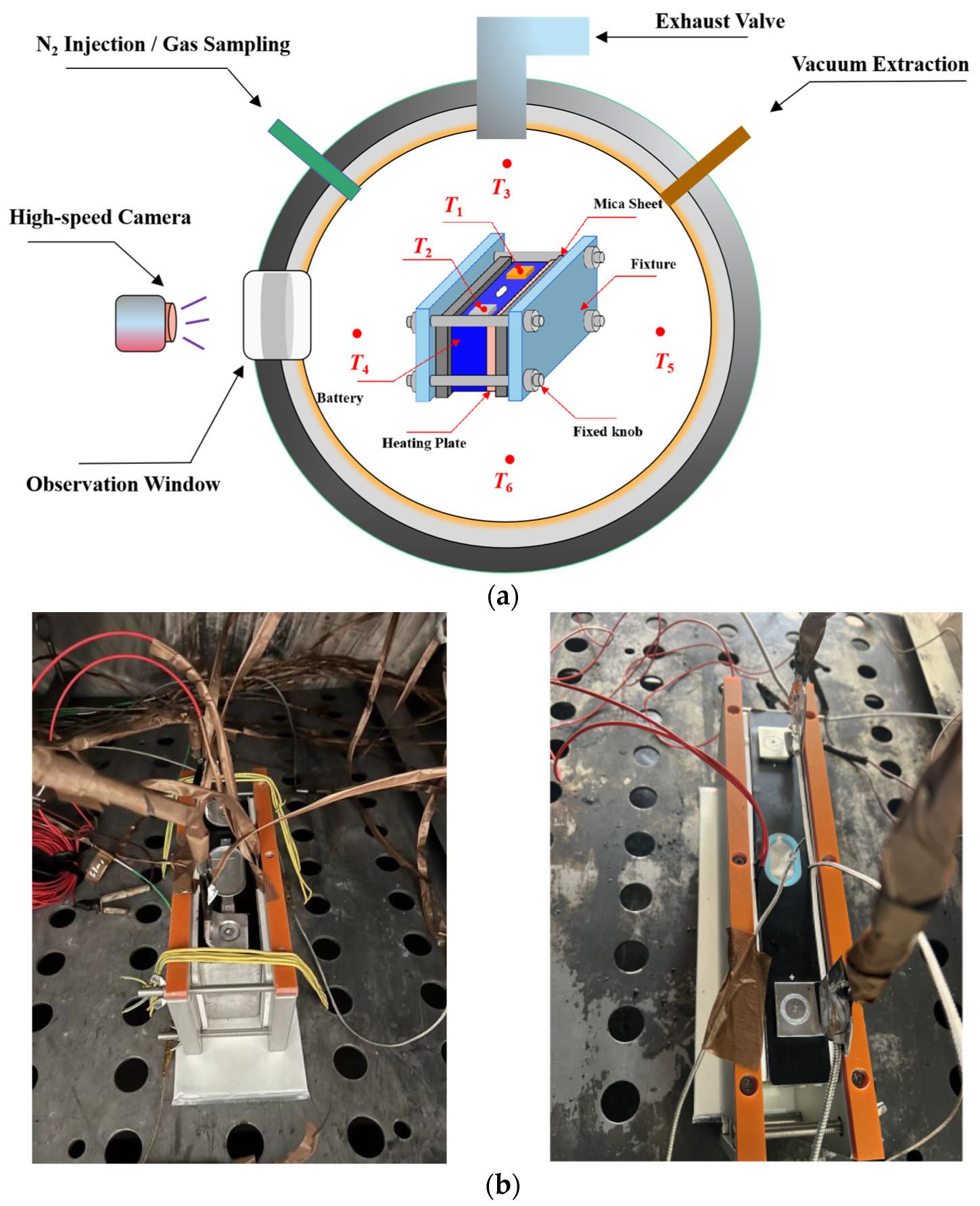
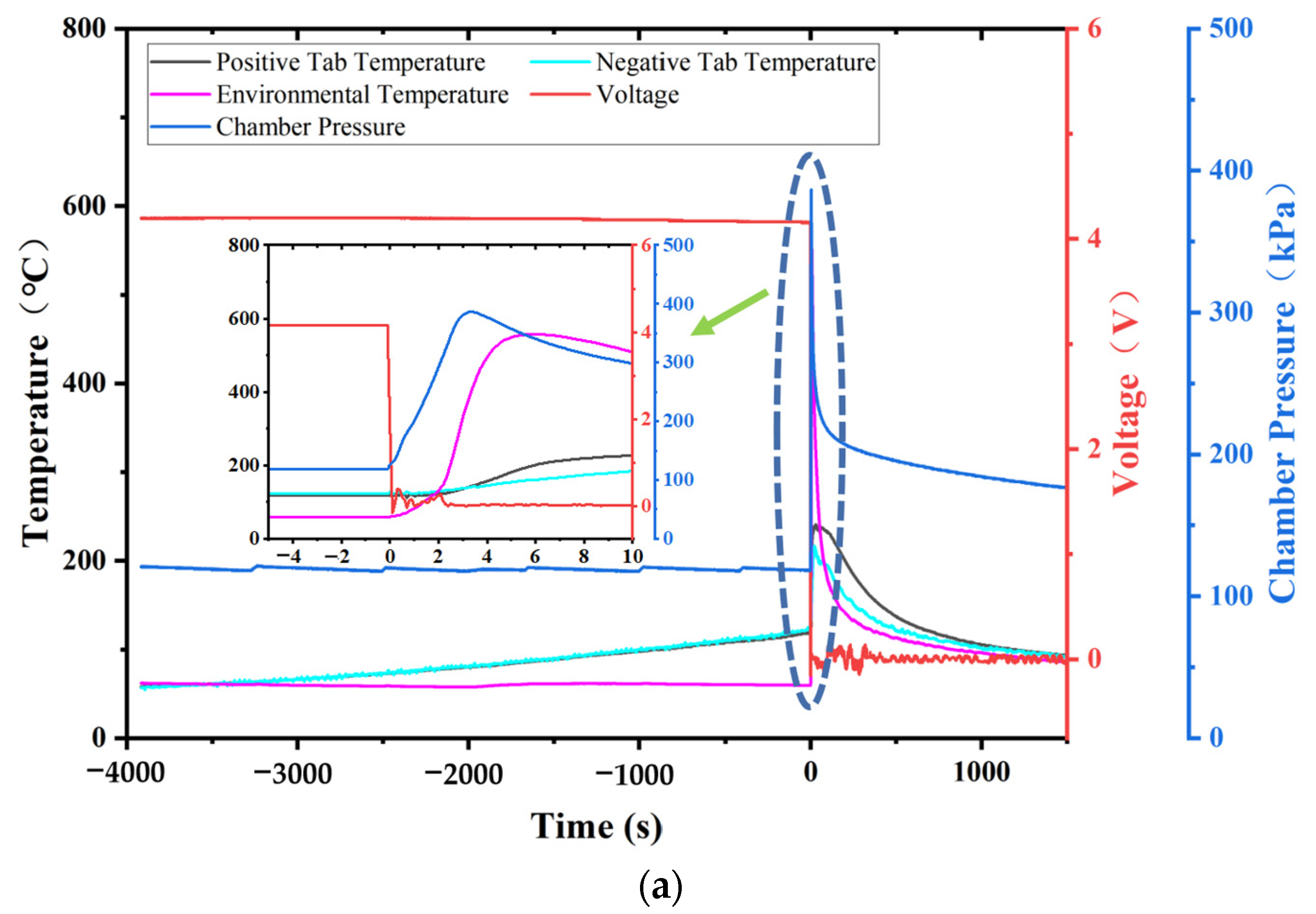
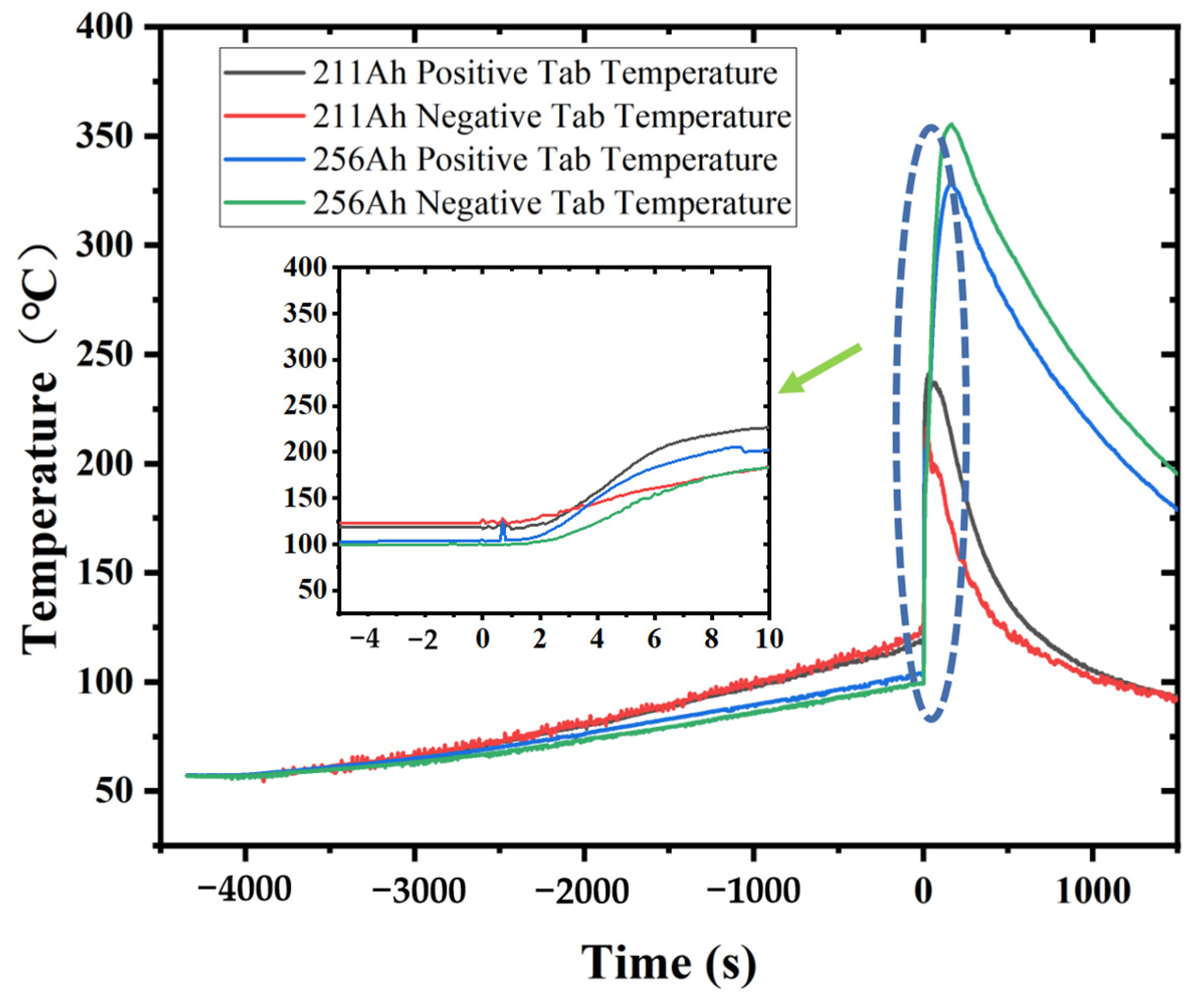
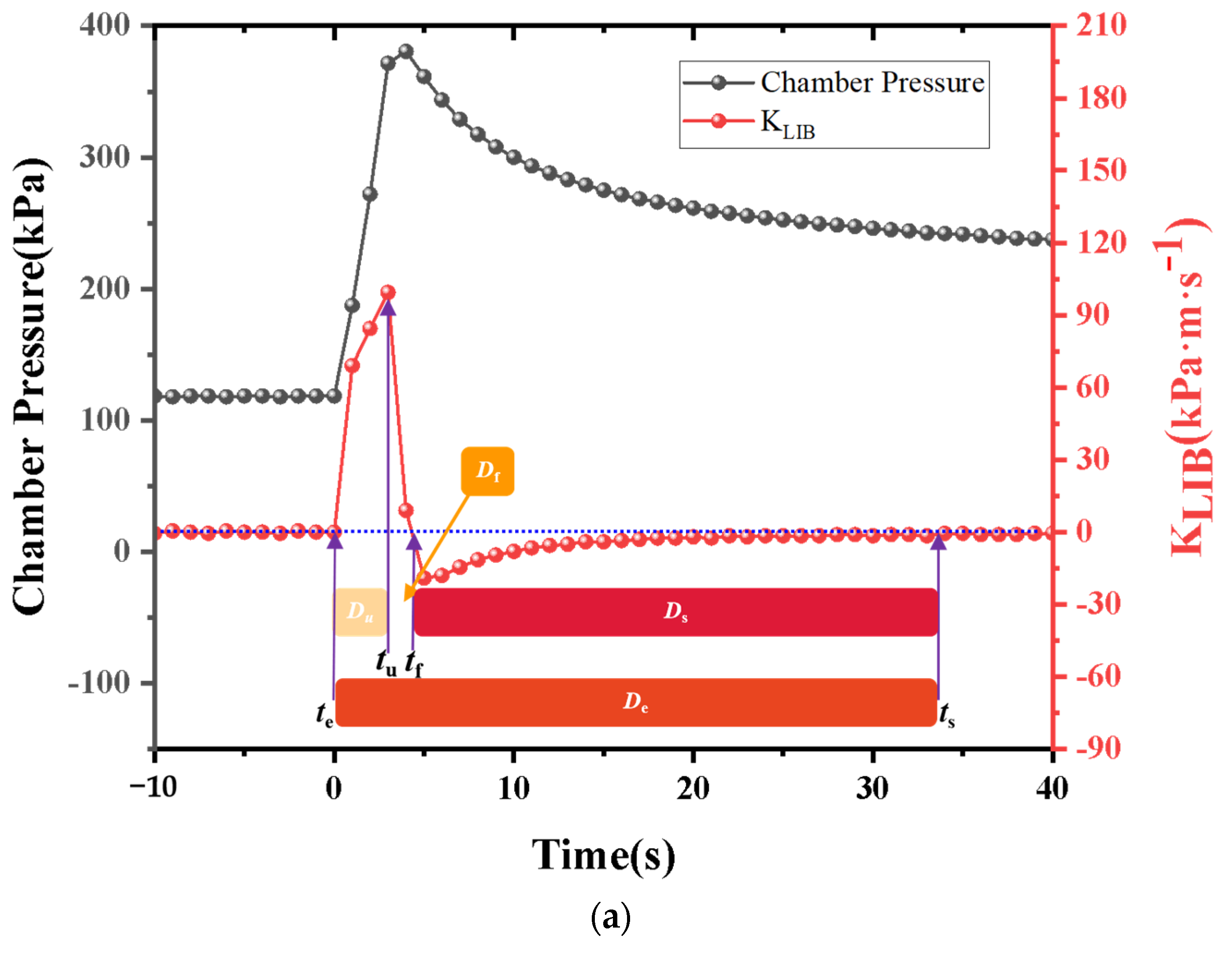
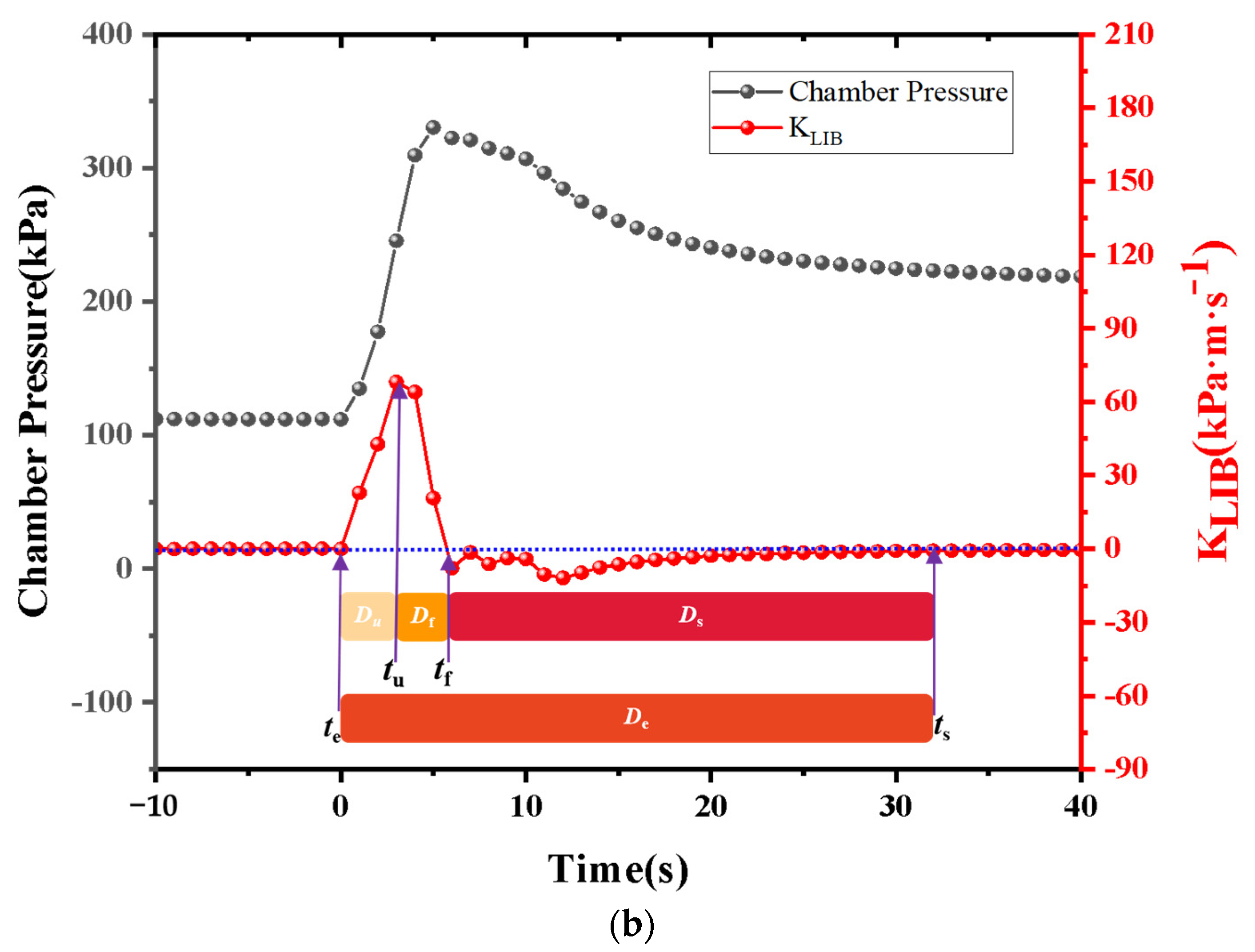
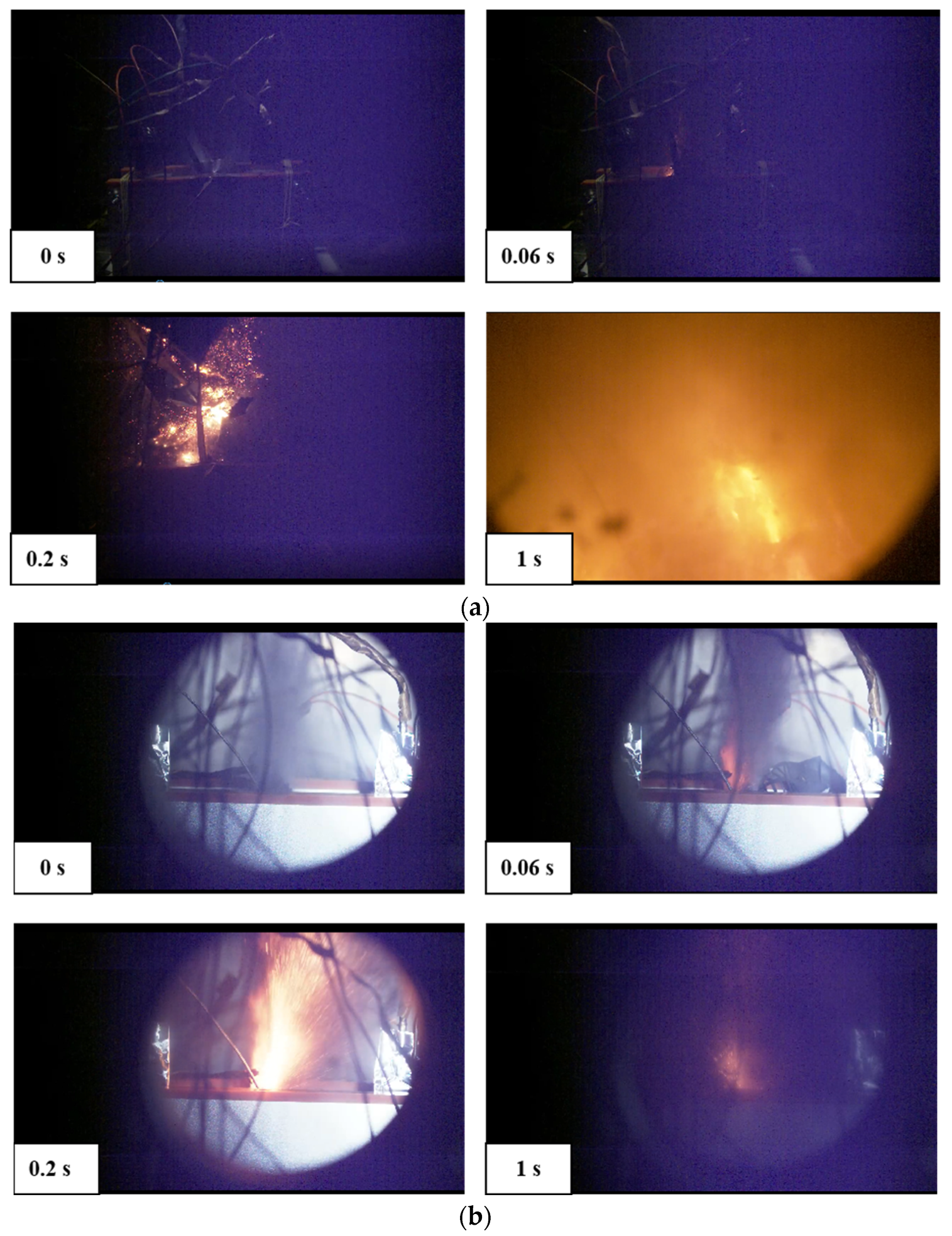
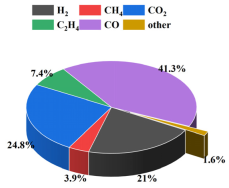
Currently, most research focuses on small-capacity batteries like the 18650 and batteries with lower energy density. There is relatively less research on high-nickel NCM 9-series and high-capacity batteries. Quantitative analysis of battery venting processes remains unclear. The research primarily concentrates on comparative analyses of TR characteristics among various battery systems or comparative analyses of different TR triggering mechanisms. This study involved two different high-nickel NCM9 0.5 0.5 lithium-ion batteries with varying capacities and energy densities. In a sealed chamber, TR was induced through lateral heating within an inert gas environment. The study monitored temperature changes at the electrode tabs during the TR process, along with variations in battery voltage and chamber pressure. High-speed cameras were used to record the TR phenomenon. It was observed that the critical temperature for TR at the electrode tabs was roughly the same for both batteries. However, the higher energy density sample exhibited significantly more intense venting during the TR process. The study employed a quantitative analysis of venting processes to compare the venting characteristics during the TR of the two batteries. Both batteries exhibited similar venting durations, but the higher energy density battery showed significantly higher fluctuations in its venting curve. Finally, the gases vented during the TR were collected and subjected to gas composition analysis.